
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kazuya Yoshida | -- | 8851 | 2022-05-28 01:29:00 | | | |
| 2 | Beatrix Zheng | -2749 word(s) | 6102 | 2022-05-30 03:16:13 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 6102 | 2022-05-30 04:29:56 | | | | |
| 4 | Beatrix Zheng | -8 word(s) | 6094 | 2022-05-30 04:54:21 | | | | |
| 5 | Beatrix Zheng | + 2757 word(s) | 8851 | 2022-06-08 08:40:34 | | | | |
| 6 | Beatrix Zheng | -2742 word(s) | 6109 | 2022-06-08 08:58:21 | | | | |
| 7 | Beatrix Zheng | Meta information modification | 6109 | 2022-06-17 08:17:52 | | | | |
| 8 | Beatrix Zheng | Meta information modification | 6109 | 2022-06-17 08:23:38 | | | | |
| 9 | Beatrix Zheng | Meta information modification | 6109 | 2022-06-17 08:27:14 | | | | |
| 10 | Beatrix Zheng | Meta information modification | 6112 | 2022-06-23 03:15:59 | | | | |
| 11 | Beatrix Zheng | -1 word(s) | 6111 | 2022-06-23 08:18:02 | | |
Video Upload Options
Botulinum neurotoxin injection is effective and safe, with few side effects in most cases when properly administered by an experienced clinician. The diagnosis and treatment of oromandibular dystonia require both neurological and dental or oral surgical knowledge and skills, and well-designed multicenter trials with a multidisciplinary team approach must be necessary to ensure accurate diagnosis and proper treatment.
1. Introduction
Botulinum neurotoxin (BoNT) is the exotoxin of Clostridium botulinum, a gram-positive, spore-forming bacterium. Kerner was the first to describe the symptoms of botulism in detail [1]. Van Ermengem isolated the microorganism Bacillus botulinus [2]. In 1979, Scott was the first to use BoNT therapeutically for strabismus via injection to the extraocular muscles [3].
The clinical applications of BoNT have since expanded to treat ophthalmic, gastrointestinal, urological, orthopedic, dermatological, dental, secretory, painful, cosmetic, and other diseases, and applications to the orofacial region have gained particular attention [4][5][6][7][8][9]. Target orofacial disorders include oromandibular dystonia (OMD) [10][11][12][13][14][15][16][17].
2. The Clinical Problem: History, Presentation, and Epidemiology
2.1. OMD
Dystonia is a hyperkinetic movement disorder characterized by sustained or intermittent muscle contractions that result in abnormal repetitive movements and/or postures [18]. Dystonia is categorized along two axes: (1) clinical characteristics, including the age at onset, body distribution (focal, multifocal, segmental, hemidystonia, generalized), temporal pattern and associated features (additional movement disorders or neurological features); and (2) etiology: inherited, acquired, and secondary [18]. The term "dystonia" was coined in 1911 by Oppenheim [19]. Before the concept of "dystonia" was published, Romberg described various cases of masticatory muscle spasms due to a variety of pathogenesis in his textbook [20]. Among the cases, a few can be diagnosed as OMD, representing the first reports of this condition. The term “oromandibular dystonia” was first introduced by Marsden in 1976 [21]. OMD is a focal type of dystonia characterized by contractions of the masticatory, lingual, pharyngeal, and/or muscles of the stomatognathic system [16][17][22][23][24][25][26][27][28][29][30][31].
2.2. Presentation
OMD symptoms include masticatory disturbances, biting of the tongue or cheek membrane, limited mouth opening, pain or discomfort of the muscles, dysphagia, dysarthria, esthetic problems, upper airway obstructions [15], and temporomandibular joint dislocations [32]. Most of these symptoms can impair daily activities, social embarrassment, cosmetic disfigurement, inability to work, and unemployment, forming a significant impact on the overall quality of life of the patient [22][23][29][30][31]. Based on the site and direction of abnormal dystonic movements, OMD is classified into six subtypes: jaw closing, jaw opening, lingual, jaw deviation, jaw protrusion, and lip dystonia [22][23][29][31].
Italian movement disorder experts developed diagnostic recommendations for OMD, pointing to patterned and repetitive oromandibular movements/postures – either spontaneous or triggered by motor tasks [33]. If present, a sensory trick (geste antagoniste) confirmed the diagnosis of OMD [33]. In patients who did not manifest a sensory trick, the active exclusion of clinical features related to the condition mimicking dystonia was necessary for diagnosis [33]. OMD is usually diagnosed based on the characteristic clinical features of focal dystonia and electromyography (EMG) findings [22][24][29]. Patient clinical features include stereotypy, task specificity, sensory tricks, morning benefit, co-contraction, and overflow phenomenon [22][23][29][34]. Patients with OMD exhibit different stereotypical patterns of muscle contraction according to subtype. Stereotypy was observed in 95.8% of 385 patients with OMD in one study [29]. OMD symptoms often appear only task-specifically, for example, while speaking or chewing, in its early phase. Notably, 69.9% of patients with OMD showed task-specificity in one study [29]. A typical example of task-specific dystonia in the oral region is embouchure dystonia, which occurs only during the performance of wind instrument players [35]. Sensory tricks are physical movements or positions that can temporarily ameliorate the symptoms of dystonia [22][23][29][31]. Such sensory tricks were observed in 51.4% of patients with OMD in one study [29]. The symptoms of dystonia tend to be milder in the morning with large inter-individual variations in their duration; this phenomenon is called ‘morning benefit’ and was reported in 47.3% of patients with OMD [29]. Another symptom, co-contraction, refers to a loss of the reciprocal inhibition of muscular activities, causing involuntary simultaneous contractions of the agonist and antagonist muscles. Jaw closing muscle contraction during mouth opening can limit the maximal mouth opening, and the occurrence of such contractions while speaking or eating can hamper speech or mastication [22][23][29][31]. The overflow phenomenon involves the activation of muscles that are unnecessary for a task, interfering with normal movement [34]. Dystonic contracture of the masticatory muscles may expand to the orbicularis oris, orbicularis oculi, or other facial, neck, and shoulder muscles [34].
Dopamine receptor–blocking agents, including antipsychotics, tricyclic antidepressants, antiemetics, and other medications for gastrointestinal disorders can induce tardive dystonia, and represent common causes of acquired dystonia [36]. Some genetic disorders (DYT-THAP1, DYT-TAF1, DYT-ATP1A3, and DYT‑KMT2B) are also characterized by OMD in the clinical spectrum [36].
2.3. Epidemiology
The mean age of OMD onset is in the 50s [27][30][31]. Notably, women are approximately twice as frequently affected as men, indicating female predominance (2:1) [27][30][31]. OMD can occur in isolation; however, it may present together as segmental or generalized dystonia. For example, comorbid OMD and blepharospasm can form together as a segmental dystonia [37][38]. Common comorbidities of OMD include cervical dystonia, writer’s cramp, and spasmodic dysphonia [22][29][30][36]. The ratio of isolated OMD among all OMD cases differs considerably as reported by neurologists (focal, 39%; segmental, 43%; generalized, 10%) [30] and an oral surgeon (focal, 90.8%; segmental, 10.4%; multifocal, 6.3%) [31]. This discrepancy occurred because neurologists assessed severe cases of OMD associated with neurological diseases, whereas the oral surgeon was able to identify numerous mild cases [31].
In a meta-analysis, Steeves et al. [39] estimated the prevalence of primary dystonia to be 16.43 per 100,000 persons, of cervical dystonia to be 4.98, of blepharospasm to be 4.24, and of OMD to be 0.52. The estimated prevalence of OMD varies from 0.1 to 6.9 per 100,000 population [39][40][41][42][43][44][45][46][47][48]. The prevalence of OMD has been postulated to be considerably higher than previously estimated [22][29]. A recent study [31]. reported the crude prevalence of OMD to be 9.8 per 100,000 persons (idiopathic dystonia, 5.7; tardive dystonia, 3.4), with an incidence of 2.0 per 100,000 person-years (idiopathic dystonia, 1.2; tardive dystonia, 0.68). The prevalence was 13.0 and 6.3 in 100,000 persons for women and men, respectively [31]. The research postulated that OMD may have an equal or even higher prevalence than cervical dystonia or blepharospasm [31]. Approximately 70% of patients with OMD visited dentists, and 60% of patients saw oral surgeons in one study [49]. However, approximately 90% of the patients had not been diagnosed correctly [49]. A vast majority of the patients had been diagnosed with temporomandibular disorders, bruxism, or psychiatric diseases [22][23][29][49]. Several patients continued the treatment of temporomandibular disorders or bruxism without effects, eventually discontinuing treatment and abandoning further consultations [22][23][29][49]. OMD has been considered a rare disorder; however, in reality, cases are simply incorrectly diagnosed.
3. Treatment Challenges and Pitfalls
OMD can be part of the clinical spectrum of various neurological diseases, including Parkinson's disease, pantothenate kinase associated neurodegeneration, Wilson’s disease, chorea-acanthocytosis, Lesch Nyhan syndrome, and Leigh syndrome; or of ischemic or hemorrhagic stroke, tumors, infarction, and brain injury [36]. If such diseases have already been diagnosed and treated, OMD must be addressed simultaneously by the attending physicians [22][23]. However, if not diagnosed yet, the patient should be referred to specialists [22][23]. Likewise, collaboration with a psychiatrist is required for the treatment of tardive dystonia.
The treatment of OMD must be multimodal and highly individualized, and methods include pharmacological [23][34][36], BoNT [10][11][12][13][14][15][16][17][26][54][55][56][57][58][59][60][61][62][63], muscle afferent block [24][64][65], occlusal splint [66][67], and surgical therapies (coronoidotomy) [68][69][70]. Due to the number of muscle spindles, muscle afferent block therapy is more effective for jaw closing muscles than for jaw opening muscles [24][64]. A sensory trick splint is particularly successful in patients with muscle hyperactivity of the jaw closing muscles. In one study, 83.7% of the responders with splints presented with jaw closing dystonia [66]. Patients who showed improvement with the use of splints and continued to wear them for at least three months were defined as responders [66]. While, patients who showed little or no effect and/or were unable to insert splints were defined as non-responders [66]. Intraoral sensory tricks were significantly more common in responders (60.2%) than in non-responders (13.3%) [66]. Coronoidotomy is only indicated for severe jaw closing dystonia [68][69]. However, one-third of the operated patients required additional BoNT injections into the masseter and/or medial pterygoid muscles [69]. This research will focus on BoNT therapy.
Chemodenervation with BoNT is considered the first line of treatment for OMD. Currently, four FDA-approved and commercially available BoNT formulations. The three types of botulinum toxin type A available are onabotulinumtoxinA (Botox; Allergan, CA, USA), abobotulinumtoxinA (Dysport; Ipsen-Pharma, UK), and incobotulinumtoxinA (Xeomin; Merz Pharma, Germany); rimabotulinumtoxinB (Myobloc in the USA; Supernus Pharmaceuticals, Inc, Rockville, MD; Neurobloc in Europe, Sloan Pharma, Switzerland) is a botulinum toxin type B preparation [9][71][72]. The following ratios are often used in clinical practice: onabotulinumtoxinA:incobotulinumtoxinA = 1:1; onabotulinumtoxinA: abobotulinumtoxinA = 1:2.5, and onabotulinumtoxinA:rimabotulinumtoxinB = 1:50 [9][72]. Although BoNT is frequently used in major countries for treatment, it has not been officially approved for OMD.
For the first injection, the dose of BoNT must be low because effects vary between individuals [23]. In the subsequent injections, the dose should be adjusted individually corresponding to the effects to reduce the risk of side effects or antibody development, and minimize the cost. A risk of developing neutralizing antibodies exists with the long-term use of BoNT [9][73]. The factors that increase the risk of developing resistance to include a high protein load in some formulations, large individual and cumulative doses, and short intervals [73][74][75][76]. Since the dose for OMD is relatively small, the risk of antibody development is low; however, a case was reported of antibody development after a large amount of BoNT was injected intensively [11]. The contraindications of BoNT include systemic neuromuscular junction disorders (myasthenia gravis, Lambert-Eaton syndrome, amyotrophic lateral sclerosis), current or possible pregnancy, and lactation.
To predict the efficacy of injected BoNT, 3–5 mL of 0.5% lidocaine was injected into the hyperactive muscles of patients in one study, and involuntary movements were carefully observed [77]. If the patients showed changes at all, BoNT was considered not likely to be effective. If the patients showed an improvement of symptoms under the effects of the local anesthetic, subsequent appointments for BoNT injections were recommended [77].
A complete understanding of the local anatomy of the stomatognathic system is a prerequisite for target muscle selection and safe injection without complications [16][23][53]. BoNT is reconstituted with normal saline to reach a final concentration of 2.5–5 units/0.1 mL. Differences in the concentration may affect diffusion; however, no difference in effect was seen in a study for blepharospasm [78]. A disposable hypodermic needle electrode (37 mm × 25 G, 50 mm × 25 G) is used for injection into the target muscles, and accurate placement of the electrode is verified by evaluating EMG activity [16][23][53]. Subsequently, after aspiration, the necessary units of BoNT are injected into the target muscles.
Sonography is important for identifying target muscles and preventing damage to other tissues and is often used for cervical dystonia [79][80]. This method is also very useful for OMD and is recommended for use in combination with other methods such as EMG.
Maximum bite force is measured bilaterally on the molars three times using an occlusal force meter [23][77][81]. The muscles and doses of BoNT are then individually determined for each patient based on their symptoms and occlusal force [23][29][77][81]. The injection is continued until the patient is satisfied with the effect [23][77]. The injection interval is three to six months, depending on patient symptoms [23][77][81].
3.1. Jaw closing dystonia
Jaw closing dystonia was observed in 59.5% of patients with OMD [29]. In severe cases, the patients cannot open the mouth at all because of the involuntary contraction of the bilateral temporalis and masseter muscles [68][69]. The recommended target muscles and doses of BoNT for jaw closing dystonia [23][77][82] are summarized in Table 1.
Table 1. Recommended target muscles and doses of BoNT (onabotulinumtoxinA: Botox®) for jaw closing dystonia [23][77][82].
|
Main muscles [Doses (units)] |
Additional muscles [Doses (units)] |
|
|
Bilateral masseter (10-50) |
Bilateral medial pterygoid (10-30) |
Insufficient cases only for masseter and temporalis, or cases in which the effect was diminished by repeated injections |
|
Bilateral temporalis (10-50) |
Contralateral or bilaterallateral pterygoid (10-30) |
With mandibular deviation, grinding, and myalgia of the lateral pterygoid muscle |
onabotulinumtoxinA:incobotulinumtoxinA = 1:1; onabotulinumtoxinA:abobotulinumtoxinA = 1:2.5 [9][72]
Masticatory disturbance may be a side effect of an excessive loss of occlusal force. The maximum chewing force should be measured before and after treatment with BoNT to prevent an excessive loss of chewing force [23][81].
3.1.1. Masseter Muscle
The main BoNT treatment for jaw closing dystonia is the masseter muscle, which comprises a superficial and a deep part [23][82]. Posteriorly, the muscle is covered by the parotid gland, and care must be taken not to damage it with a needle [23][82]. An ultrasound-guided injection makes it possible to distinguish the masseter muscle from the parotid gland [23][82]. BoNT is injected 10–15 mm into the most prominent region during jaw clenching, with 10–50 units (depending on the condition) at three points (Figure 1) [23][82].
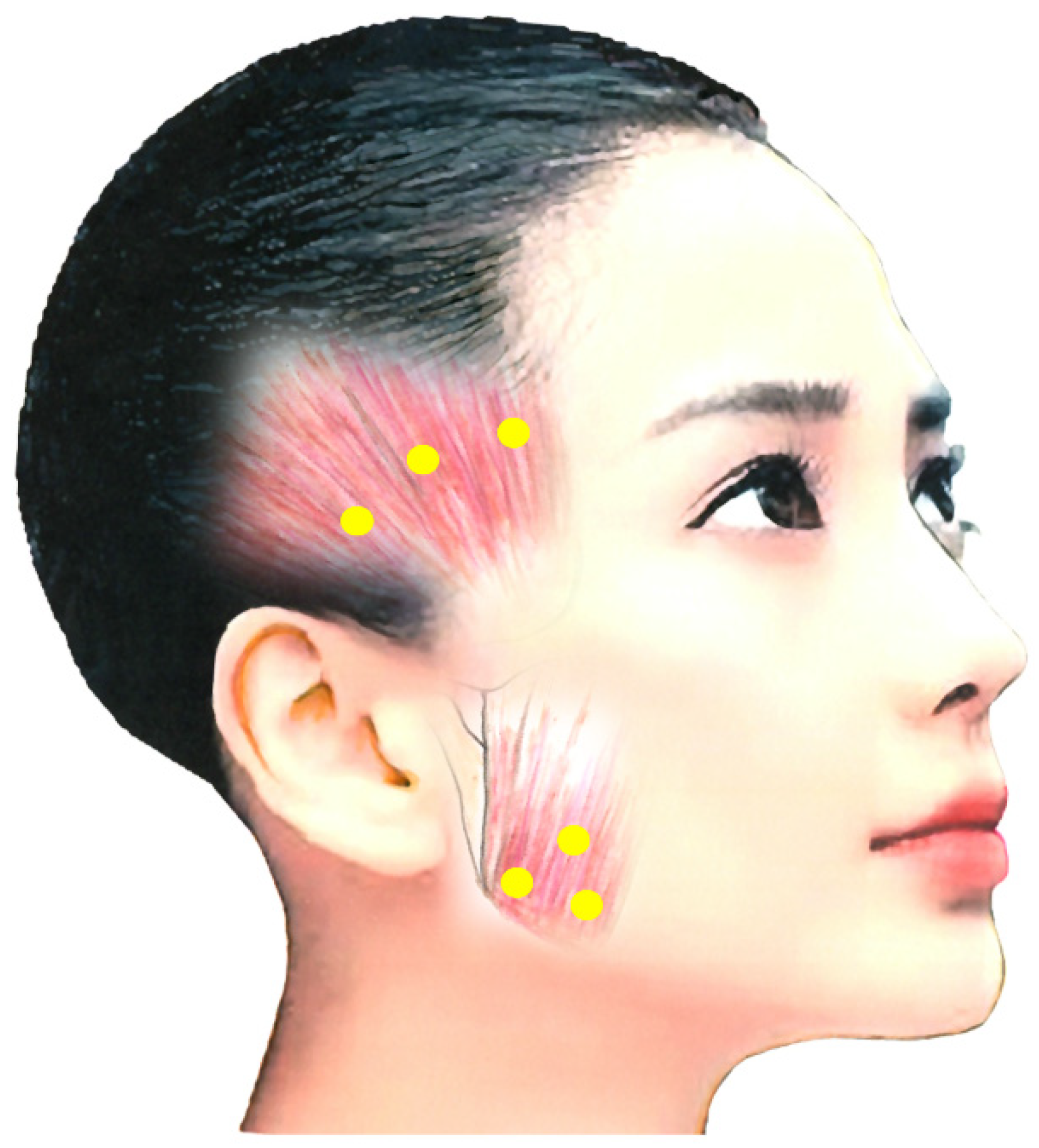
Figure 1. Injection sites for the masseter and temporalis muscles [23][77][82].
3.1.2. Temporalis Muscle
After careful examination including EMG, occlusal force and palpation, BoNT is often injected into the temporalis muscle at the same time. This muscle, particularly anterior fibers, elevates the mandible, and pulls it back with posterior fibers. Notably, 10–50 units of BoNT are injected 10–15 mm deep at three sites (Figure 1) [23][82].
3.1.3. Medial Pterygoid Muscle
Repeated injections into the masseter and temporalis muscles can cause tension on the medial pterygoid muscle due to the “whack-a-mole phenomenon” [69][83]. This phenomenon can be explained as follows: When a dystonic muscle has been improved by BoNT treatment, one of the other muscles with the same function becomes more active [69][83]. Subsequently, when BoNT is injected into the latter muscle, another muscle with a similar function gradually becomes dystonic [69][83]. It is therefore necessary to inject BoNT into the medial (internal) pterygoid muscle. The medial pterygoid muscle is accessible via intraoral and extraoral approaches (Figure 2). With the intraoral method, the patient must gargle with a mouthwash solution, then the needle should be positioned at an angle of 20° to the rear and upward, then to the side by 20° after palpation of the muscle in relation to the occlusal plane (Figure 2) [69][82][83]. The needle should be inserted up to a depth of 15–20 mm. BoNT is injected in 10–30 units. The correct placement of the needle in the target muscle should be monitored by EMG [69][82][83]. For the extraoral method, the patient's head should be tilted towards the contralateral side. The needle is then inserted into the submandibular skin, 10 mm forward the angle of the lower jaw, parallel to the inner side of the mandible, and at a depth of 15–20 mm (Figure 2) [69][82][83].
Patients who mainly exhibit grinding also often report severe tenderness in the lateral pterygoid muscle. The details of this muscle are described in section 3.2.1.
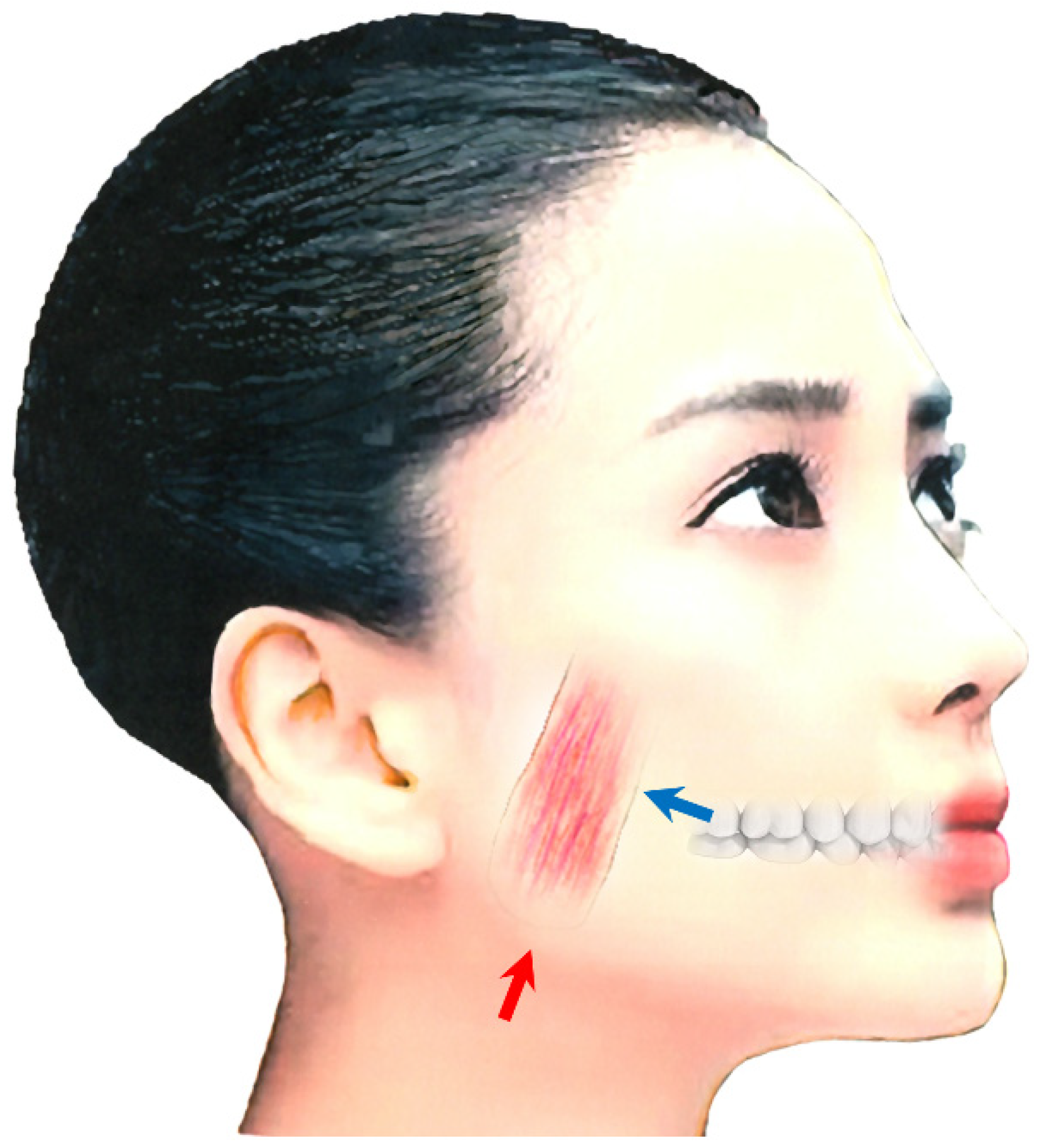
Figure 2. Injection methods for the medial pterygoid muscle; intraoral approach (blue arrow) and extraoral oral approach (red arrow) [23][32][50][82][83].
3.2. Jaw opening, deviation, and protrusion dystonia
The occurrence rates of jaw opening, jaw deviation, and jaw protrusion dystonia in patients with OMD are 12.7%, 5.5%, and 3.1%, respectively [29]. In jaw opening and protrusion dystonia, the bilateral lateral pterygoid muscles show involuntary hyperactivity [50][82][83]. In contrast, in jaw deviation dystonia, the muscle on the contralateral side of the deviation exhibits abnormal contractions [50][82][83]. In the most severe cases, the temporomandibular joint is dislocated [15][32]. The recommended target muscles and doses of BoNT for jaw opening, jaw deviation, and jaw protrusion dystonia [23][32][50][82][83] are summarized in Table 2.
Table 2. Recommended muscles and doses of BoNT (onabotulinumtoxinA: Botox®) for jaw opening, deviation, and protrusion dystonia [23][32][50][82][83].
|
Subtypes |
Main muscles [Doses (units)] |
Additional muscles [Doses (units)] |
|
|
Jaw opening dystonia |
Bilateral lateral pterygoid (10-50) |
Anterior digastric (5-10) |
Insufficient cases only for lateral pterygoid |
|
Platysma (10-20) |
With anterior neck tension |
||
|
Genioglossus (10-20) |
With tongue protrusion |
||
|
Jaw deviation dystonia |
Contralateral lateral pterygoid (10-50) |
Ipsilateral posterior temporalis (10-20) |
Insufficient cases only for lateral pterygoid |
|
Platysma (10-20) |
With anterior neck tension |
||
|
Jaw protrusion dystonia |
Bilateral lateral pterygoid (10-40) |
Anterior digastric (5-10) |
Insufficient cases only for lateral pterygoid |
|
Platysma (10-20) |
With anterior neck tension |
||
onabotulinumtoxinA:incobotulinumtoxinA = 1:1; onabotulinumtoxinA:abobotulinumtoxinA = 1:2.5 [9][72]
3.2.1. Lateral Pterygoid Muscle
The lateral (external) pterygoid muscle is a two-headed muscle comprising a superior (upper) and an inferior (lower) head [50][82][83]. As the bilateral inferior heads contract, the condyle is pulled forward and slightly downward. If the muscle is only activated on one side, the inferior jaw rotates around a vertical axis that runs through the contralateral condyle, and it is pulled medially to the contralateral side [50][82][83]. The superior and inferior heads are activated alternately during chewing, such that the inferior head contracts during mouth opening while the superior head relaxes. Upon closing of the mouth, the actions are reversed [50][82][83][84][85].
The maxillary artery arises behind the neck of the mandible and is initially embedded in the parotid gland [50][82][83]. Known anatomical differences exist between Japanese and Caucasian populations in the course of the maxillary artery in relation to the lateral pterygoid muscle [50][82][83]. In 92.7% of Japanese individuals, the maxillary artery runs laterally to the lower head [50][82][83]. However, in Caucasians, the artery more often runs medially to the muscle (38%) [50][82][83]. Injury to the maxillary artery during needle insertion can result in arterial bleeding, swelling, and bruising. Both intraoral and extraoral methods are available for BoNT injection; when injecting into the lateral pterygoid muscle, medical practitioners must be aware of these anatomical differences (Figure 3) [50][82][83].
The extraoral injection is easy to administer; however, BoNT can only be injected in a limited area [50][82][83]. Further, a risk of mouth dryness exists [55]. In contrast, the intraoral injection can be applied directly into the region with muscle tension with EMG monitoring. With the intraoral method, the needle is inserted 20–30 mm into the inferior head of the mucobuccal fold next to the upper second molar [50][82][83]. The angle of needle insertion is 30° upwards against the occlusal surface and 20° inwards against the sagittal plane (Figure 3). BoNT is injected in 10–50 units, depending on the condition. With the extraoral method, after palpating the infratemporal fossa, the needle can be inserted perpendicular to the skin, 20–30 mm deep through the notch in the mandible (Figure 3) [50][82][83].
Computer-aided design and manufacturing process was used to develop a needle guide to reliably administer BoNT into the inferior head of the lateral pterygoid muscle [50][82][83]. Computed tomography and scan data of the upper jaw model are transferred to a computer and the two most suitable points on the lower head are determined [50]. The needle guide is subsequently mounted in the oral cavity and the needle is inserted to the planned depth. Experiments showed that the needle was easily inserted without any complications in all the procedures [50].
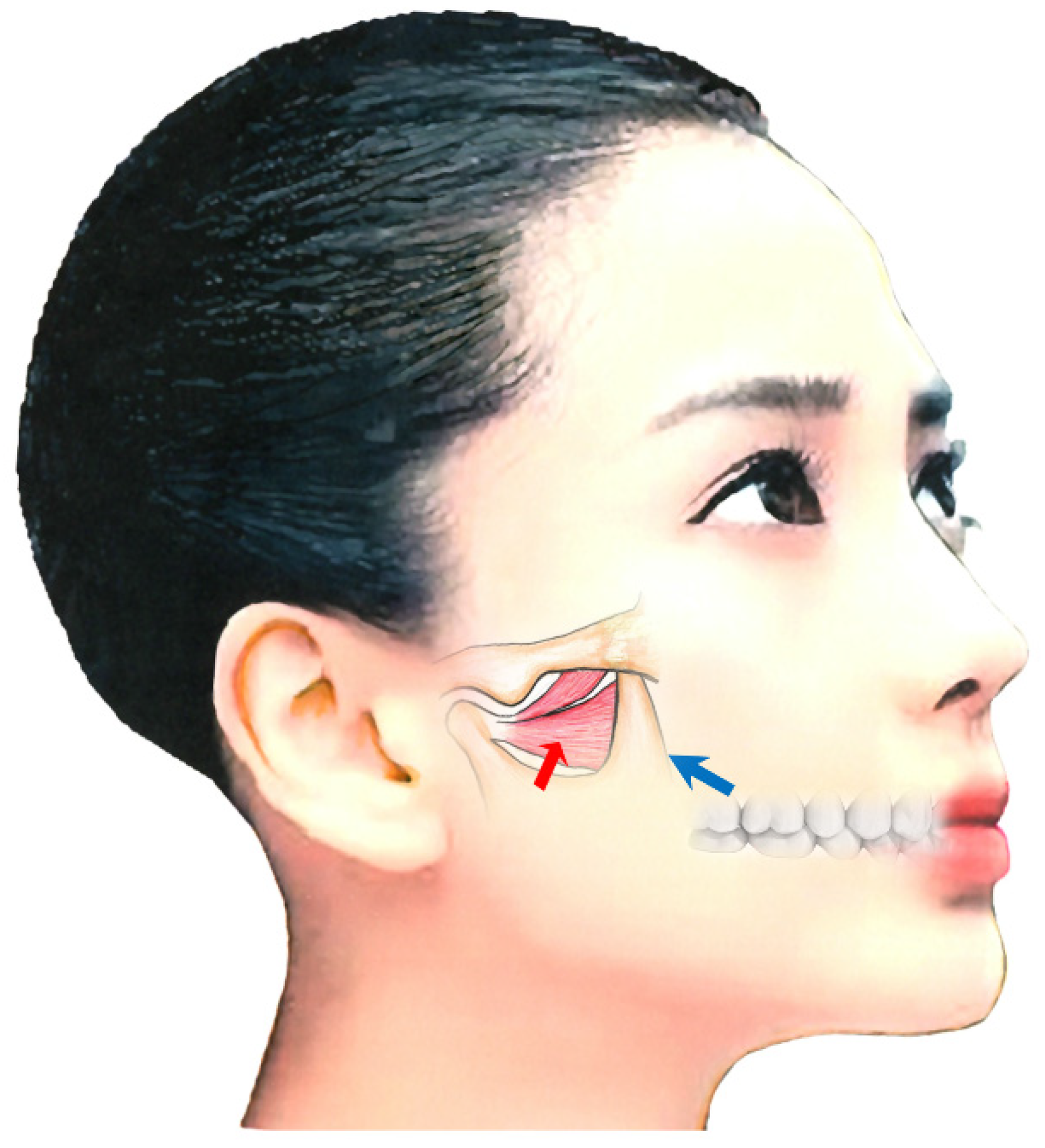
Figure 3. Injection methods for the lateral pterygoid muscle; intraoral approach (blue arrow) and extraoral oral approach (red arrow) [23][32][50][82][83].
3.2.2. Digastric Muscle
The digastric muscle comprises two muscle bellies (anterior and posterior) connected by an intermediate tendon [23]. The anterior belly of the digastric muscle acts in the early phase of opening the mouth by lowering the chin [84][85]. Injection into the anterior belly can cause dysphagia and should be carefully monitored. BoNT is injected in 5–10 units [23]. Patients with OMD or bruxism have often reported tenderness in the posterior belly of the digastric muscle. If the patients have tenderness in the muscle, the muscle can be easily palpable. Because blood vessels and nerves run nearby, approximately 2.5–5 units of BoNT should be carefully injected under EMG guidance [23].
3.2.3. Temporalis Muscle and Platysma
If the effect of BoNT injections not satisfactory for patients with jaw deviation dystonia, BoNT (10–20 units) should be injected into the posterior fiber of the ipsilateral temporalis muscle. In some patients with jaw opening dystonia, the platysma may become hyperactive and require injection (10–20 units).
3.3. Lingual dystonia
Lingual (tongue) dystonia is characterized by involuntary, often task-specific contractions of the tongue muscle [86]. Lingual dystonia was found in 25.5% of patients with OMD [29]. Lingual dystonia is divided into four subtypes: protrusion, retraction, laterotrusion, and curling [53].
The tongue comprises four extrinsic (genioglossus, hyoglossus, styloglossus, palatoglossus muscles) and four intrinsic (superior longitudinal, inferior longitudinal, transverse, vertical muscles) muscles [53][87]. The extrinsic muscles control tongue position, while the intrinsic muscles control tongue shape [53][87]. Although serious complications of BoNT injection, including life-threatening dysphagia, aspiration pneumonia, and breathing difficulties were reported in previous studies [88][89], the therapy has been recognized as a promising option in recent years [53][90][91].
The subjective improvement after the injection of BoNT was 77.6% in 136 patients with lingual dystonia [53]. The greatest improvement was seen for curling type dystonia (81.9%), whereas the retraction type exhibited the lowest improvement rate (67.9%) [53]. Mild dysphagia occurred in 12.5% of the patients; however, this resolved spontaneously within a few days to two weeks. No serious side effects were observed [53]. Lingual dystonia is often accompanied by jaw opening dystonia. For such patients, injection into the lateral pterygoid muscle is necessary [23]. The recommended target muscles and doses of BoNT for lingual dystonia [23][53][82] are summarized in Table 3.
Table 3. Recommended muscles and doses of BoNT (onabotulinumtoxinA: Botox®) for lingual dystonia [23][53][82].
|
Subtypes |
Doses [units] |
Main muscles [Doses (units)] |
Additional muscles |
|
|
Protrusion type |
15-60 |
Bilateral genioglossus (50-100% of total dose) |
Ipsilateral superior and inferior longitudinal (0-50%) |
With laterotrusion |
|
Bilateral superior longitudinal (0-50%) |
With curling |
|||
|
Bilateral vertical (0-50%) |
With flattening |
|||
|
Bilateral transverse (0-50%) |
With elongation |
|||
|
Bilateral lateral pterygoid (0-50%) |
With jaw opening |
|||
|
Retraction type |
15-50 |
Bilateral genioglossus (30-70% of total dose) |
Intrinsic and geniohyoid (30-70%) |
Insufficient cases only for genioglossus |
|
Laterotrusion type |
10-40 |
Ipsilateral superior and inferior longitudinal (70-100% of total dose) |
Contralateral genioglossus (0-30%) |
Insufficient cases only for superior and inferior longitudinal |
|
Curling type |
10-40 |
Bilateral superior longitudinal (80-100% of total dose) |
Bilateral genioglossus (0-20%) |
With protrusion |
onabotulinumtoxinA:incobotulinumtoxinA = 1:1; onabotulinumtoxinA:abobotulinumtoxinA = 1:2.5 [9][72]
3.3.1. Genioglossus and Other Tongue Muscles
The genioglossus muscle is the dominant muscle for tongue protrusion [87]. BoNT injection into the lingual muscle occasionally causes life-threatening complications, such as serious dysphagia [88], aspiration pneumonia [88], and swallowing or breathing difficulties [89]. Investigators have reported various methods for BoNT injection, primarily the submandibular approach [53][88][89][90][91][92][93][94], with intraoral approaches also reported [95]. In the submandibular method, injection is performed in one or two sites, bilaterally.
A more detailed injection method for the four subtypes of lingual dystonia has been described depending on the direction of the tongue deviation [53][82]. The dose of BoNT therapy should be started at 10–20 units and gradually increase to 40–50 units corresponding to patient symptoms [53][82]. The appropriate doses of BoNT (15–60 units) is then determined based on EMG examination and patient symptoms (Table 3).
For protrusion type dystonia, approximately 50–100% of the total doses are injected into the bilateral genioglossus percutaneously through the submandibular region. The insertion points are defined as two sites 25–30 mm posterior from the midline of the body of the mandible and 15–20 mm apart from each other (Figure 4A) [53]. If tongue protrusion occurs while simultaneously curling up or down, the remaining doses are injected into the superior longitudinal muscle (5-mm depth; injection to counteract tongue protrusion) or into the inferior longitudinal muscle (10–15 mm depth; injection to counteract curling) [53]. If laterotrusion occurs with protrusion, BoNT is administered into the superior and inferior longitudinal muscles on the deviated side (Figure 4B) [53]. If the tongue shows flattening or narrowing, the remaining BoNT is injected into the bilateral vertical muscle (10 mm in depth; to counteract protrusion) and the bilateral transverse muscle (10 mm in depth; to counteract curling) [53].
For retraction type dystonia, target muscles are identified after careful EMG examination. These include a wide range of tongue muscles that may undergo contraction, such as the genioglossus, intrinsic muscles, geniohyoid, and hyoglossus [53]. Appropriate doses of BoNT are in the range of 15–50 units (Table 3).
For laterotrusion type dystonia, the appropriate dose of BoNT is between 10–40 units (Table 3). BoNT is injected into the superior (5 mm in depth) and inferior (10–15 mm in depth) longitudinal muscles on the deviated side (Figure 4B) [53]. The inferior longitudinal muscle is more accessible from the inferior aspect of the tongue (5 mm in depth) than from the dorsum. If the genioglossus on the opposite side exhibits EMG activity, an additional injection is administered [53].
For curling type dystonia, the appropriate dose of BoNT is between 10 and 40 units (Table 3). The toxin is injected bilaterally at two or three sites from the dorsum of the tongue, approximately 5 mm in depth, into the superior longitudinal muscle (Figure 4B) [53]. BoNT is injected into the superior longitudinal muscle near the apex if curling up of the apex occurs (Figure 4B).
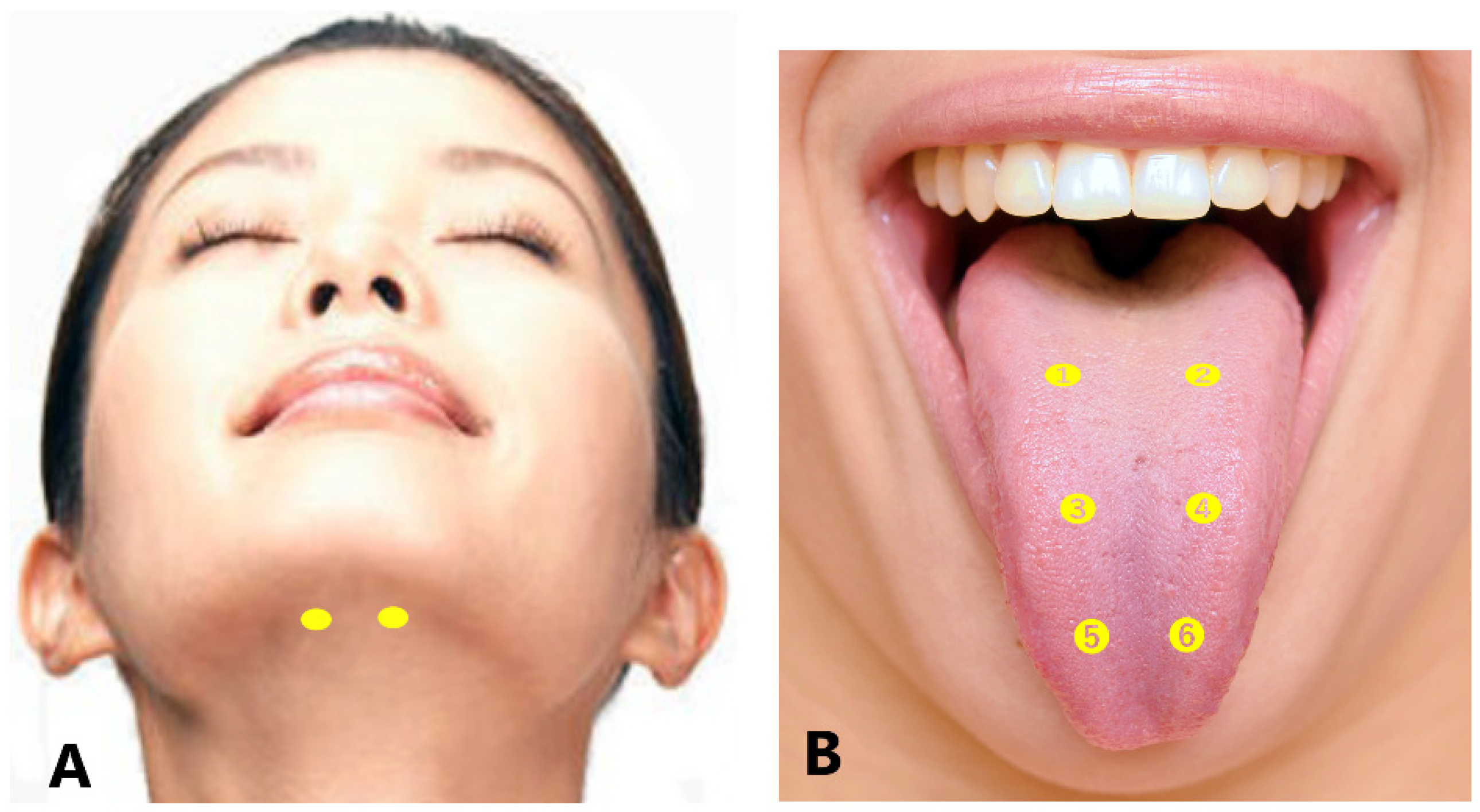
Figure 4. Submandibular sites of BoNT injection for lingual dystonia (A). Intraoral sites for BoNT injection (B); for protrusion type (➊-➍), laterotrusion type [right deviation (➊, ➌), left deviation (➋, ➍)], and curling type (➊-➏) [23][53][82].
3.4. Lip dystonia
Lip dystonia was observed in 3.6% of the reported OMD cases [29]. The orbicularis oris muscle is a sphincter that surrounds the oral orifice. According to the symptoms, BoNT should be injected into the orbicularis oris, risorius, depressor anguli oris, depressor labii inferioris, mentalis, and platysma. Since the fibers of the orbicularis oris muscle close to the orifice are responsible for pursing the lips, and the fibers distant from the orifice press the lips against the teeth [57], the dose and site should be adjusted according to the symptoms [23]. The dose of BoNT should be low (2.5–5 units) because of risk of the labial incompetence and asymmetric smile.
Lip dystonia, especially unilateral traction, can occur with functional involuntary movements [96]. Functional movement disorders often show distinguishable clinical features in the orofacial area. Treatment with BoNT is effective in some cases, but complete treatment often requires psychotherapy or physiotherapy [96].
3.5. Pitfalls
The largest clinical pitfall of OMD treatment seems to be a misdiagnosis. Most patients with OMD see a dentist or oral surgeon and are diagnosed with bruxism, temporomandibular disorders, and/or psychiatric disorders [29][49]. OMD is considered a blind spot between medical science and dentistry [29]. Neurologists may diagnose temporomandibular disorder as OMD, (i.e., bruxism as jaw closing dystonia, and anterior disc displacement without reduction as jaw deviation dystonia) [29]. The knowledge and experience of both neurology and dental medicine are needed for proper diagnosis of OMD from temporomandibular disorders, but these two perspectives are nearly impossible to obtain simultaneously. Therefore, a simple diagnostic tool was developed to enable primary care physicians, neurologists, dentists, and oral surgeons to differentiate OMD from temporomandibular disorder and to initiate appropriate treatments rapidly [29].
A subsequent pitfall of BoNT treatment is the method of injection into the masticatory muscles. Dressler et al. [63] described in their consensus guidelines, “The Mm. pterygoidei can easily be injected through the incisura mandibulae. EMG requiring thick combination needles seems unnecessary as dystonic involvement is usually affecting both, the lateral and the medial pterygoid muscles.” Nevertheless, it is risky to inject BoNT into the lateral and medial pterygoid muscles without EMG guidance [50][82][83]. The lateral pterygoid muscle should be injected in jaw opening dystonia [50][82][83]. In contrast, the medial pterygoid muscle should be injected for patients with jaw closing dystonia [17][25][55][82]. If BoNT is erroneously injected into the lateral pterygoid muscle for jaw closing dystonia, or into the medial pterygoid muscle for jaw opening dystonia, adverse effects are expected. Because the needle is inserted at the notch by percutaneous lateral pterygoid muscle injection, penetrating the parotid gland, mouth dryness may occur due to the spread of BoNT [55]. Most OMD specialists recommend an intraoral approach [16][17][25][32][50][55].
In a systematic review [28] involving 387 patients with OMD treated with BoNT, 27.1% of the patients had side effects, most frequently dysphagia. Previously reported adverse effects of BoNT include temporary regional weakness, tenderness over the injection sites, minor discomfort during chewing, asymmetric smile, loss of smile, lip numbness, muscle atrophy, paresthesia, difficulty in swallowing, mouth dryness, speech changes, nasal speech, headache, hematoma, nasal regurgitation, swelling, bruising, facial asymmetry, transient edema, itching, and pain at the injection area [11][12][14][17][55][58][97]. Most of these effects were observed to be transient and spontaneously disappeared. Moreover, the majority of these side effects are thought to be related to the injection technique and avoided by accurate knowledge of the local anatomy of muscles, nerves, and other tissues, and accurate injection procedures. The more accurately BoNT is administered into the target muscles, the more likely the improvement in patient symptoms, and the lower the risk of complications [32][50][53][77]. Empirical differences in injection techniques may be associated with adverse effects [77]. Selected dose should be as high as necessary, but as low as possible.
Several authors have reported changes in the mandibular bone after BoNT injections [98][99]. In contrast, a retrospective study in adult women with masseter hypertrophy found no significant change in whole mandible volume or in cortical thickness of the mandibular ramus three months after BoNT therapy in the masseter muscles [100]. Changes in bone after BoNT therefore may not be an adverse effect, but a normal physiological response related to corrected occlusal force [77]. Injection with BoNT improves the excessive tension of the jaw elevator muscles and allows the hypertrophied muscles and bones to return to their original shape [77].
Because the symptoms of OMD can vary from patient to patient, it is difficult to objectively measure disease severity and changes after treatment [22]. In 2002, 44 patients with OMD were evaluated before and after muscle afferent block therapy using a simple clinical scoring system according to subscores for pain, mastication, speech, and discomfort [64]. In 2010, Merz et al. [101] developed and validated the Oromandibular Dystonia Questionnaire. Recently, a comprehensive measurement tool for OMD, the ‘Oromandibular Dystonia Rating Scale’ was developed and validated [22]. The scale can be useful for the comprehensive evaluation of severity, disability, psychosocial functioning, and impact on the quality of life as well as therapeutic changes in patients with OMD [22].
4. Reported Trials – Evidence-Based Medicine
In a double-blind, placebo-controlled study of BoNT treatment for cranial-cervical dystonia in 10 patients with oromandibular-cervical dystonia, only 37.5% of patients reported improved symptoms [10]. However, the low number of participants and combined phenotype of cervical dystonia and OMD in this research limit the conclusions [9]. Tan and Jankovic [14] studied 162 patients with OMD for approximately 10 years. BoNT was administered in the submentalis muscle complex of patients with jaw opening dystonia, and in the masseter muscles patients with jaw clenching with or without bruxism. A total of 31.5% of patients experienced adverse effects (dysphagia, 10.2%; dysarthria, 0.9%) [14]. A retrospective chart review included 59 patients with OMD (jaw closing, 47.5%; jaw opening, 35.6%; and jaw deviation, 16.9%) [17]. Bakke et al. [16] reported functional and clinical characteristics in 21 patients with OMD. Fourteen patients received BoNT for OMD for 8–10 years, and 9 of 14 patients continued with BoNT therapy [62]. Intraoral BoNT injection into the lateral pterygoid muscle in six of the eight patients with OMD led to significant symptom improvement, and only one patient experienced nasal speech [58].
More recently, a pilot single-blind study was published evaluating BoNT dosing and efficacy in 18 patients with OMD (jaw opening, 9; jaw closing, 3; intermixed tongue protrusion, 6) [51]. In an initial dose-finding phase, three subjects were injected with BoNT in prespecified fixed doses [55] assigned to each of the identified target muscles (anterior digastric, genioglossus, masseter, medial pterygoid, and lateral pterygoid muscles). Two of the three patients experienced mild adverse effects even at these doses, so no further dose escalation was performed, and the low dose scheme was used for a subsequent single injection session. All patients received injections tailored to their symptoms using fixed doses of 7.5 to 50 units BoNT in muscles selected from the same set used in phase 1 [55]. Efficacy parameters, including the jaw/tongue portions of the Global Dystonia Severity Rating Scale and Unified Dystonia Rating Scale, showed significant improvements compared to baseline levels at 6 and 12 weeks in unblinded ratings, but not in blinded video assessments. Measures of quality of life and speech in addition to the Clinical Global Impression improvement and severity also significantly improved. A total of nine patients experienced mild to moderate side effects, most commonly dysphagia. Five patients received injections for lingual dystonia; four patients developed dysphagia. Subsequent dosing of the genioglossus muscle was decreased from 15 units to 7.5 units [55].
In a retrospective study of 172 patients with lingual dystonia, BoNT was administered to 136 patients, most of whom noted a marked improvement in mastication, pain, and phonation [53]. Transient trouble with swallowing occurred in 12.5% of patients. In another study, 50 units of BoNT injected into each genioglossus muscle was effective in treating lingual-dystonia-related tardive dyskinesia [94]. In a study of 30 patients with lingual dystonia who participated in a survey of quality of life evaluated by the OMD questionnaire-25, average scores dropped from 46.8 to 38.2 after BoNT therapy [91]. Dysphagia occurred in 16.7% of patients. A retrospective chart review reported 17 patients with lingual dystonia, and nine of the patients had received BoNT injections; 55.6% reported symptom improvement, but one patient developed dysphagia requiring gastrostomy tube placement [90].
The comparisons of total Oromandibular Dystonia Rating Scale scores before and after BoNT therapy in 92 patients revealed a significant decrease after treatment (135.3 vs. 55.2) [22]. Symptoms improved significantly from the baseline to four weeks after BoNT therapy in all Oromandibular Dystonia Rating Scale subscales, including examiner- (severity, disability, and pain) and patient-rated parameters (general, eating, speech, cosmetic, social/family life, sleep, annoyance, mood, psychosocial functioning) [22]. Comella [26] performed a systematic review of BoNT in OMD and concluded that BoNT may be the most effective treatment available, improving movement and quality of life in patients with OMD.
5. Practical Guidelines for Treatment
OMD was not addressed in the 2008 American Academy of Neurology guidelines, and evidence for its treatment with BoNT comes primarily from open-label case series, observational studies, and retrospective chart reviews [102]. In 2016, the American Academy of Neurology published practice guidelines for BoNT administration in the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache [103]. However, OMD was not included in the guideline. In 2011, the European Federation of the Neurological Societies guidelines on the diagnosis and treatment of primary dystonia were published and included recommendations and good practice points regarding BoNT treatment of focal dystonia [104]. The guidelines present level A evidence for the use of BoNT as a first-line treatment for primary cranial or cervical dystonia, but OMD was excluded [71]. A guideline is usually produced after the careful evaluation of previously reported studies by experts in the field. Therefore, the guidelines both in 2008 and 2016 were unable to address OMD.
Although few well-designed trial data exist, BoNT injections are considered the preferred treatment for OMD by several investigators [9][17][25][26][53]. In 2021, Dressler et al. [63] reported consensus guidelines for BoNT therapy, including general algorithms and dosing tables for dystonia and spasticity. These guidelines also addressed OMD, despite the lack of evidence. However, the authors made no distinction between the lateral pterygoid and the medial pterygoid muscles without EMG guidance, and the present method differs completely from the well-recognized methods used by other OMD specialists [17][25][32][55][82][83].
Treatment algorithms or treatment strategies for OMD have been reported. Sinclair et al. [17] reported a treatment algorithm for OMD, and Bakke et al. [25] presented clinical strategies for BoNT injection in the oromandibular region. More recently, Yoshida reported therapeutic strategies for OMD, including medication, muscle afferent block therapy, splint therapy, BoNT therapy, and surgery [23][83].
Moreover, a clinical practice statement has been released by the American Academy of Oral Medicine, encouraging the identification and referral of patients with suspected OMD to the appropriate oral and/or medical health provider for further evaluation and management [105].
In clinical practice, an experienced clinician would select target muscles and injection sites and determine the dose and allocation for each BoNT injection, corresponding to patient satisfaction and results of palpation and EMG measurements [23][77][82][83]. The dosing of BoNT should be limited to the necessary minimum amount to minimize the risk of adverse effects and cost and to prevent antibody development. Personalized adjustment of target muscles, sites, and doses will result in much better outcomes than standard methods without individualized planning [23][77][82][83].
6. Proposals for Research and Future Studies
In the future, well-designed, randomized controlled trials with larger sample sizes and longer follow-up periods are required to determine the therapeutic efficacy, optimal dose, duration of effect, adverse effects, brand-specific differences, definite indications, and establishment of a protocol for BoNT therapy. However, the presence of disabilities in patients with OMD places constraints on traditional placebo-control trial design [51]. As patients seeking with OMD specialists visit from very long distances with very high expectations, making a control group ethically difficult to set [77].
It is important to differentially diagnose patients for BoNT injection. Because BoNT is expensive, it is crucial to predict and exclude non-responders for economic reasons [77]. Discrepant results and adverse effects seen in previous studies may have been related to the injection techniques. Experienced clinicians should inject BoNT at an adequate, personalized dose for each patient. Further, clinicians should comprehensively evaluate disease severity, disability, psychosocial functioning, and impact on the quality of life using the recently developed OMD rating scale [22].
The approaches to OMD treatment should be taken seriously not only by neurologists or neurosurgeons but also by oral and maxillofacial surgeons or dentists. Patients with OMD have been successfully treated with BoNT. However, those with severe trismus related to this disease, or for whom treatment with BoNT injections, muscle afferent block therapy, or sensory trick splint [66] was insufficient, successfully underwent coronoidotomy [68][69][70]. Although a large number of patients worldwide wish to visit the researchers' department, only a few can actually make the visit. In reality, it costs an enormous amount of money and only a few wealthy patients can afford it [49]. Furthermore, overseas travel was prohibited due to coronavirus disease 2019 restrictions, making it impossible to receive medical examinations from abroad. Telemedicine is one of the solutions for treatment accessibility problems [49]. After the direct import and fusion of patient computed tomography scans with a plaster cast model of the maxilla, the optimal needle insertion site over the lateral pterygoid muscle can be determined using software [50]. Such data can be transmitted over the internet from anywhere in the world. As telemedicine for OMD using digital technology in the era of coronavirus disease 2019, computer-aided design and manufacturing of needle guides for lateral pterygoid muscle injection should be applied in response to the demands of overseas patients with OMD.
References
- Kerner, J. Vergiftung durch verdorbene Wurste. Tübinger Blätter f. Naturwissensch. u Arzneykunde. 1817, 3, 1–25.
- Van Ermengem, E. Über eine neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Z. Hyg. Infektionskrankh. 1897, 26, 1–56.
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Opthalmol. 1980, 87, 1044–1049.
- Jankovic, J.; Brin, M.F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [PubMed]
- Truong, D.D.; Stenner, A.; Reichel, G. Current clinical applications of botulinum toxin. Curr. Pharm. Des. 2009, 15, 3671–3680. [CrossRef] [PubMed]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [CrossRef] [PubMed]
- Persaud, R.; Garas, G.; Silva, S.; Stamatoglou, C.; Chatrath, P.; Patel, K. An evidence-based review of botulinum toxin (Botox) applications in non-cosmetic head and neck conditions. JRSM Short. Rep. 2013, 4, 10. doi: 10.1177/2042533312472115
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. doi: 10.1016/j.toxicon.2017.09.003 [CrossRef] [PubMed]
- Anandan, C.; Jankovic, J. Botulinum toxin in movement disorders: An update. Toxins 2021, 13, 42.doi: 10.3390/toxins13010042
- Jankovic, J.; Orman, J. Botulinum a toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology 1987, 37, 616–623. [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.; Greene, P.E.; Fahn, S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann. Otol. Rhinol. Laryngol. 1989, 98, 93–97. [CrossRef] [PubMed]
- Jankovic, J.; Schwartz, K.; Donovan, D.T. Botulinum toxin treatment of cranial-cervical dystonia, spasmodic dysphonia, other focal dystonias and hemifacial spasm. J. Neurol. Neurosurg. Psychiatry 1990, 53, 633–639. [CrossRef] [PubMed]
- Hermanowicz, N.; Truong, D.D. Treatment of oromandibular dystonia with botulinum toxin. Laryngoscope 1991, 101, 1216–1218. doi: 10.1288/00005537-199111000-00010
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology 1999, 53, 2102–2107. [CrossRef] [PubMed]
- Yoshida, K.; Iizuka, T. Botulinum toxin treatment for upper airway collapse resulting from temporomandibular joint dislocation due to jaw-opening dystonia. Cranio 2006, 24: 217–222. doi: 10.1179/crn.2006.035.
- Bakke, M.; Larsen, B.M.; Dalager, T.; Møller, E. Oromandibular dystonia–functional and clinical characteristics: a report on 21 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e21–e26. doi: 10.1016/j.oooo.2012.04.023
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus up date. Mov. Disord. 2013, 28, 863–873. doi: 10.1002/mds.25475.
- Oppenheim, H. Über eine eigenartige Krampfkrankheit des kindlichen und jugendlichen Alters (Dysbasia lordotica progressiva, Dystonia musculorum deformans). Neurologie Centralblatt 1911, 30, 1090–1107.
- Romberg, M.H. Krampf im Muskelgebiete der Pars minor Quinti. Masticatorischer Gesichtskrampf. Trismus. In: Lehrbuch der Nervenkrankheiten des Menschen, Alexander Duncker, Berlin, 1846; pp. 308–316.
- Marsden, C.D. The problem of adult-onset idiopathic torsion dystonia and other isolated dyskinesias in adult life (including blepharospasm, oromandibular dystonia, dystonic writer’s cramp, and torticollis, or axial dystonia). Adv. Neurol. 1976, 14, 259–276.
- Yoshida, K. Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front. Neurol. 2020, 11, 583177; doi: 10.3389/fneur.2020.583177
- Yoshida, K. Behandlungsstrategien bei oromandibulärer Dystonie. Fortschr. Neurol. Psychiatr. 2021, 89, 1562–572. doi: 10.1055/a-1375-0669.
- Yoshida, K.; Kaji, R.; Kubori, T. Kohara, N.; Iizuka, T.; Kimura, J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998; 13: 699–705. doi: 10.1002/mds.870130416.
- Bakke, M. ; Dalager, T. ; Møller, E. What clinical strategies are applied for botulinum toxin injection in the oromandibular region? In Botulinum Toxin therapy manual for dystonia and spasticity. Editors Rosales, R.L.; Dressler, D. 2016; pp. 79-95, IntechOpen. https://doi.org/10.5772/64271
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [CrossRef]
- Slaim, L.; Cohen, M.; Klap, P.; Vidailhet, M.; Perrin, A.; Brasnu, D.; Ayache, D.; Mailly, M. Oromandibular dystonia: demographics and clinical data from 240 Patients. J. Mov. Disord. 2018, 11, 78–81. [CrossRef]
- Dadgardoust, P.D.; Rosales, R.L.; Asuncion, R.M.; Dressler, D. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: A meta-analysis. J. Neural Transm. 2019, 126, 141–148. [CrossRef]
- Yoshida, K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin. Oral Investing. 2019, 23, 405–411; doi: 10.1007/s00784-018-2449-3.
- Scorr, L.M.; Factor, S.A.; Parra, S.P.; Kaye, R.; Paniello, R.C.; Norris, S.A.; Perlmutter, J.S.; Bäumer, T.; Usnich, T.; Berman, B.D. et al. Oromandibular dystonia: A clinical examination of 2,020 cases. Front. Neurol. 2021, 12, 700714. doi: 10.3389/fneur.2021.700714. eCollection 2021.
- Yoshida, K. Prevalence and incidence of oromandibular dystonia: an oral and maxillofacial surgery service-based study. Clin. Oral Investig. 2021, 25, 5755–5764, doi:10.1007/s00784-021-03878-9.
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hyperactivity. Toxins 2018, 10, 174; doi: 10.3390/toxins10050174.
- Defazio, G.; Albanese, A.; Pellicciari, R; Scaglione, C.L.; Esposito, M.; Morgante, F.; Abbruzzese, G.; Bentivoglio, A.R.; Bono, F. et al. Expert recommendations for diagnosing cervical, oromandibular, and limb dystonia. Neurol. Sci. 2019, 40, 89-95. doi: 10.1007/s10072-018-3586-9.
- Yoshida, K. Involuntary movements of the stomatognathic region. https://sites.google.com/site/oromandibulardystoniaenglish. Accessed 1 March 2022.
- Frucht, S.J. Embouchure dystonia--Portrait of a task-specific cranial dystonia. Mov. Disord. 2009, 24, 1752-1762. doi: 10.1002/mds.22550.
- Saraf, U.; Chandarana, M.; Divya, K.P.; Krishnan, S. Oromandibular dystonia – A systematic review. Ann. Indian Acad. Neurol. 2022, 25, 26-34. doi: 10.4103/aian.aian_242_21
- Meige, H. Les convulsions de la face, une forme clinique de convulsion faciale bilatérale et médiane. Revue Neurol. 1910, 20, 437–443.
- Tolosa, E.S.; Klawans,K.H. Meige’s Disease: A clinical form of facial convulsion, bilateral and medial. Arch. Neurol. 1979, 36, 635–637. [CrossRef] [PubMed]
- Steeves, T.D.; Day, L.; Dykeman, J.; Jette, N.; Pringsheim, T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov. Disord, 2012, 27, 1789–1796. https://doi.org/10.1002/mds.25244
- Nutt, J.G; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton 3rd, L.J. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. https://doi.org/10.1002/mds.870030302
- Duffey, P.O.; Butler, A.G.; Hawthorne, M.R.; Barnes, M.P. The epidemiology of the primary dystonias in the north of England. Ad. Neurol. 1998, 78, 121–125.
- Le, K-D.; Nilsen, B.; Dietrichs, E. Prevalence of primary focal and segmental dystonia in Oslo. Neurology 2003, 61, 1294–1296. https://doi.org/10.1212/01.wnl.0000090463.05980.59
- Castelon Konkiewitz, E.; Trender-Gerhard, I.; Kamm, C.; Warner, T.; Ben-Shlomo, Y.; Gasser, T.; Conrad, B.; Ceballos-Baumann, A.O. Service-based survey of dystonia in Munich. Neuroepidemiology 2002, 21, 202–206. https://doi.org/10.1159/000059525
- Matsumoto, S.; Nishimura, M.; Shibasaki, H.; Kaji, R. Epidemiology of primary dystonias in Japan: comparison with Western countries. Mov. Disord. 2003, 18, 1196–1198. https://doi.org/10.1002/mds.10480
- Pekmezovic, T.; Ivanovic, N.; Svetel, M.; Nalić, D.; Smiljković, T.; Raicević, R.; Kostić, V.S. Prevalence of primary late-onset focal dystonia in the Belgrade population. Mov. Disord. 2003, 18, 1389–1392. https://doi.org/10.1002/mds.10615
- Asgeirsson, H.; Jakobsson, F.; Hjaltason, H.; Jonsdottir, H.; Sveinbjornsdottir, S. Prevalence study of primary dystonia in Iceland. Mov. Disord. 2006, 21, 293–298. https://doi.org/10.1002/mds.20674
- Joensen, P. High prevalence of primary focal dystonia in the Faroe Islands. Acta Neurol. Scand. 2016, 133, 55–60. https://doi.org/10.1111/ane.12438
- Williams, L.; McGovern, E.; Kimmich, O.; Molloy, A.; Beiser, I.; Butler, J.S.; Molloy, F.; Logan, P.; Healy, D.G.; T Lynch, T. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur. J. Neurol. 2017, 24, 73–81. https://doi.org/10.1111/ene.13133
- Yoshida, K. Multilingual website and cyberconsultations for oromandibular dystonia. Neurol. Int. 2018, 10, 7536. doi: 10.4081/ni.2018.7536.
- Yoshida, K. Computer-aided design/computer-assisted manufacture-derived needle guide for injection of botulinum toxin into the lateral pterygoid muscle in patients with oromandibular dystonia. J. Oral facial Pain Headache 2018, 32, e13–e21. doi: 10.11607/ofph.1955.
- Scorr, L.M.; Silver, M.R.; Hanfelt, J.; Sperin, E.; Freeman, A.; Jinnah, H.A. Factor, S.A. Pilot single-blind trial of AbobotulinumtoxinA in oromandibular dystonia. Neurotherapeutics 2018, 15, 452–458. doi: 10.1007/s13311-018-0620-9
- Skármeta, N.P.; Espinoza-Mellado, P.; Chana, P. Orofacial dystonia and other oromandibular movement disorders. In Dystonia, Editor, Rizk, T.M.G. IntecOpen, 2018; doi: 10.5772/intechopen.78607
- Yoshida, K. Botulinum neurotoxin therapy for lingual dystonia using an individualized injection method based on clinical features. Toxins 2019, 11, 51. doi: 10.3390/toxins11010051.
- Singer, C.; Papapetropoulos, S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat. Disord. 2006, 12, 115–118. doi: 10.1016/j.parkreldis.2005.07.007
- Rosales, R.L.; Ng, A.R.; Santos, M.M.D-D.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (Part II): an open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121 Suppl 1, 44–56.doi: 10.3109/00207454.2011.558260.
- Teive, H.A.; Kluppel, L.E.; Munhoz, R.P.; Becker, N.; Muller, P.R.; Werneck, L.C. Jaw-opening oromandibular dystonia secondary to Wilson’s disease treated with botulinum toxin type A. Arq. Neuropsiquiatr. 2012, 70, 407–409. doi: 10.1590/S0004-282X2012000600 005
- Jost, W. Pictorial Atlas of Botulinum Toxin Injection: Dosage, Localization, Application. 2nd ed.; Quintessence, Berlin, Germany, 2013.
- Moscovich, M.; Chen, Z.P.; Rodriguez, R. Successful treatment of open jaw and jaw deviation dystonia with botulinum toxin using a simple intraoral approach. J. Clin. Neurosci. 2015, 22, 594–596. doi: 10.1016/j.jocn.2014.08.027
- Teemul, T.A.; Patel, R.; Kanatas, A.; Carter, L.M. Management of oromandibular dystonia with botulinum A toxin: a series of cases. Br. J. Oral Maxillofac. Surg. 2016, 54, 1080–1084. doi: 10.1016/j.bjoms.2016.06.028
- Page, A.D.; Siegel, L.; Jog, M. Self-Rated communication-related quality of life of individuals with oromandibular dystonia receiving botulinum toxin injections. Am. J. Speech Lang. Pathol. 2017, 26, 674–681. [CrossRef] [PubMed]
- Bakke, M. ; Henriksen, T.; Biernat, H.B.; Dalager, T. ; Møller, E. Interdisciplinary recognizing and managing of drug-induced tardive oromandibular dystonia: two case reports. Clin. Case Rep. 2018, 6, 2150–2155.doi: 10.1002/ccr3.1548
- Bakke, M.; Baram, S.; Dalager, T.; Biernat, H.B.; Møller, E. Oromandibular dystonia, mental distress and oro-facial dysfunction— A follow-up 8–10 years after start of treatment with botulinum toxin. Oral Rehabil. 2019, 46, 441–449. [CrossRef]
- Dressler, D.; Altavista, M.C.; Altenmueller, E.; Bhidayasiri, R.; Bohlega, S.; Chana, P.; Chung, T.M.; Colosimo, C.; Fheodoroff, K.; Garcia-Ruiz, PJ. et al. Consensus guidelines for botulinum toxin therapy: general algorithms and dosing tables for dystonia and spasticity. J. Neural. Transm. (Vienna). 2021, 128, 321–335. doi: 10.1007/s00702-021-02312-4.Epub 2021 Feb 26.
- Yoshida, K.; Kaji, R.; Shibasaki, H.; Iizuka , T. Factors influencing the therapeutic effect of muscle afferent block for oromandibular dystonia and dyskinesia: implications for their distinct pathophysiology. Int. J. Oral Maxillofac. Surg. 2002; 31: 499–505. doi: 10.1054/ijom.2002.0291.
- Yoshida, K.; Kaji, R.; Takagi, A.; Iizuka, T. Customized EMG needle insertion guide for the muscle afferent block of jaw-deviation and jaw-opening dystonias. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 664–669. doi: 10.1016/s1079-2104(99)70006-5.
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244; doi: 10.1016/j.jpor.2017.09.004.
- De Meyer, M.; Vereecke, L.; Bottenberg, P.; Jacquet, W.; Sims, A.B.; Santens, P. Oral appliances in the treatment of oromandibular dystonia: a systematic review. Acta Neurol. Belg. 2020, 120, 831–836. doi: 10.1007/s13760-020-01404-4.
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031; doi: 10.1002/mds.20859.
- Yoshida, K. Surgical intervention for oromandibular dystonia-related limited mouth opening: long-term follow-up. J. Cranio-Maxillofac. Surg. 2017, 45, 56–62; doi: 10.1016/j.jcms.2016.10.009.
- Yoshida, K. Mouth opening retaining appliance after coronoidotomy for the treatment of trismus: effects on pain during postoperative training and maximal extent of mouth opening. Clin. Surg. 2020, 5, 2737.
- Spiegel, L.L.; Ostrem, J.L.; Bledsoe, I.O. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins 2020, 12, 332.doi: 10.3390/toxins12050332.
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, E48–E54. [CrossRef]
- Jankovic, J.; Schwartz, K. Response and immunoresistance to botulinum toxin injections. Neurology 1995, 45, 1743–1746. [CrossRef]
- Brin, M.F.; Comella, C.L.; Jankovic, J.; Lai, F.; Naumann, M.; Ahmed, F.; Brashear, A.; Chehrenama, M.; Erjanti, H.; Evatt, M.; et al. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov. Disord. 2008, 23, 1353–1360. [CrossRef]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120,275–290. [CrossRef]
- Yoshida, K. Effects of botulinum toxin type A on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins 2021, 13, 605, https://doi.org/10.3390/toxins13090605
- Boyle,H.; McGwin Jr, G.; Flanagan, C.E.; Vicinanzo, M.G.; Long, J.A. High versus low concentration botulinum toxin A for benign essential blepharospasm: does dilution make a difference? Ophthalmic Plast. Reconstr. Surg. 2009, 25, 81–84. doi: 10.1097/IOP.0b013e31819946c4.
- Jost, W.H. How Do I treat cervical dystonia with botulinum toxin by using ultrasound? Mov. Disord. Clin. Pract. 2017, 4, 647. doi: 10.1002/mdc3.12468.
- Fietzek, U.M.; Nene, D.; Schramm, A.; Appel-Cresswell, S.; Košutzká, Z.; Walter, U.; Wissel, J.; Berweck, S.; Chouinard, S.; Bäumer, T. The role of ultrasound for the personalized botulinum toxin treatment of cervical dystonia. Toxins 2021, 13, 365.doi: 10.3390/toxins13050365.
- Yoshida, K. Is botulinum toxin therapy effective for bruxism? Anti-Ageng Med. 2017, 13, 394–398.
- Yoshida, K. Clinical application of botulinum neurotoxin for diseases in the stomatognathic system. J. Jpn. Dent. Soc. Anesthesiol. 2020, 48, 33‒40.
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2016, 4, 285. doi: 10.1002/mdc3.12460.
- Yoshida, K. An electromyographic study on the superior head of the lateral pterygoid muscle during mastication from the standpoint of condylar movement. J. Jpn. Prosthodont. Soc. 1992, 36, 110–120. [CrossRef]
- Yoshida, K. Masticatory muscle responses associated with unloading of biting force during food crushing. J. Oral Rehabil. 1998, 25, 830–837. [CrossRef] [PubMed]
- Yoshida, K. Clinical and phenomenological characteristics of patients with task-specific lingual dystonia: Possible association with occupation. Front. Neurol. 2017, 8, 649. [CrossRef] [PubMed]
- Berkovitz, B.K.B. Tongue. In Gray’s Anatomy, 41st ed.; Standring, S., Ed.; Elsevier: Amsterdam,The Netherlands, 2016; pp. 511–517.
- Blitzer, A.; Brin, M.F.; Fahn, S. Botulinum toxin injections for lingual dystonia. Laryngoscope 1991, 101, 799. [CrossRef] [PubMed]
- Schneider, S.A.; Aggarwal, A.; Bhatt, M.; Dupont, E.; Tisch, S.; Limousin, P.; Lee, P.; Quinn, N.; Bhatia, K.P. Severe tongue protrusion dystonia Clinical syndromes and possible treatment. Neurology 2006, 67, 940–943. [CrossRef]
- Esper, C.D.; Freeman, A.; Factor, S.A. Lingual protrusion dystonia: Frequency, etiology and botulinum toxin therapy. Park. Relat. Disord. 2010, 16, 438–441. [CrossRef] [PubMed]
- Nastasi, L.; Mostile, G.; Nicoletti, A.; Zappia, M.; Reggio, E.; Catania, S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J. Neurol. 2016, 263, 1702–1708. [CrossRef] [PubMed]
- Charles, P.D.; Davis, T.L.; Shannon, K.M.; Hook, M.A.; Warner, J.S. Tongue protrusion dystonia: Treatment with botulinum toxin. South Med. J. 1997, 90, 522–525. [CrossRef] [PubMed]
- Ondo,W. Lingual dystonia following electrical injury. Mov. Disord. 1997, 12, 253. [CrossRef] [PubMed]
- Hennings, J.M.H.; Krause, E.; Bötzel, K.; Wetter, T.C. Successful treatment of tardive lingual dystonia with botulinum toxin: Case report and review of the literature. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1167–1171. [CrossRef] [PubMed]
- Kasravi, N.; Jog, M.S. Botulinum toxin in the treatment of lingual movement disorders. Mov. Disord. 2009, 24, 2199–2202. [CrossRef] [PubMed]
- Yoshida, K. Clinical characteristics of functional movement disorders in the stomatognathic system. Front. Neurol. 2020, 11: 23, doi: 10.3389/fneur.2020.00123.
- Sankhla, C.; Lai, E.C.; Jankovic, J. Peripherally induced oromandibular dystonia. J. Neurol. Neurosurg. Psychiatry 1998, 65, 722–728. doi: 10.1136/jnnp.65.5.722
- Raphael, K.G.; Tadinada, A.; Bradshaw, J.M.; Janal, M.N.; Sirois, D.A.; Chan, K.C.; Lurie, A.G. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: A pilot study. J. Oral Rehabil. 2014, 41, 555–563. doi: 10.1111/joor.12180.
- Lee, H.J.; Kim, S.J.; Lee, K.J.; Yu, H.S.; Baik, H.S. Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: a longitudinal study. Korean J. Orthod. 2017, 47, 222–228. doi: 10.4041/kjod.2017.47.4.222.
- Chang, C.S.; Bergeron, L.; Yu, C.C.; Chen, P.K.; Chen, Y.R. Mandible changes evaluated by computed tomography following Botulinum Toxin A injections in square-faced patients. Aesthetic Plast. Surg. 2011, 35, 452–455. doi: 10.1007/s00266-010-9624-5.
- Merz, R.I.; Deakin, J.; Hawthorne, M.R. Oromandibular dystonia questionnaire (OMDQ-25): a valid and reliable instrument for measuring health-related quality of life. Clin. Otolaryngol. 2010, 35, 390–396. doi: 10.1111/j.1749-4486.2010.02194.x
- Simpson, D.M.; Blitzer, A.; Brashear, A.; Comella, C.; Dubinsky, R.; Hallett, M.; Jankovic, J.; Karp, B.; Ludlow, C.L.; Miyasaki, J.M. et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1699–1706. [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J. et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [CrossRef]
- Albanese, A.; Asmus, F.; Bhatia, K.P.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; Valls-Sole, J. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur, J. Neurol. 2011, 18, 5–18.
- France, K.; Stoopler, E.T. The American Academy of Oral Medicine Clinical Practice Statement: Oromandibular dystonia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 283–285. doi: 10.1016/j.oooo.2018.01.023.




