
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhao DongXu | -- | 4453 | 2022-05-26 11:55:50 | | | |
| 2 | Beatrix Zheng | -108 word(s) | 4345 | 2022-05-27 07:57:31 | | | | |
| 3 | Beatrix Zheng | -1 word(s) | 4344 | 2022-06-02 07:56:41 | | | | |
| 4 | Beatrix Zheng | -104 word(s) | 4240 | 2022-06-02 08:23:59 | | | | |
| 5 | Beatrix Zheng | + 2 word(s) | 4242 | 2022-06-02 08:25:22 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) is the sixth most commonly malignant tumor and the third leading cause of cancer-related death in the world, and the early diagnosis and treatment of patients with HCC is core in improving its prognosis. The early diagnosis of HCC depends largely on magnetic resonance imaging (MRI). MRI has good soft-tissue resolution, which is the international standard method for the diagnosis of HCC. However, MRI is still insufficient in the diagnosis of some early small HCCs and malignant nodules, resulting in false negative results. With the deepening of research on HCC, researchers have found many specific molecular biomarkers on the surface of HCC cells, which may assist in diagnosis and treatment. On the other hand, molecular imaging has progressed rapidly in recent years, especially in the field of cancer theranostics. Hence, the preparation of molecular imaging probes that can specifically target the biomarkers of HCC, combined with MRI testing in vivo, may achieve the theranostic purpose of HCC in the early stage.
1. Alpha-Fetoprotein
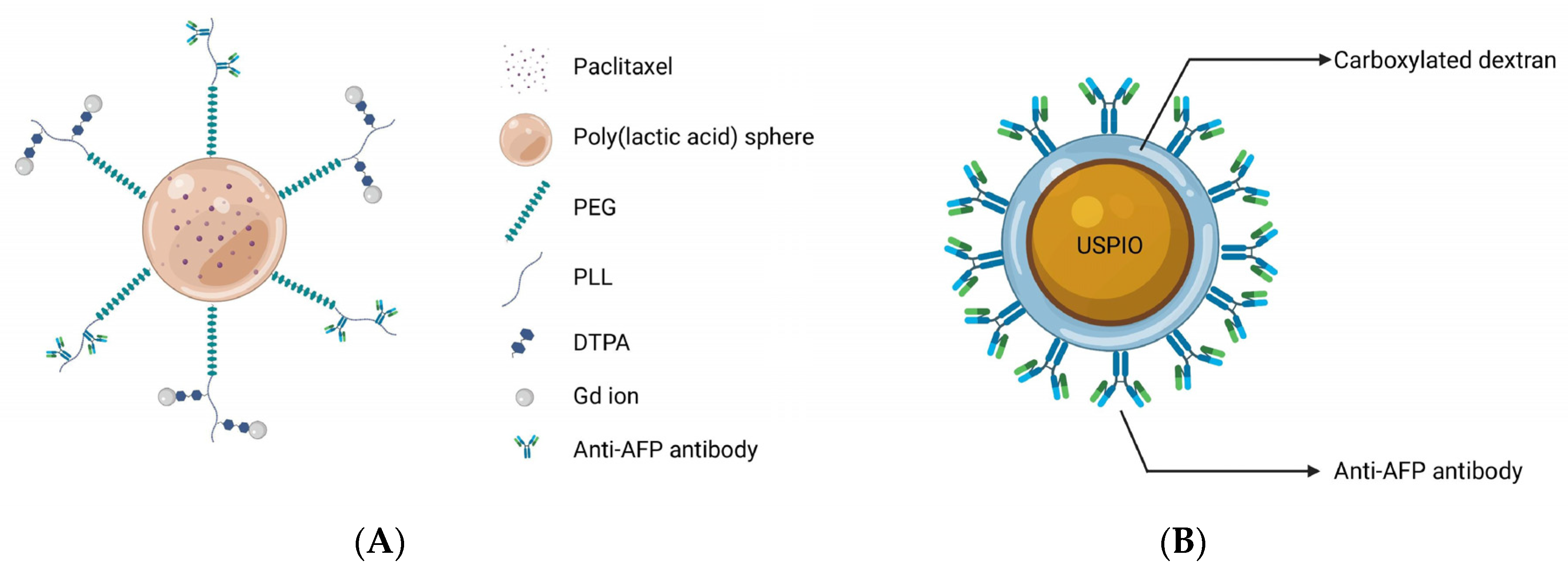
2. Glypican-3
3. Folate Receptors
4. VEGF/VEGFR
5. Integrin
6. Endoglin (CD105)
7. Asialoglycoprotein Receptor
8. CD44
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236.
- Tatarinov, Y.S.; Afanaseva, A.V.; Parfenova, L.F. Formation of serum proteins in human ontogeny. Fed. Proc. Transl. Suppl. 1964, 23, 437–440.
- Peterson, M.L.; Ma, C.; Spear, B.T. Zhx2 and Zbtb20: Novel regulators of postnatal alpha-fetoprotein repression and their potential role in gene reactivation during liver cancer. Semin. Cancer Biol. 2011, 21, 21–27.
- Liu, Y.; Li, J.; Liu, F.; Feng, L.; Yu, D.; Zhang, N. Theranostic Polymeric Micelles for the Diagnosis and Treatment of Hepatocellular Carcinoma. J. Biomed. Nanotechnol. 2015, 11, 613–622.
- Li, Y.W.; Chen, Z.G.; Zhao, Z.S.; Li, H.L.; Wang, J.C.; Zhang, Z.M. Preparation of magnetic resonance probes using one-pot method for detection of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 4275–4283.
- Chen, Y.; Lu, J.; Yang, J.; Hao, K.; Li, M. Investigation of Alpha-Fetoprotein Antibody Modified Fluorescent Magnetic Probe on HepG2 Cell and Cancer Model Mouse. J. Nanosci. Nanotechnol. 2020, 20, 5147–5150.
- Zhang, Q.; Lu, Y.; Xu, X.; Li, S.; Du, Y.; Yu, R. MR molecular imaging of HCC employing a regulated ferritin gene carried by a modified polycation vector. Int. J. Nanomed. 2019, 14, 3189–3201.
- Lu, C.Y.; Ji, J.S.; Zhu, X.L.; Tang, P.F.; Zhang, Q.; Zhang, N.N.; Wang, Z.H.; Wang, X.J.; Chen, W.Q.; Hu, J.B.; et al. T2-Weighted Magnetic Resonance Imaging of Hepatic Tumor Guided by SPIO-Loaded Nanostructured Lipid Carriers and Ferritin Reporter Genes. ACS Appl. Mater. Interfaces 2017, 9, 35548–35561.
- Yuan, C.; An, Y.; Zhang, J.; Li, H.; Zhang, H.; Wang, L.; Zhang, D. Magnetic nanoparticles for targeted therapeutic gene delivery and magnetic-inducing heating on hepatoma. Nanotechnology 2014, 25, 345101.
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97.
- Miao, H.L.; Pan, Z.J.; Lei, C.J.; Wen, J.Y.; Li, M.Y.; Liu, Z.K.; Qiu, Z.D.; Lin, M.Z.; Chen, N.P.; Chen, M. Knockdown of GPC3 inhibits the proliferation of Huh7 hepatocellular carcinoma cells through down-regulation of YAP. J. Cell. Biochem. 2013, 114, 625–631.
- Zittermann, S.I.; Capurro, M.I.; Shi, W.; Filmus, J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int. J. Cancer 2010, 126, 1291–1301.
- Sun, B.; Huang, Z.; Wang, B.; Yu, Y.; Lin, S.; Luo, L.; Wang, Y.; Huang, Z. Significance of Glypican-3 (GPC3) Expression in Hepatocellular Cancer Diagnosis. Med. Sci. Monit. 2017, 23, 850–855.
- Zhou, F.; Shang, W.; Yu, X.; Tian, J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med. Res. Rev. 2018, 38, 741–767.
- Iglesias, B.V.; Centeno, G.; Pascuccelli, H.; Ward, F.; Peters, M.G.; Filmus, J.; Puricelli, L.; de Kier Joffé, E.B. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol. Histopathol. 2008, 23, 1333–1340.
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224.
- Hsu, H.C.; Cheng, W.; Lai, P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: Biological significance and temporospatial distribution. Cancer Res. 1997, 57, 5179–5184.
- Zhu, Z.W.; Friess, H.; Wang, L.; Abou-Shady, M.; Zimmermann, A.; Lander, A.D.; Korc, M.; Kleeff, J.; Büchler, M.W. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001, 48, 558–564.
- Park, J.O.; Stephen, Z.; Sun, C.; Veiseh, O.; Kievit, F.M.; Fang, C.; Leung, M.; Mok, H.; Zhang, M. Glypican-3 targeting of liver cancer cells using multifunctional nanoparticles. Mol. Imaging 2011, 10, 69–77.
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 antibody functionalized Prussian blue nanoparticles for targeted MR imaging and photothermal therapy of hepatocellular carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696.
- Primus, F.J.; Bennett, S.J.; Kim, E.E.; DeLand, F.H.; Zahn, M.C.; Goldenberg, D.M. Circulating immune complexes in cancer patients receiving goat radiolocalizing antibodies to carcinoembryonic antigen. Cancer Res. 1980, 40, 497–501.
- Milenic, D.E.; Detrick, B.; Reynolds, J.C.; Colcher, D. Characterization of primate antibody responses to administered murine monoclonal immunoglobulin. Int. J. Biol. Markers 1990, 5, 177–187.
- Hofmeister, L.H.; Lee, S.H.; Norlander, A.E.; Montaniel, K.R.; Chen, W.; Harrison, D.G.; Sung, H.J. Phage-display-guided nanocarrier targeting to atheroprone vasculature. ACS Nano 2015, 9, 4435–4446.
- Ye, Y.; Zhu, L.; Ma, Y.; Niu, G.; Chen, X. Synthesis and evaluation of new iRGD peptide analogs for tumor optical imaging. Bioorg. Med. Chem. Lett. 2011, 21, 1146–1150.
- Song, X.; Shang, W.; Peng, L.; Jiang, H.; Wang, K.; Fang, C.; Tian, J. Novel GPC3-binding WS2-Ga3+-PEG-peptide nanosheets for in vivo bimodal imaging-guided photothermal therapy. Nanomedicine 2018, 13, 1681–1693.
- Tian, R.; Zhu, L.; Qin, Z.; Wang, G.; Wang, J.; Zhang, H. Glypican-3 (GPC3) targeted Fe3O4 core/Au shell nanocomplex for fluorescence/MRI/photoacoustic imaging-guided tumor photothermal therapy. Biomater. Sci. 2019, 7, 5258–5269.
- Niu, Z.S.; Niu, X.J.; Wang, W.H.; Zhao, J. Latest developments in precancerous lesions of hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 3305–3314.
- Kim, H.; Park, Y.N. Role of biopsy sampling for diagnosis of early and progressed hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 813–829.
- Schütte, K.; Schulz, C.; Link, A.; Malfertheiner, P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J. Hepatol. 2015, 7, 139–149.
- Li, B.; Liu, H.; Shang, H.W.; Li, P.; Li, N.; Ding, H.G. Diagnostic value of glypican-3 in alpha fetoprotein negative hepatocellular carcinoma patients. Afr. Health Sci. 2013, 13, 703–709.
- He, L.; Zhang, Y.; Chen, J.; Liu, G.; Zhu, J.; Li, X.; Li, D.; Yang, Y.; Lee, C.S.; Shi, J.; et al. A multifunctional targeted nanoprobe with high NIR-II PAI/MRI performance for precise theranostics of orthotopic early-stage hepatocellular carcinoma. J. Mater. Chem. B 2021, 9, 8779–8792.
- Deng, H.; Shang, W.; Lu, G.; Guo, P.; Ai, T.; Fang, C.; Tian, J. Targeted and Multifunctional Technology for Identification between Hepatocellular Carcinoma and Liver Cirrhosis. ACS Appl. Mater. Interfaces 2019, 11, 14526–14537.
- Cai, M.; Li, B.; Lin, L.; Huang, J.; An, Y.; Huang, W.; Zhou, Z.; Wang, Y.; Shuai, X.; Zhu, K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater. Sci. 2020, 8, 3485–3499.
- Ma, X.H.; Wang, S.; Liu, S.Y.; Chen, K.; Wu, Z.Y.; Li, D.F.; Mi, Y.T.; Hu, L.B.; Chen, Z.W.; Zhao, X.M. Development and in vitro study of a bi-specific magnetic resonance imaging molecular probe for hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3030–3043.
- Yan, Y.; Yang, C.; Dai, G.; Zhang, Y.; Tu, G.; Li, Y.; Yang, L.; Shu, J. Folic Acid-Conjugated CuFeSe2 Nanoparticles for Targeted T2-Weighted Magnetic Resonance Imaging and Computed Tomography of Tumors In Vivo. Int. J. Nanomed. 2021, 16, 6429–6440.
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994, 73, 2432–2443.
- Ebrahimnejad, P.; Sodagar Taleghani, A.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2022, 27, 471–489.
- Zhao, X.; Li, H.; Lee, R.J. Targeted drug delivery via folate receptors. Expert Opin. Drug Deliv. 2008, 5, 309–319.
- Bahrami, B.; Mohammadnia-Afrouzi, M.; Bakhshaei, P.; Yazdani, Y.; Ghalamfarsa, G.; Yousefi, M.; Sadreddini, S.; Jadidi-Niaragh, F.; Hojjat-Farsangi, M. Folate-conjugated nanoparticles as a potent therapeutic approach in targeted cancer therapy. Tumour Biol. 2015, 36, 5727–5742.
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424.
- Maghsoudinia, F.; Tavakoli, M.B.; Samani, R.K.; Hejazi, S.H.; Sobhani, T.; Mehradnia, F.; Mehrgardi, M.A. Folic acid-functionalized gadolinium-loaded phase transition nanodroplets for dual-modal ultrasound/magnetic resonance imaging of hepatocellular carcinoma. Talanta 2021, 228, 122245.
- Liu, J.; Wang, D.; Wang, G. Magnetic-gold theranostic nanoagent used for targeting quad modalities T1 & T2-MRI/CT/PA imaging and photothermal therapy of tumours. RSC Adv. 2021, 11, 18440–18447.
- Ling, D.; Xia, H.; Park, W.; Hackett, M.J.; Song, C.; Na, K.; Hui, K.M.; Hyeon, T. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano 2014, 8, 8027–8039.
- Chi, X.; Zhang, R.; Zhao, T.; Gong, X.; Wei, R.; Yin, Z.; Lin, H.; Li, D.; Shan, H.; Gao, J. Targeted arsenite-loaded magnetic multifunctional nanoparticles for treatment of hepatocellular carcinoma. Nanotechnology 2019, 30, 175101.
- Zhou, Z.; Lu, Z.R. Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 113, 24–48.
- Wang, S.W.; Liu, S.C.; Sun, H.L.; Huang, T.Y.; Chan, C.H.; Yang, C.Y.; Yeh, H.I.; Huang, Y.L.; Chou, W.Y.; Lin, Y.M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114.
- Pfister, N.T.; Fomin, V.; Regunath, K.; Zhou, J.Y.; Zhou, W.; Silwal-Pandit, L.; Freed-Pastor, W.A.; Laptenko, O.; Neo, S.P.; Bargonetti, J.; et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015, 29, 1298–1315.
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920.
- Liu, Y.; Chen, Z.; Liu, C.; Yu, D.; Lu, Z.; Zhang, N. Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials 2011, 32, 5167–5176.
- Huang, H.; Li, Y.; Li, C.; Wang, Y.; Sun, Y.; Wang, J. A novel anti-VEGF targeting and MRI-visible smart drug delivery system for specific diagnosis and therapy of liver cancer. Macromol. Biosci. 2013, 13, 1358–1368.
- Liu, D.; Liu, F.; Liu, Z.; Wang, L.; Zhang, N. Tumor specific delivery and therapy by double-targeted nanostructured lipid carriers with anti-VEGFR-2 antibody. Mol. Pharm. 2011, 8, 2291–2301.
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138.
- Liu, Y.; Wu, X.; Sun, X.; Wang, D.; Zhong, Y.; Jiang, D.; Wang, T.; Yu, D.; Zhang, N. Design, synthesis, and evaluation of VEGFR-targeted macromolecular MRI contrast agent based on biotin-avidin-specific binding. Int. J. Nanomed. 2017, 12, 5039–5052.
- Liu, Y.; Feng, L.; Liu, T.; Zhang, L.; Yao, Y.; Yu, D.; Wang, L.; Zhang, N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231–3242.
- Cabodi, S.; del Pilar Camacho-Leal, M.; Di Stefano, P.; Defilippi, P. Integrin signalling adaptors: Not only figurants in the cancer story. Nat. Rev. Cancer 2010, 10, 858–870.
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215.
- Becchetti, A.; Arcangeli, A. Integrins and ion channels in cell migration: Implications for neuronal development, wound healing and metastatic spread. Adv. Exp. Med. Biol. 2010, 674, 107–123.
- Li, J.; Zhou, P.; Li, L.; Zhang, Y.; Shao, Y.; Tang, L.; Tian, S. Effects of Cationic Microbubble Carrying CD/TK Double Suicide Gene and αVβ3 Integrin Antibody in Human Hepatocellular Carcinoma HepG2 Cells. PLoS ONE 2016, 11, e0158592.
- Kumar, C.C. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr. Drug Targets 2003, 4, 123–131.
- Lin, B.Q.; Zhang, W.B.; Zhao, J.; Zhou, X.H.; Li, Y.J.; Deng, J.; Zhao, Q.; Fu, G.; Xie, C.M.; Xu, Y.K.; et al. An Optimized Integrin α6-Targeted Magnetic Resonance Probe for Molecular Imaging of Hepatocellular Carcinoma in Mice. J. Hepatocell. Carcinoma 2021, 8, 645–656.
- Xu, Y.H.; Yang, J.; Meng, J.; Wang, H. Targeted MR Imaging Adopting T1-Weighted Ultra-Small Iron Oxide Nanoparticles for Early Hepatocellular Carcinoma: An in vitro and in vivo Study. Chin. Med. Sci. J. 2020, 35, 142–150.
- Jia, Z.; Song, L.; Zang, F.; Song, J.; Zhang, W.; Yan, C.; Xie, J.; Ma, Z.; Ma, M.; Teng, G.; et al. Active-target T1-weighted MR Imaging of Tiny Hepatic Tumor via RGD Modified Ultra-small Fe3O4 Nanoprobes. Theranostics 2016, 6, 1780–1791.
- Khatik, R.; Wang, Z.; Zhi, D.; Kiran, S.; Dwivedi, P.; Liang, G.; Qiu, B.; Yang, Q. Integrin alphavbeta3 Receptor Overexpressing on Tumor-Targeted Positive MRI-Guided Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 163–176.
- Wu, C.; Gong, F.; Pang, P.; Shen, M.; Zhu, K.; Cheng, D.; Liu, Z.; Shan, H. An RGD-modified MRI-visible polymeric vector for targeted siRNA delivery to hepatocellular carcinoma in nude mice. PLoS ONE 2013, 8, e66416.
- Shen, J.M.; Li, X.X.; Fan, L.L.; Zhou, X.; Han, J.M.; Jia, M.K.; Wu, L.F.; Zhang, X.X.; Chen, J. Heterogeneous dimer peptide-conjugated polylysine dendrimer-Fe3O4 composite as a novel nanoscale molecular probe for early diagnosis and therapy in hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 1183–1200.
- Yang, H.; Zhuang, Y.; Sun, Y.; Dai, A.; Shi, X.; Wu, D.; Li, F.; Hu, H.; Yang, S. Targeted dual-contrast T1- and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593.
- Chen, Q.; Shang, W.; Zeng, C.; Wang, K.; Liang, X.; Chi, C.; Liang, X.; Yang, J.; Fang, C.; Tian, J. Theranostic imaging of liver cancer using targeted optical/MRI dual-modal probes. Oncotarget 2017, 8, 32741–32751.
- Zhang, Y.; Zhao, J.; Cai, J.; Ye, J.C.; Xiao, Y.T.; Mei, Y.; Zeng, M.S.; Xie, C.M.; Jiang, Y.; Feng, G.K. Integrin alpha6-Targeted Magnetic Resonance Imaging of Hepatocellular Carcinoma in Mice. Mol. Imaging Biol. 2020, 22, 864–872.
- Brooks, D.L.; Schwab, L.P.; Krutilina, R.; Parke, D.N.; Sethuraman, A.; Hoogewijs, D.; Schörg, A.; Gotwald, L.; Fan, M.; Wenger, R.H.; et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol. Cancer 2016, 15, 26.
- Kariya, Y.; Kariya, Y.; Gu, J. Roles of Integrin α6β4 Glycosylation in Cancer. Cancers 2017, 9, 79.
- Krebsbach, P.H.; Villa-Diaz, L.G. The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017, 26, 1090–1099.
- Feng, G.K.; Ye, J.C.; Zhang, W.G.; Mei, Y.; Zhou, C.; Xiao, Y.T.; Li, X.L.; Fan, W.; Wang, F.; Zeng, M.S. Integrin α6 targeted positron emission tomography imaging of hepatocellular carcinoma in mouse models. J. Control. Release 2019, 310, 11–21.
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019, 567, 257–261.
- Ke, A.W.; Shi, G.M.; Zhou, J.; Huang, X.Y.; Shi, Y.H.; Ding, Z.B.; Wang, X.Y.; Devbhandari, R.P.; Fan, J. CD151 amplifies signaling by integrin α6β1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology 2011, 140, 1629–1641.e1615.
- Ozaki, I.; Yamamoto, K.; Mizuta, T.; Kajihara, S.; Fukushima, N.; Setoguchi, Y.; Morito, F.; Sakai, T. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 1998, 43, 837–842.
- Ahmed, N.; Riley, C.; Rice, G.; Quinn, M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin. Exp. Metastasis 2005, 22, 391–402.
- Yang, L.Y.; Lu, W.Q.; Huang, G.W.; Wang, W. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer 2006, 6, 110.
- Dallas, N.A.; Samuel, S.; Xia, L.; Fan, F.; Gray, M.J.; Lim, S.J.; Ellis, L.M. Endoglin (CD105): A marker of tumor vasculature and potential target for therapy. Clin. Cancer Res. 2008, 14, 1931–1937.
- Gougos, A.; Letarte, M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J. Immunol. 1988, 141, 1925–1933.
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887.
- Zhong, L.; Zou, H.; Huang, Y.; Gong, W.; He, J.; Tan, J.; Lai, Z.; Li, Y.; Zhou, C.; Zhang, G.; et al. Magnetic Endoglin Aptamer Nanoprobe for Targeted Diagnosis of Solid Tumor. J. Biomed. Nanotechnol. 2019, 15, 352–362.
- Rosen, L.S.; Gordon, M.S.; Robert, F.; Matei, D.E. Endoglin for targeted cancer treatment. Curr. Oncol. Rep. 2014, 16, 365.
- Tang, S.L.; Bai, M.Y.; Wang, J.Y.; Hong, P.D. Development and application of micro-polysaccharide drug carriers incorporating doxorubicin and superparamagnetic iron oxide for bimodality treatment of hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 151, 304–313.
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. AntiCancer Res. 2011, 31, 2283–2290.
- Zhou, Z.; Wang, Z.; Zhao, L.; Li, G. Preparation and properties of magnetic-fluorescent endoglin aptamer nanoprobe. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 022035.
- Yan, H.; Gao, X.; Zhang, Y.; Chang, W.; Li, J.; Li, X.; Du, Q.; Li, C. Imaging Tiny Hepatic Tumor Xenografts via Endoglin-Targeted Paramagnetic/Optical Nanoprobe. ACS Appl. Mater. Interfaces 2018, 10, 17047–17057.
- Ashwell, G.; Harford, J. Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 1982, 51, 531–554.
- Ueno, S.; Mojic, M.; Ohashi, Y.; Higashi, N.; Hayakawa, Y.; Irimura, T. Asialoglycoprotein receptor promotes cancer metastasis by activating the EGFR-ERK pathway. Cancer Res. 2011, 71, 6419–6427.
- Kato, Y.; Onishi, H.; Machida, Y. Biological characteristics of lactosaminated N-succinyl-chitosan as a liver-specific drug carrier in mice. J. Control. Release 2001, 70, 295–307.
- Zhang, M.; Zhou, X.; Wang, B.; Yung, B.C.; Lee, L.J.; Ghoshal, K.; Lee, R.J. Lactosylated gramicidin-based lipid nanoparticles (Lac-GLN) for targeted delivery of anti-miR-155 to hepatocellular carcinoma. J. Control. Release 2013, 168, 251–261.
- Liu, H.; Wang, H.; Xu, Y.; Guo, R.; Wen, S.; Huang, Y.; Liu, W.; Shen, M.; Zhao, J.; Zhang, G.; et al. Lactobionic acid-modified dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of human hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 6944–6953.
- Liang, J.; Zhang, X.; Miao, Y.; Li, J.; Gan, Y. Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 2033–2044.
- Zeng, Y.; Zhang, D.; Wu, M.; Liu, Y.; Zhang, X.; Li, L.; Li, Z.; Han, X.; Wei, X.; Liu, X. nanohybrid for MRI/CT imaging and photothermal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 14266–14277.
- Cai, C.; Xie, Y.; Wu, L.; Chen, X.; Liu, H.; Zhou, Y.; Zou, H.; Liu, D.; Zhao, Y.; Kong, X.; et al. PLGA-based dual targeted nanoparticles enhance miRNA transfection efficiency in hepatic carcinoma. Sci. Rep. 2017, 7, 46250.
- Saravanakumar, G.; Deepagan, V.G.; Jayakumar, R.; Park, J.H. Hyaluronic acid-based conjugates for tumor-targeted drug delivery and imaging. J. Biomed. Nanotechnol. 2014, 10, 17–31.
- Spadea, A.; Rios de la Rosa, J.M.; Tirella, A.; Ashford, M.B.; Williams, K.J.; Stratford, I.J.; Tirelli, N.; Mehibel, M. Evaluating the Efficiency of Hyaluronic Acid for Tumor Targeting via CD44. Mol. Pharm. 2019, 16, 2481–2493.
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A.; Lee, S.; et al. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061–1077.e1066.
- Wang, R.; Luo, Y.; Yang, S.; Lin, J.; Gao, D.; Zhao, Y.; Liu, J.; Shi, X.; Wang, X. Hyaluronic acid-modified manganese-chelated dendrimer-entrapped gold nanoparticles for the targeted CT/MR dual-mode imaging of hepatocellular carcinoma. Sci. Rep. 2016, 6, 33844.
- Moon, M.; Thomas, R.G.; Heo, S.U.; Park, M.S.; Bae, W.K.; Heo, S.H.; Yim, N.Y.; Jeong, Y.Y. A Hyaluronic Acid-Conjugated Gadolinium Hepatocyte-Specific T1 Contrast Agent for Liver Magnetic Resonance Imaging. Mol. Imaging Biol. 2015, 17, 497–503.
- Yang, H.; Wang, N.; Mo, L.; Wu, M.; Yang, R.; Xu, X.; Huang, Y.; Lin, J.; Zhang, L.M.; Jiang, X. Reduction sensitive hyaluronan-SS-poly(epsilon-caprolactone) block copolymers as theranostic nanocarriers for tumor diagnosis and treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 9–18.
- Lee, J.; Gordon, A.C.; Kim, H.; Park, W.; Cho, S.; Lee, B.; Larson, A.C.; Rozhkova, E.A.; Kim, D.H. Targeted multimodal nano-reporters for pre-procedural MRI and intra-operative image-guidance. Biomaterials 2016, 109, 69–77.
- Zhang, F.; Jia, Y.; Zheng, X.; Shao, D.; Zhao, Y.; Wang, Z.; Dawulieti, J.; Liu, W.; Sun, M.; Sun, W.; et al. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019, 100, 352–364.




