1. Association between Obesity and Hematologic Cancers with COVID-19 Outcomes
1.1. COVID-19 and Obesity
Since the beginning of the pandemic, obesity was recognized as a major factor associated with SARS-CoV-2 infection adverse outcomes
[1][2][55,56]. From a meta-analysis of 30 early studies that reported specific outcomes with respect to measures of adiposity, an overall adjusted odds ratio (OR) of 2.09 (1.67, 2.62) for severe COVID-19 among patients with a “high” compared with those with a low BMI was noted. The adjusted ORs for individual outcomes were 2.36 for hospitalization, 2.32 for intensive care unit (ICU) admission, 2.63 for mechanical ventilation and 1.49 for death
[3][5]. Additionally, a considerably higher visceral adipose tissue (VAT) accumulation was ascertained among those experiencing adverse outcomes compared with those with mild disease by three studies
[4][5][6][57,58,59]. It should be noted that even though most included studies used the conventional cut off of 30 kg/m
2, there were others that deviated from this criterion to discriminate between patients with high and low BMI, whereas no clear definition was given in six studies
[3][5]. Later studies confirmed these findings, further highlighting a dose-dependent relationship between the severity of obesity and risk for most adverse COVID-19 outcomes
[7][8][9][60,61,62]. Interestingly, the overall relationship of BMI with serious SARS-CoV-2 infection conforms to a J-shaped curve, with those at the lowest adiposity extreme being more prone to an adverse course, hence, highlighting another manifestation of the “Obesity paradox” in the frame of COVID-19
[7][8][60,61].
1.2. COVID-19 Outcomes in Obesity-Related Hematologic Malignancies
An overview of the studies on the impact of hematologic malignancies on SARS-CoV-2 outcomes is presented in Table 12.
Table 12.
Overview of key studies addressing the impact of hematologic malignancies on COVID-19 outcomes.
The earliest evidence regarding SARS-CoV-2 infection in patients with hematologic malignancies originated from a small cohort study among hospitalized patients in hematologic wards at the time of the initial outbreak in Wuhan, China (
Table 12). There were similar SARS-CoV-2 acquisition rates among patients compared with a matched controlled group of health care workers, but a higher incidence of severe disease and death
[14][4].
Furthermore, an analysis of the data of the openSAFELY platform in the United Kingdom pointed towards increased mortality among patients with hematologic malignancies. The risk was highest (2.5-fold) among those who had received the diagnosis in the last 1–5 years and slightly decreased thereafter, either reflecting the acute detrimental effects of treatment or resulting from survivalist bias among patients with greater survival rates
[18][70]. A subsequent meta-analysis of 39 reports (5 pediatric and 34 among adults) attempted a more comprehensive evaluation of the effects of age, treatment and specific diagnosis on the risk of death from COVID-19. The risk of death in adults was found to be higher than in pediatric hematologic patients (34% vs. 4%), and among those, higher for those aged >60 (relative risk: RR 1.8) as well as lower among white compared to non-white patients (RR 2.2). The pooled risk was substantially higher than this observed in the general population. The decisive effect of age on COVID-19 outcomes observed in this study is in agreement with the vast majority of available reports in diverse populations. The relationship between age and the risk of hospitalization or death due to SARS-CoV-2 infection appears to be linear, without distinct age cut-offs, above which an additional risk for severe disease is conferred
[19][71]. Furthermore, it is likely that the effect of age is not mediated by the increased co-morbidity burden in the elderly population
[20][72]. Although the factors that drive this relationship are not fully elucidated, a crucial role of the age-related immune senescence may be hypothesized
[21][73]. Likewise, an increased risk for severe disease among black patients and racial minorities compared to white patients has been also observed in numerous cohorts. There are likely to be numerous and diverse factors that could explain the racial disparities regarding COVID-19 outcomes. These include a compromised socioeconomic status and poorer living/working conditions, limited or delayed access to health care services, higher prevalence of relevant comorbidities, poor nutritional habits, more frequent smoking and increased psychosocial stress
[22][23][74,75]. Interestingly, the presence of any or solely cytotoxic recent anticancer therapy was not shown to have an effect on mortality compared to the absence of treatment. It is also noteworthy that, excluding patients with acquired bone marrow failure syndrome (commonly in the frame of MDS or aplastic anemia) which showed a peak mortality rate of 53%, the risk of death was relatively homogenous for the rest of the diagnosis, ranging from 31–33% for those with CLL, lymphomas and MM to 41% for those with acute leukemias
[15][67].
2. Obesity, Related Hematologic Malignancies and Severe COVID-19: Pathogenetic Considerations
It may not come as a surprise that two epidemiologically- and possibly causally-related conditions—obesity and hematologic malignancies—both belong to the known factors predisposing individuals to adverse COVID-19 prognoses (
Figure 1). The organ-specific, obesity-related comorbidities—such as diabetes mellitus and arterial hypertension—that established cardiovascular and restrictive pulmonary disease on one hand, and the overall frailty that characterizes certain groups of individuals affected with hematologic cancers on the other, predispose to adverse outcomes in acute illnesses of various causes, including sepsis
[24][25][26][27][28][76,77,78,79,80]. Nonetheless, specific features of the systemic immune response in the frame of SARS-CoV-2 infection can be hypothesized to influence the course of the disease among individuals affected with obesity and/or hematologic malignancies.
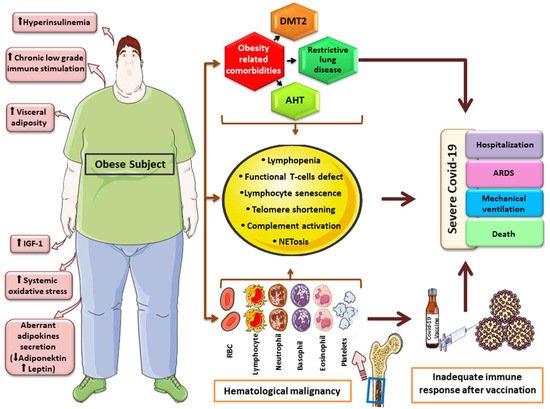
Figure 1. Graphical overview of factors and mechanisms linking obesity, hematologic malignancy and severe COVID-19 course. Obesity has been linked to carcinogenesis through aberrant cytokine and adipokine production, hyperinsulinemia, increase growth-factor levels (IGF-1), elevated oxidative-stress and chronic low-grade inflammation. Obesity-related comorbidities, along with features accompanying hematologic malignancies, such as quantitative and qualitative immune system defects and telomere shortening, may lead to an inadequate immune response after COVID-19 vaccination and severe clinical outcomes. * This image was derived from the free medical site
http://smart.servier.com/ (accessed on 31 March 2022) by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.
Successful host defense and viral clearance requires a multifaceted innate and acquired cellular immune response, orchestrated by a complex variety of cellular and humoral components. Of crucial importance are a timely pro- and anti-inflammatory cytokine cascade and the balanced involvement of CD4+/CD8+ and B lymphocytes. An aberrantly exaggerated, uncoordinated immune response may drive the elusive manifestation of the hyperinflammation syndrome
[29][81].
Quantitative and qualitative defects of the immune system are most prominent in hematologic malignancies but are encountered in obesity as well
[30][82]. Lymphopenia is a prominent feature of a broad variety of hematologic malignancies such as MDS
[31][83], Hodgkin disease, NHL
[32][84] and MM
[33][85], and can complicate therapy. ALL can be viewed as a state of “functional” lymphopenia despite the occasionally extremely high lymphocyte counts. Quantitative and qualitative T-lymphocyte defects have also been described in obesity and may be partially reversible following successful weight loss
[34][86]. Interestingly, the association between lymphopenia and adverse SARS-CoV-2 outcomes, including hospitalization, ARDS, mechanical ventilation, and death, has been observed in numerous studies
[35][36][37][87,88,89]. Besides being a consequence of SARS-CoV-2 infection itself
[38][90], which may imply a component of reverse causality for this observation, pre-existing lymphopenia has also been shown to predispose people to severe COVID-19
[39][91], which advocates for a crucial role of T cell-mediated immune response for successful viral clearance and infection resolution. Furthermore, lymphopenia, either preceding or developing in the course of SARS-CoV-2 infection, may facilitate the development and/or increase the severity of secondary bacterial and/or fungal infections that may further adversely affect prognosis
[40][41][92,93]. Alternatively, a prominent reduction in regulatory T cells could predispose people to an exaggerated immune response and cytokine storm which are major features of the COVID-19-related hyperinflammation syndrome
[42][94]. A state of systemic chronic low-grade inflammation is a feature of both obesity and various hematologic malignant states, such as MDS, acute leukemias, MM and NHL
[43][44][45][46][95,96,97,98]. This feature can promote or be inherent in “inflammaging”, which refers to a state of immune cell senescence and dysregulation
[47][99], predisposing to adverse COVID-19-related outcomes
[47][48][99,100]. Lymphocyte telomere shortening constitutes an important component of lymphocyte senescence and dysfunction, which is typically encountered in hematologic malignancies, occasionally in conjunction with an adverse prognosis
[49][101]. Telomere length has been also shown to inversely associate with BMI
[50][102] and correlates with the negative metabolic consequences of excess adiposity
[51][103], while weight loss causes a dose-dependent elongation
[47][99]. A shorter telomere length has been suggested as the epidemiologic link between known clinical and epidemiologic risk factors for severe COVID-19, implicating impaired lymphocyte replication and recruitment capacity as well as the senescence-associated pro-inflammatory lymphocyte phenotype in severe disease pathogenesis
[52][53][104,105]. Indeed, a shorter telomere length has been associated with poor SARS-CoV-2 infection outcomes
[54][55][106,107], while the infection itself may have an accelerating impact on telomere shortening
[56][108].
The release of extracellular traps (NETs) by neutrophils is increased in certain hematologic cancers such as DLBCL
[57][109] or CLL
[58][110] as well as in severe obesity
[59][111]. NETosis has been shown to be a prominent feature of COVID-19 immunothrombosis, which is implicated in target organ damage
[60][112]. Likewise, aberrant complement activation constitutes another common feature of the three entities
[60][61][62][112,113,114].
3. Vaccination against SARS-CoV-2 in Patients with Hematologic Malignancy
Upon the market release of COVID-19 vaccines in December 2020 and in the face of initial relative shortages, there was an urgent need for a rationalized prioritization of vaccine supplies. The aim was to maximize the efficacy of vaccination strategies with respect to maximal prevention of COVID-19-related deaths, reduction in hospitalization burden and societal and economic disruption. The World Health Organization issued corresponding guidance to direct available vaccine resources to the most vulnerable patient groups
[63][115]. Patients with comorbidities rendering them vulnerable to severe disease were categorized in the second priority group; hence, these patients were scheduled to be vaccinated in the first 11–20% of the population. Although individual national programs occasionally deviated from this scheme, both obesity as well as hematologic malignancy fell into this category, the latter corresponding to “cancer” as well as to “conditions or therapies associated with immune suppression” in the issued document
[63][115]. Similar approaches were used for the prioritization of booster vaccinations and are likely to be repeated in the future updated versions of vaccination programs as the pandemic continues.
The vaccines critically altered the course of the pandemic, being highly efficacious against symptomatic SARS-CoV-2 infection and, even after the appearance of altered viral variants, for the prevention of severe course, hospitalization and death. Despite initial considerations, vaccine efficacy was shown to be essentially unaffected by the presence of obesity
[64][65][116,117]. Vaccine efficacy among those with obesity-related hematologic cancers is inherently more challenging since it is expected that the disturbed immune function originating from the conditions themselves or their therapies may hinder the development of adequate immunity. Indeed, inadequate antibody and/or T-cellular responses have been documented after the vaccination of patients with monoclonal gammopathies (particularly for MM rather than smoldering myeloma
[66][118]), acute leukemias, Hodgkin disease and NHL, MDS, acute leukemias and chronic myeloproliferative disorders
[66][67][68][118,119,120]. Seroconversion rates appear substantially lower among hematologic cancer patients compared with those affected by solid neoplasms
[68][69][120,121], and may be particularly low in specific diseases, depending on their pathophysiological and cytogenetic characteristics as well as mode of therapy. Greenberger et al. investigated the antibody responses following two mRNA-based platform doses in 1445 hematologic patients from the USA, approximately 40 days following the second dose. Seropositivity rates were found to be minimal across a variety of NHL histological subtypes or CLL and, in contrast, were satisfactory in Hodgkin disease, acute leukemias, monoclonal gammopathies and myeloproliferative disorders
[70][122]. Unsurprisingly, B-cell-targeted therapies such as anti CD20-monoclonal antibodies, chimeric antigen receptor (CAR) T cells against CD19, or Bruton’s tyrosine kinase (BTK) inhibitors have been associated with diminished antibody responses
[68][69][70][120,121,122]. Regarding receivers of allogeneic hematopoietic stem cell transplantation (HSCT) and despite some concerns
[69][121], in a cohort of 117 patients vaccinated with BNT162b2, antibody response rates were satisfactory among patients who had received the transplantation more than one or two years ago, compared with less than 12 months. Response rates after two doses were 89%, 96%, 52%, respectively, and were greater among those with lymphocyte counts of >1000/μL compared with those with a lower lymphocyte count (91% vs. 64%, respectively) and those who were not under ongoing treatment (91% vs. 64%, respectively)
[71][123]. Likewise, a study among 67 lymphoma or MM patients who received autologous HSCT showed that 56 (87%) developed humoral
[72][124] immunity following the vaccination of the BNT162b2 mRNA vaccine. Similar findings have been ascertained in a case series of scleroderma patients who received autologous stem cell transplantation
[73][125]. Apart from diagnosis and mode of therapy, other factors acknowledged to positively affect the immune response include vaccination outside the frame of active therapy, a longer time interval since the last anti-CD20 antibody administration, and vaccination during a complete remission status
[68][74][120,126].
Regarding vaccine type, there have been occasional reports of favorable efficacy profiles of specific platforms; between the two available mRNA-based preparations, higher antibody titers may be observed among patients with monoclonal gammopathies after receiving the mRNA-1273 than the BNT162b2 vaccine
[66][118]. Likewise, in the study by Greenberger et al., the vaccination with mRNA-1273 was associated with greater odds of seropositivity status compared with BNT162b2, independent of multiple confounders, including the type of hematologic malignancy
[70][122]. Although there is a limited number of studies to address specific vaccines among hematologic patients and available reports vary with respect to participant composition and studied circumstances, there were no significant differences in seropositivity rates across the different available vaccine platforms according to a recent meta-analysis
[68][120]. Subsequently, although it does not seem justified based on the current data to prioritize one vaccine platform over another for its use among hematologic patients, more information in hematologic populations according to specific underlying diagnosis and treatment is needed to optimize vaccination strategies. In any case, adherence to a timely vaccination schedule irrespective of a specific platform for all affected patients is strongly warranted.
It should be noted that most available data regarding vaccine immunogenicity have been based on induced neutralizing antibody titers. Undoubtfully, even among individuals who show suboptimal or even negative antibody levels following vaccination or SARS-CoV-2 infection, a certain cellular immunity-mediated protection against symptomatic infection and severe disease may be assumed. However, based on available evidence, it seems likely that neutralizing antibody responses themselves correlate satisfactorily with the probability of SARS-CoV-2 infection
[75][76][127,128].
At least a third (booster) vaccine dose is recommended in most countries for individuals who have successfully undergone a full vaccination course to overcome the waning of immunity and prevent breakthrough infections, especially by new variants. Immunocompromised individuals are to be prioritized in this strategy, while local guidelines occasionally advise in favor of a second booster (fourth) dose for these patients
[77][129], despite the extremely scarce currently available evidence to support the effectiveness of this recommendation
[78][79][130,131]. Patients with hematologic malignancy would fall into this category of high-risk patients. Available data on booster dosing demonstrate that despite the fact that a third dose of BNT162b2 strengthens humoral immunity among antibody-positive hematologic patients, it does not alter the serological status among those that remained seronegative despite prior immunization; nevertheless, it does seem to augment T cell-mediated cellular immunity irrespective of antibody status
[80][132], thus, demonstrating a potential benefit of booster immunization even among those with a prior insufficient humoral response.
4. Blood Product Transfusion in the Era of COVID-19
Individuals with hematologic malignancies constitute a pool of patients with urgent and frequent dependency on the transfusion of blood products. Voluminal requirements greatly vary depending on underlying diagnosis, type of treatment and local standard operating procedures of hematology oncology units; nonetheless, transfusion of erythrocytes and/or platelets is frequently necessary in the setting of active cytotoxic therapy, while in the case of certain background diagnoses (e.g., particularly low-risk MDS), erythrocyte and/or platelet transfusions constitute the core of long-term maintenance therapy
[81][133].
Concerns regarding the potential infectivity of blood units from asymptomatic or pre-symptomatic infected donors originate from the ascertainment of viral RNAemia in hospitalized patients in some reports, although this finding is not universal among available studies
[82][134]. Nevertheless, in a broad screening of plasma minipools from 258,000 blood donations between March and September 2020 in the US, positive SARS-CoV-2 PCRs were remarkably uncommon and generally in very low titers
[82][134]. Real-world data from cases of blood product transfusion donated by pre-symptomatic infected patients also support the notion that even though transfusion as a mode of SARS-CoV-2 transmission may be theoretically plausible, it is extremely rare
[83][84][85][86][87][135,136,137,138,139].
Particularly during the initial outbreak of the pandemic, but also during every subsequent accelerated phase leading to a new surge in infections, there was a visible danger of blood supply shortages. Shortages may develop depending on geographical region, hospital level of care and resource availability as a result of reduced donation rates due to a fear of donors being in contact with healthcare services, physician concerns in the face of the presence of COVID-19-suspicious donor symptoms, increased demand for blood products to cover the needs of hospitalized COVID-19 patients, or an overall reassignment of personnel- and material-related resources to counter the pandemic waves. In the case of a development of extreme shortages, it is likely that updated guidelines by competent authorities would be necessary regarding a re-evaluation of transfusion intervals and/or thresholds
[88][89][140,141] with the aim of rationalizing the use of available resources.
Despite initial concerns
[89][90][141,142], and even though the collective volume of blood donations and success of donor recruitment campaigns reduced during the first pandemic waves
[91][92][93][94][143,144,145,146], this was counterbalanced by a reduction in blood product demand, chiefly through a reduction in elective surgical procedures and medical transfusions
[95][147]. Although some blood product supply shortage did develop in some regions
[96][97][148,149], there is no available evidence that this phenomenon had a significant impact on planned or acute transfusions among patients with hematologic disease
[91][143].
Nevertheless, the degree of shortages and resulting initial uncertainties brought about by COVID-19 have provided further reasons for the reappraisal and optimization of current transfusion strategies. This would concern not only the regular transfusion programs among hematologic patients, but also the transfusions for practically any medical indication in a variety of, at least non-acute, circumstances. With respect to chronic hematologic diseases, caution should be taken in the detection of reversible causes of anemia, such as iron, folic acid, or vitamin B12 deficiency, as well as their treatment through adequate oral or parenteral substitution. Furthermore, the indications for erythropoietin therapy should be meticulously followed or even broadened in the face of emerging new evidence in the long term, since its administration may reduce volume requirements in transfusion-dependent patients
[98][150]. Importantly, lower transfusion thresholds than commonly utilized seem to be safe and well-tolerated among asymptomatic patients
[99][151], thus, restrictive rather than liberal transfusion strategies may be preferred in order to optimally portion erythrocyte supplies. A similar strategy could also apply for stable, non-bleeding patients with chronic thrombocytopenia dependent on thrombocyte transfusions. Furthermore, accessory measures could apply to transfusions for surgical indications, such as the correction of disorders of hemostasis prior to elective surgical procedures, intra- or postoperative use of coagulation factor concentrates, antifibrinolytic agents such as tranexamic acid and utilization of autologous blood transfusion where indicated. Lastly, frequency and volume of blood draws in blood supply storage should be limited to the absolute minimum that may affect clinical decision making, both in the acute and chronic setting.