
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimitrios Tsilingiris | -- | 3707 | 2022-05-25 09:54:28 | | | |
| 2 | Catherine Yang | Meta information modification | 3707 | 2022-05-25 10:20:37 | | |
Video Upload Options
Obesity is epidemiologically and likely, causally related to various hematological cancers, while both conditions may predispose to severe SARS-CoV-2 infection. The COVID-19 pandemic brought about a variety of obstacles with respect to numerous aspects in the management of hematological malignancies. Patients with hematologic malignancies faced a variety of challenges, pertinent to the nature of an underlying hematologic disorder itself as well as its therapy as a risk factor for severe SARS-CoV-2 infection, suboptimal vaccine efficacy and the need for uninterrupted medical observation and continued therapy. Obesity constitutes another factor which was acknowledged since the early days of the pandemic that predisposed people to severe COVID-19, and shares a likely causal link with the pathogenesis of a broad spectrum of hematologic cancers.
1. Association between Obesity and Hematologic Cancers with COVID-19 Outcomes
1.1. COVID-19 and Obesity
1.2. COVID-19 Outcomes in Obesity-Related Hematologic Malignancies
| Data Source, Reference | Population of Interest | Main Outcomes |
|---|---|---|
| Hematologic cancer registry of India [10] | 565 reports of patients of all ages from tertiary Indian centers with HM and laboratory-confirmed COVID-19 between 21 March 2020–20 March 2021 | ↑ mortality (aHR 2.85, 1.58–5.13) and severe disease (aOR 2.73, 1.45–5.12 for AML vs. ALL) No differences between AML and other hematologic diagnoses ↑ mortality among those not in remission (aOR 1.85, 1.18–2.89) No effects of corticosteroid treatment or exposure to monoclonal antibodies |
| European Hematology Association Survey [11] | 3,801 patients with HM and laboratory-confirmed COVID-19 from 132 hematology centers across Europe between March 2020–December 2020 | Highest death rates in AML (40%) and MDS (42.3%) Active malignancy associated with ↑mortality (aHR 1.86, 1.62–2.14) Among different HL diagnoses, only AML independently associated with ↑mortality (aHR 2.046, 1.18–3.56 vs. NHL) |
| Nationwide retrospective study in Israel [12] | 313 patients with HM and COVID-19 from 16 medical centers | Age > 70 years, arterial hypertension, active treatment associated with adverse outcomes Remdesivir treatment linked to ↓ mortality no effects of other treatment modalities (corticosteroids, enoxaparin, convalescent plasma) |
| Data from population-based registry in Madrid, Spain [13] | 833 patients with HM and COVID-19 from 27 medical centers between 28 February 2020 and 25 May 2020 | Overall, 62% severe/critical disease, 33% mortality (highest among AML and MDS patients, 40% and 42.3%, respectively) ↑ risk of death > 60 years, no effect of gender ↑ mortality for AML (aHR 2.22, 1.31–3.74 vs. NHL), Monoclonal antibody treatment and conventional chemotherapy (aHRs vs. nontreatment (aHRs 2.02, 1.14–3.60 and 1.50, 0.99–2.29 vs. no treatment, respectively) ↓ mortality for Ph-negative myeloproliferative disorders and treatment with hypomethylating agents (aHRs 0.33, 0.14–0.81 vs. NHL and 0.47, 0.23–0.94 vs. no treatment, respectively) |
| Case–control study from 2 Hospital in Wuhan province, China [14] | 13 cases among 128 hospitalized patients with HM and 16 HCWs with COVID-19 | ↑ mortality for those with HM vs. controls (62% vs. 0, p = 0.002) |
| Meta-analysis of 34 studies in adult and 5 in pediatric populations [15] | 3377 patients with HM from 39 studies in total | No effects of recent systemic overall antineoplastic or cytotoxic therapy (RRs 1.17, 0.83–1.64 and 1.29, 0.78–2.15 vs. no treatment, respectively) on COVID-19 mortality |
| Case control study from a nationwide database of patient electronic health records in the US [16] | 73 million patients, 517.580 with 8 types of HMs, 420 with SARS-CoV-2 infection up to 1 September 2020 | Significantly ↑ SARS-CoV-2 acquisition rates for HM vs. controls (overall aOR 11.9, 11.3–12.5 for diagnosis < 1 year, 2.3, 2.2–2.4 for prior diagnosis), highest among ALL, ET, MM, AML and lowest for PV ↑ Higher hospitalization and death rates for HM vs. non-HM |
| Prospective cohort study among patients enrolled UK Coronavirus Cancer Monitoring project [17] | 227 patients with HM (Leukemia, Lymphoma, MM, others) among 1044 with active cancer and documented SARS-CoV-2 infection between 18 March 2020–8 May 2020 |
↑ risk for adverse outcomes for HM vs. solid tumor patients (aORs for high flow oxygen therapy 1.82, 1.11–2.94, NIV 2.10, 1.14–3.76, ICU 2.73, 1.43–5.11, severe/critical disease 1.57, 1.15–2.15) ↑ in-hospital mortality for HM patients who recently received chemotherapy (1.57, 1.15–2.15 vs. no recent chemotherapy) |
2. Obesity, Related Hematologic Malignancies and Severe COVID-19: Pathogenetic Considerations
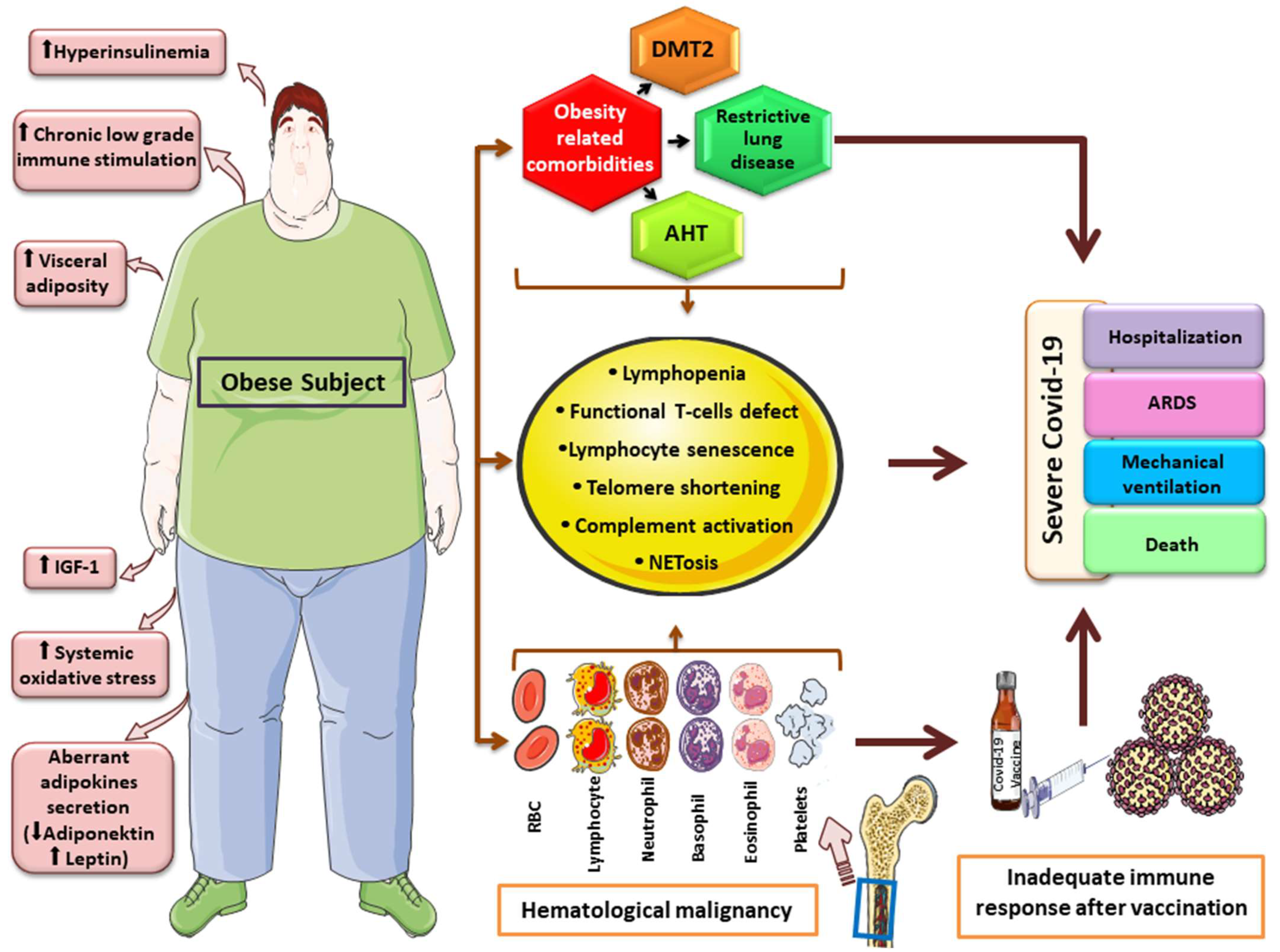
3. Vaccination against SARS-CoV-2 in Patients with Hematologic Malignancy
4. Blood Product Transfusion in the Era of COVID-19
References
- Tamara, A.; Tahapary, D.L. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes Metab. Syndr. 2020, 14, 655–659.
- Tsilingiris, D.; Dalamaga, M.; Liu, J. SARS-CoV-2 adipose tissue infection and hyperglycemia: A further step towards the understanding of severe COVID-19. Metabol. Open 2022, 13, 100163.
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378.
- Battisti, S.; Pedone, C.; Napoli, N.; Russo, E.; Agnoletti, V.; Nigra, S.G.; Dengo, C.; Mughetti, M.; Conte, C.; Pozzilli, P.; et al. Computed Tomography Highlights Increased Visceral Adiposity Associated With Critical Illness in COVID-19. Diabetes Care 2020, 43, e129–e130.
- Chandarana, H.; Dane, B.; Mikheev, A.; Taffel, M.T.; Feng, Y.; Rusinek, H. Visceral adipose tissue in patients with COVID-19: Risk stratification for severity. Abdom. Radiol. 2021, 46, 818–825.
- Watanabe, M.; Risi, R.; Tuccinardi, D.; Baquero, C.J.; Manfrini, S.; Gnessi, L. Obesity and SARS-CoV-2: A population to safeguard. Diabetes Metab. Res. Rev. 2020, 36, e3325.
- Gao, M.; Piernas, C.; Astbury, N.M.; Hippisley-Cox, J.; O’Rahilly, S.; Aveyard, P.; Jebb, S.A. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021, 9, 350–359.
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body Mass Index and Risk for COVID-19-Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March-December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361.
- Sjogren, L.; Stenberg, E.; Thuccani, M.; Martikainen, J.; Rylander, C.; Wallenius, V.; Olbers, T.; Kindblom, J.M. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden-A cohort study. PLoS ONE 2021, 16, e0257891.
- Jain, A.; Nayak, L.; Kulkarni, U.P.; Mehra, N.; Yanamandra, U.; Kayal, S.; Damodar, S.; John, J.M.; Mehta, P.; Singh, S.; et al. Outcomes of patients with hematologic malignancies and COVID-19 from the Hematologic Cancer Registry of India. Blood Cancer J. 2022, 12, 2.
- Pagano, L.; Salmanton-Garcia, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168.
- Levy, I.; Lavi, A.; Zimran, E.; Grisariu, S.; Aumann, S.; Itchaki, G.; Berger, T.; Raanani, P.; Harel, R.; Aviv, A.; et al. COVID-19 among patients with hematological malignancies: A national Israeli retrospective analysis with special emphasis on treatment and outcome. Leuk. Lymphoma 2021, 62, 3384–3393.
- Garcia-Suarez, J.; de la Cruz, J.; Cedillo, A.; Llamas, P.; Duarte, R.; Jimenez-Yuste, V.; Hernandez-Rivas, J.A.; Gil-Manso, R.; Kwon, M.; Sanchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133.
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645.
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martin-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892.
- Wang, Q.; Berger, N.A.; Xu, R. When hematologic malignancies meet COVID-19 in the United States: Infections, death and disparities. Blood Rev. 2021, 47, 100775.
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316.
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Romero Starke, K.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The isolated effect of age on the risk of COVID-19 severe outcomes: A systematic review with meta-analysis. BMJ Glob. Health 2021, 6, e006434.
- Henkens, M.; Raafs, A.G.; Verdonschot, J.A.J.; Linschoten, M.; van Smeden, M.; Wang, P.; van der Hooft, B.H.M.; Tieleman, R.; Janssen, M.L.F.; Ter Bekke, R.M.A.; et al. Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study. BMC Geriatr. 2022, 22, 184.
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2020, 11, 571416.
- Buikema, A.R.; Buzinec, P.; Paudel, M.L.; Andrade, K.; Johnson, J.C.; Edmonds, Y.M.; Jhamb, S.K.; Chastek, B.; Raja, H.; Cao, F.; et al. Racial and ethnic disparity in clinical outcomes among patients with confirmed COVID-19 infection in a large US electronic health record database. EClinicalMedicine 2021, 39, 101075.
- Fu, J.; Reid, S.A.; French, B.; Hennessy, C.; Hwang, C.; Gatson, N.T.; Duma, N.; Mishra, S.; Nguyen, R.; Hawley, J.E.; et al. Racial Disparities in COVID-19 Outcomes Among Black and White Patients With Cancer. JAMA Netw. Open 2022, 5, e224304.
- Arnautovic, J.; Mazhar, A.; Souther, B.; Mikhijan, G.; Boura, J.; Huda, N. Cardiovascular Factors Associated with Septic Shock Mortality Risks. Spartan Med. Res. J. 2018, 3, 6516.
- Mahalingam, M.; Moore, J.X.; Donnelly, J.P.; Safford, M.M.; Wang, H.E. Frailty Syndrome and Risk of Sepsis in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. J. Intensive Care Med. 2019, 34, 292–300.
- Nunes, J.P. Arterial hypertension and sepsis. Rev. Port. Cardiol. 2003, 22, 1375–1379.
- Papadimitriou-Olivgeris, M.; Aretha, D.; Zotou, A.; Koutsileou, K.; Zbouki, A.; Lefkaditi, A.; Sklavou, C.; Marangos, M.; Fligou, F. The Role of Obesity in Sepsis Outcome among Critically Ill Patients: A Retrospective Cohort Analysis. BioMed. Res. Int. 2016, 2016, 5941279.
- Wang, Z.; Ren, J.; Wang, G.; Liu, Q.; Guo, K.; Li, J. Association Between Diabetes Mellitus and Outcomes of Patients with Sepsis: A Meta-Analysis. Med. Sci. Monit. 2017, 23, 3546–3555.
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642.
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75.
- Silzle, T.; Blum, S.; Schuler, E.; Kaivers, J.; Rudelius, M.; Hildebrandt, B.; Gattermann, N.; Haas, R.; Germing, U. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prognostic value in IPSS-R-low-risk patients. Blood Cancer J. 2019, 9, 63.
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391.
- Sewell, R.L. Lymphocyte abnormalities in myeloma. Br. J. Haematol. 1977, 36, 545–551.
- Tanaka, S.; Isoda, F.; Ishihara, Y.; Kimura, M.; Yamakawa, T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: Adequate weight reduction can be corrective. Clin. Endocrinol. 2001, 54, 347–354.
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36.
- Li, W.; Lin, F.; Dai, M.; Chen, L.; Han, D.; Cui, Y.; Pan, P. Early predictors for mechanical ventilation in COVID-19 patients. Ther. Adv. Respir. Dis. 2020, 14, 1753466620963017.
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target Ther. 2020, 5, 33.
- Rahimmanesh, I.; Kouhpayeh, S.; Azizi, Y.; Khanahmad, H. Conceptual Framework for SARS-CoV-2-Related Lymphopenia. Adv. Biomed. Res. 2022, 11, 16.
- Garbo, R.; Valent, F.; Gigli, G.L.; Valente, M. Pre-Existing Lymphopenia Increases the Risk of Hospitalization and Death after SARS-CoV-2 Infection. Infect. Dis. Rep. 2022, 14, 20–25.
- Goupil, R.; Brachemi, S.; Nadeau-Fredette, A.C.; Deziel, C.; Troyanov, Y.; Lavergne, V.; Troyanov, S. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin. J. Am. Soc. Nephrol. 2013, 8, 416–423.
- Ortega-Loubon, C.; Cano-Hernandez, B.; Poves-Alvarez, R.; Munoz-Moreno, M.F.; Roman-Garcia, P.; Balbas-Alvarez, S.; de la Varga-Martinez, O.; Gomez-Sanchez, E.; Gomez-Pesquera, E.; Lorenzo-Lopez, M.; et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. J. Clin. Med. 2019, 8, 1512.
- Alahyari, S.; Rajaeinejad, M.; Jalaeikhoo, H.; Amani, D. Regulatory T Cells in Immunopathogenesis and Severity of COVID-19: A Systematic Review. Arch. Iran. Med. 2022, 25, 127–132.
- Banerjee, T.; Calvi, L.M.; Becker, M.W.; Liesveld, J.L. Flaming and fanning: The Spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019, 36, 57–69.
- Bosseboeuf, A.; Allain-Maillet, S.; Mennesson, N.; Tallet, A.; Rossi, C.; Garderet, L.; Caillot, D.; Moreau, P.; Piver, E.; Girodon, F.; et al. Pro-inflammatory State in Monoclonal Gammopathy of Undetermined Significance and in Multiple Myeloma Is Characterized by Low Sialylation of Pathogen-Specific and Other Monoclonal Immunoglobulins. Front. Immunol. 2017, 8, 1347.
- Craver, B.M.; El Alaoui, K.; Scherber, R.M.; Fleischman, A.G. The Critical Role of Inflammation in the Pathogenesis and Progression of Myeloid Malignancies. Cancers 2018, 10, 104.
- Purdue, M.P.; Hofmann, J.N.; Kemp, T.J.; Chaturvedi, A.K.; Lan, Q.; Park, J.H.; Pfeiffer, R.M.; Hildesheim, A.; Pinto, L.A.; Rothman, N. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood 2013, 122, 951–957.
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12.
- Pietrobon, A.J.; Teixeira, F.M.E.; Sato, M.N. I mmunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front. Immunol. 2020, 11, 579220.
- Nogueira, B.M.D.; Machado, C.B.; Montenegro, R.C.; MEA, D.E.M.; Moreira-Nunes, C.A. Telomere Length and Hematological Disorders: A Review. In Vivo 2020, 34, 3093–3101.
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; de Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475.
- Johnscher, G.; Woenckhaus, C.; Nicolas, J.C.; Pons, M.; Descomps, B.; Crastes de Paulet, A. Specific modification of 17 beta-estradiol dehydrogenase from human placenta by nicotinamide- (5-bromoacetyl-4-methylimidazole) dinucleotide. FEBS Lett. 1976, 61, 176–179.
- Aviv, A. Telomeres and COVID-19. FASEB J. 2020, 34, 7247–7252.
- Tsilingiris, D.; Tentolouris, A.; Eleftheriadou, I.; Tentolouris, N. Telomere length, epidemiology and pathogenesis of severe COVID-19. Eur. J. Clin. Investig. 2020, 50, e13376.
- Sanchez-Vazquez, R.; Guio-Carrion, A.; Zapatero-Gaviria, A.; Martinez, P.; Blasco, M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging 2021, 13, 1–15.
- Wang, Q.; Codd, V.; Raisi-Estabragh, Z.; Musicha, C.; Bountziouka, V.; Kaptoge, S.; Allara, E.; Angelantonio, E.D.; Butterworth, A.S.; Wood, A.M.; et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: A cohort study in UK Biobank. EBioMedicine 2021, 70, 103485.
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int. J. Mol. Sci. 2021, 22, 6151.
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879.
- Podaza, E.; Sabbione, F.; Risnik, D.; Borge, M.; Almejun, M.B.; Colado, A.; Fernandez-Grecco, H.; Cabrejo, M.; Bezares, R.F.; Trevani, A.; et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol. Immunother 2017, 66, 77–89.
- D’Abbondanza, M.; Martorelli, E.E.; Ricci, M.A.; De Vuono, S.; Migliola, E.N.; Godino, C.; Corradetti, S.; Siepi, D.; Paganelli, M.T.; Maugeri, N.; et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 2019, 9, 14678.
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157.
- Luo, S.; Wang, M.; Wang, H.; Hu, D.; Zipfel, P.F.; Hu, Y. How Does Complement Affect Hematological Malignancies: From Basic Mechanisms to Clinical Application. Front. Immunol. 2020, 11, 593610.
- Shim, K.; Begum, R.; Yang, C.; Wang, H. Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World J. Diabetes 2020, 11, 1–12.
- WHO. Who Sage Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply. Available online: https://www.who.int/docs/default-source/immunization/sage/covid/sage-prioritization-roadmap-covid19-vaccines.pdf (accessed on 11 February 2022).
- Butsch, W.S.; Hajduk, A.; Cardel, M.I.; Donahoo, W.T.; Kyle, T.K.; Stanford, F.C.; Zeltser, L.M.; Kotz, C.M.; Jastreboff, A.M. COVID-19 vaccines are effective in people with obesity: A position statement from The Obesity Society. Obesity 2021, 29, 1575–1579.
- Townsend, M.J.; Kyle, T.K.; Stanford, F.C. COVID-19 Vaccination and Obesity: Optimism and Challenges. Obesity 2021, 29, 634–635.
- Stampfer, S.D.; Goldwater, M.S.; Jew, S.; Bujarski, S.; Regidor, B.; Daniely, D.; Chen, H.; Xu, N.; Li, M.; Green, T.; et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021, 35, 3534–3541.
- Malard, F.; Gaugler, B.; Gozlan, J.; Bouquet, L.; Fofana, D.; Siblany, L.; Eshagh, D.; Adotevi, O.; Laheurte, C.; Ricard, L.; et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021, 11, 142.
- Teh, J.S.K.; Coussement, J.; Neoh, Z.C.F.; Spelman, T.; Lazarakis, S.; Slavin, M.A.; Teh, B.W. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: A systematic review and meta-analysis. Blood Adv. 2022, 6, 2014–2034.
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090.e2.
- Greenberger, L.M.; Saltzman, L.A.; Senefeld, J.W.; Johnson, P.W.; DeGennaro, L.J.; Nichols, G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021, 39, 1031–1033.
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Bene, M.C.; Chevallier, P. Safety and Antibody Response After 1 and 2 Doses of BNT162b2 mRNA Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant. JAMA Netw. Open 2021, 4, e2126344.
- Salvini, M.; Maggi, F.; Damonte, C.; Mortara, L.; Bruno, A.; Mora, B.; Brociner, M.; Mattarucchi, R.; Ingrassia, A.; Sirocchi, D.; et al. Immunogenicity of anti-SARS-CoV-2 Comirnaty vaccine in patients with lymphomas and myeloma who underwent autologous stem cell transplantation. Bone Marrow Transpl. 2022, 57, 137–139.
- Rimar, D.; Slobodin, G.; Paz, A.; Henig, I.; Zuckerman, T. SARS-COV-2 vaccination after stem cell transplantation for scleroderma. Ann. Rheum. Dis. 2021, 80, 1354–1355.
- Tamariz-Amador, L.E.; Battaglia, A.M.; Maia, C.; Zherniakova, A.; Guerrero, C.; Zabaleta, A.; Burgos, L.; Botta, C.; Fortuno, M.A.; Grande, C.; et al. Immune biomarkers to predict SARS-CoV-2 vaccine effectiveness in patients with hematological malignancies. Blood Cancer J. 2021, 11, 202.
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428.
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211.
- CDC. COVID-19 Vaccines for Moderately or Severely Immunocompromised People. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 2 March 2022).
- Benotmane, I.; Bruel, T.; Planas, D.; Fafi-Kremer, S.; Schwartz, O.; Caillard, S. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. 2022, 101, 1073–1076.
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. 4th Dose COVID mRNA Vaccines’ Immunogenicity & Efficacy Against Omicron VOC. MedRxiv 2022.
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graca, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat. Commun. 2022, 13, 864.
- Wood, E.M.; McQuilten, Z.K. Outpatient transfusions for myelodysplastic syndromes. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 167–174.
- Bakkour, S.; Saa, P.; Groves, J.A.; Montalvo, L.; Di Germanio, C.; Best, S.M.; Grebe, E.; Livezey, K.; Linnen, J.M.; Strauss, D.; et al. Minipool testing for SARS-CoV-2 RNA in United States blood donors. Transfusion 2021, 61, 2384–2391.
- Cappy, P.; Candotti, D.; Sauvage, V.; Lucas, Q.; Boizeau, L.; Gomez, J.; Enouf, V.; Chabli, L.; Pillonel, J.; Tiberghien, P.; et al. No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood 2020, 136, 1888–1891.
- Cho, H.J.; Koo, J.W.; Roh, S.K.; Kim, Y.K.; Suh, J.S.; Moon, J.H.; Sohn, S.K.; Baek, D.W. COVID-19 transmission and blood transfusion: A case report. J. Infect. Public. Health 2020, 13, 1678–1679.
- Kwon, S.Y.; Kim, E.J.; Jung, Y.S.; Jang, J.S.; Cho, N.S. Post-donation COVID-19 identification in blood donors. Vox. Sang. 2020, 115, 601–602.
- Lee, C.K.; Leung, J.N.S.; Cheng, P.; Lung, D.C.; To, K.K.W.; Tsang, D.N.C. Absence of SARS-CoV-2 viraemia in a blood donor with COVID-19 post-donation. Transfus. Med. 2021, 31, 223–224.
- Liapis, K.; Papoutselis, M.; Vrachiolias, G.; Misidou, C.; Spanoudakis, E.; Bezirgiannidou, Z.; Pentidou, A.; Konstantinidis, T.; Kotsianidis, I. Blood and platelet transfusion from a donor with presymptomatic COVID-19. Ann. Hematol. 2021, 100, 2133–2134.
- Sekeres, M.A.; Steensma, D.P.; DeZern, A.; Roboz, G.; Garcia-Manero, G.; Komrokji, R. COVID-19 and Myelodysplastic Syndromes: Frequently Asked Questions. Available online: https://www.hematology.org/covid-19/covid-19-and-myelodysplastic-syndromes (accessed on 26 February 2022).
- Carson, J.L.; Stanworth, S.J.; Dennis, J.A.; Trivella, M.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.; Doree, C.; Hebert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst. Rev. 2021, 12, CD002042.
- Hammami, E.; Hadhri, M.; Fekih Salem, S.; Ben Lakhal, F.; El Borgi, W.; Gouider, E. COVID-19 induced blood supply shortage: A Tunisian blood deposit perspective. Transfus Clin. Biol. 2022, 29, 102–104.
- Delabranche, X.; Kientz, D.; Tacquard, C.; Bertrand, F.; Roche, A.C.; Tran Ba Loc, P.; Humbrecht, C.; Sirlin, F.; Pivot, X.; Collange, O.; et al. Impact of COVID-19 and lockdown regarding blood transfusion. Transfusion 2021, 61, 2327–2335.
- Tripathi, P.P.; Kumawat, V.; Patidar, G.K. Donor’s Perspectives on Blood Donation During COVID-19 Pandemic. Indian J. Hematol. Blood Transfus. 2021, 30, 1–10.
- Velazquez-Kennedy, K.; Luna, A.; Sanchez-Tornero, A.; Jimenez-Chillon, C.; Jimenez-Martin, A.; Valles Carboneras, A.; Tenorio, M.; Garcia Garcia, I.; Lopez-Jimenez, F.J.; Moreno-Jimenez, G. Transfusion support in COVID-19 patients: Impact on hospital blood component supply during the outbreak. Transfusion 2021, 61, 361–367.
- Yuan, Z.; Chen, D.; Chen, X.; Wei, Y. Estimation of the number of blood donors during the COVID-19 incubation period across China and analysis of prevention and control measures for blood transfusion transmission. Transfusion 2020, 60, 1778–1784.
- Stanworth, S.J.; New, H.V.; Apelseth, T.O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020, 7, e756–e764.
- Al-Riyami, A.Z.; Abdella, Y.E.; Badawi, M.A.; Panchatcharam, S.M.; Ghaleb, Y.; Maghsudlu, M.; Satti, M.; Lahjouji, K.; Merenkov, Z.; Adwan, A.; et al. The impact of COVID-19 pandemic on blood supplies and transfusion services in Eastern Mediterranean Region. Transfus. Clin. Biol. 2021, 28, 16–24.
- Loua, A.; Kasilo, O.M.J.; Nikiema, J.B.; Sougou, A.S.; Kniazkov, S.; Annan, E.A. Impact of the COVID-19 pandemic on blood supply and demand in the WHO African Region. Vox. Sang. 2021, 116, 774–784.
- Osaro, E.; Charles, A.T. The challenges of meeting the blood transfusion requirements in Sub-Saharan Africa: The need for the development of alternatives to allogenic blood. J. Blood Med. 2011, 2, 7–21.
- Mirski, M.A.; Frank, S.M.; Kor, D.J.; Vincent, J.L.; Holmes, D.R., Jr. Restrictive and liberal red cell transfusion strategies in adult patients: Reconciling clinical data with best practice. Crit. Care 2015, 19, 202.




