
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabrin R. M. Ibrahim | -- | 2638 | 2022-05-25 07:10:27 | | | |
| 2 | Lindsay Dong | -28 word(s) | 2610 | 2022-05-25 10:10:55 | | |
Video Upload Options
Pluchea indica (L.) Less. (Asteraceae) commonly-known as Indian camphorweed, pluchea, or marsh fleabane has gained great importance in various traditional medicines for its nutritional and medicinal benefits. It is utilized to cure several illnesses such as lumbago, kidney stones, leucorrhea, inflammation, gangrenous and atonic ulcer, hemorrhoids, dysentery, eye diseases, itchy skin, acid stomach, dysuria, abdominal pain, scabies, fever, sore muscles, dysentery, diabetes, rheumatism, etc. The plant or its leaves in the form of tea are commonly used for treating diabetes and rheumatism.
1. Introduction
The Asteraceae (Compositae) family is one of the largest plant families, which includes about 24,000–30,000 species and 1600–1700 genera [1]. Its plants are shrubs and herbs, which have been commonly used since ancient times as herbal medicines and diets all over the world [2]. It contains well-known species, such as sunflower, chicory, coreopsis, lettuce, daisy, and dahlias, as well as several significant medicinal plants, such as chamomile, wormwood, and dandelion [3].
Pluchea is a flowering plants genus of the Asteraceae family, comprising about 80 species [4]. Its members are known as plucheas, camphorweeds, or fleabanes, and some are called sour bushes [5]. Pluchea indica (L.) Less. is an evergreen shrub found abundantly in salt marshes and mangrove swamps with a 1 to 2 m height that has an important role in maintaining the ecological balance in the coastal areas [6]. It is known as Indian camphorweed, pluchea, or marsh fleabane, Khlu (Thai), Kukrakonda, Kakronda, or Munjhu rukha (Bengali), Kuo bao ju (Chinese), luntas (Javanese), Beluntas (Malaysia, Indonesia, Bahasa), and kalapini (Philippines) [7]. This plant is mainly found in the subtropical and tropical zones of Asia, Africa, Australia, and America and in warm temperature regions of countries such as Indonesia, Malaysia, Taiwan, Australia, Mexico, and India [6][7][8]. A chemical investigation of this plant revealed the existence of various phytoconstituents: thiophenes, sesquiterpenes, quinic acids, sterols, lignans, and flavonoids. Additionally, this plant has wide folk uses and diverse bioactivities: anti-inflammatory, anti-cancer, antioxidant, anti-microbial, and insecticidal activities.
2. Phytochemistry
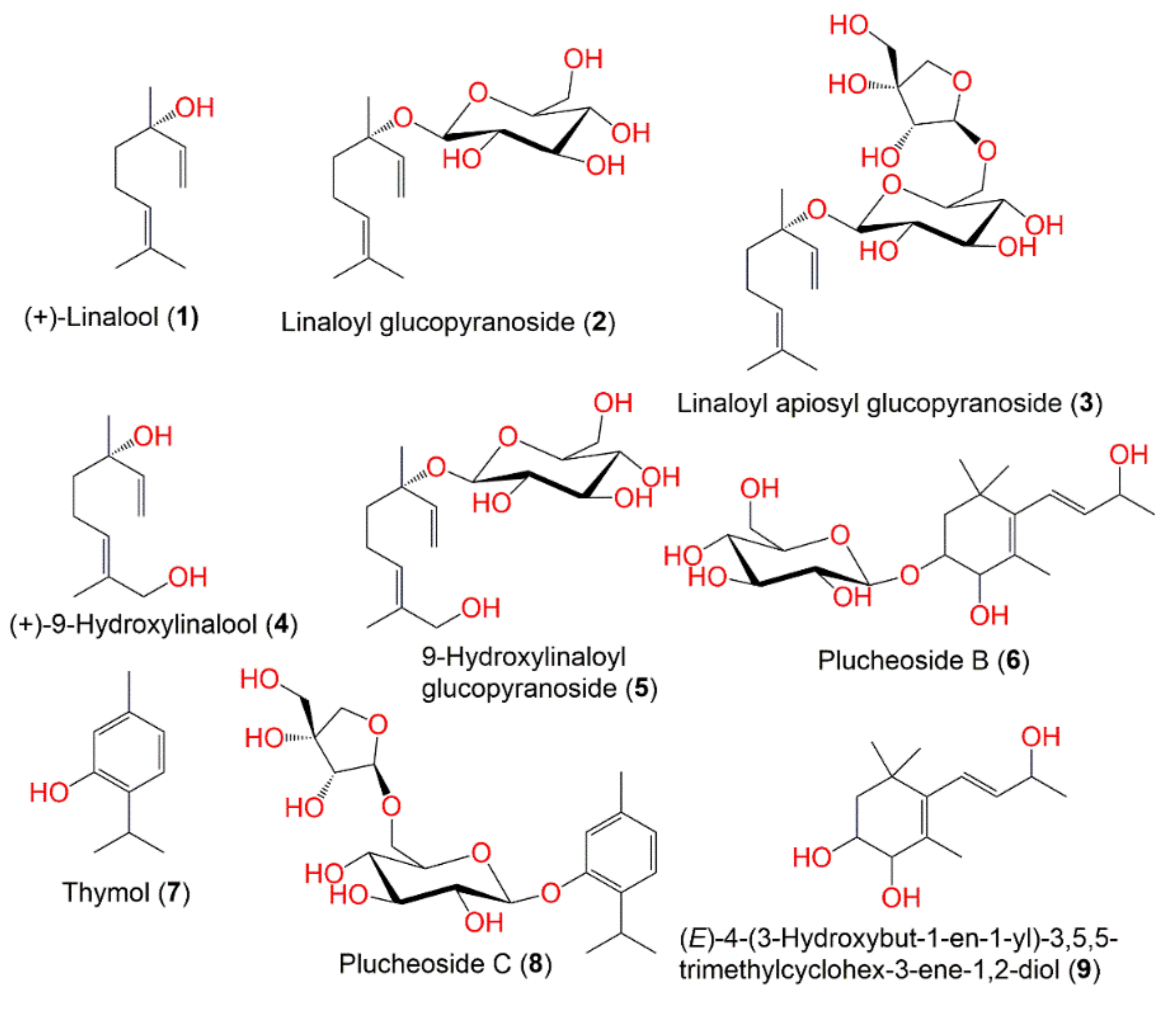
3. Ethnomedicinal Uses
All parts of P. indica can be used medicinally, and it has a long tradition in alternative medicine for a wide variety of ailments. In Indochina, the roots’ decoction is prescribed for fever as a diaphoretic, and its leaves’ infusion is taken internally in lumbago. The leaves and roots are utilized as an astringent and antipyretic in Patna [13]. It was reported that the overconsumption of the leaves for long periods of time may cause health problems, especially for individuals suffering from cardiovascular disease and hypertension due to its high contents of chloride and sodium [14]. In Indonesia, leave infusion/decoction is utilized as an appetite stimulant, antipyretic, digestive aid, deodorant, diarrhea solution, antitussive, and emollient [15][16]. The root decoction is utilized as an astringent and antipyretic [17]. In Thailand, bark and stem decoctions are utilized to treat kidney stones and hemorrhoids, respectively, and leaves are used to cure leucorrhea, inflammation, and lumbago [18]. Leaf tea is widely consumed in Thailand as a health-promoting drink; however, drinking this tea in large amounts increases the feeling of needing to urinate due to its diuretic effect [13]. Additionally, a poultice of the fresh leaf is used for a gangrenous and atonic ulcer [19]. Arial parts are used as an ointment to treat eye diseases and itchy skin. The plant is used for treating acid stomach, dysuria, hemorrhoids, abdominal pain, stomach cramps, scabies, fever, sore muscles, dysentery, menstruation, and rheumatism [20][21][22][23]. In Malaysia, the leaves are prescribed for leucorrhea, dysentery, rheumatism, bad body odor and breath, boils, and ulcers, while roots are used to treat lumbago, fever, headache, and indigestion [24]. The chopped stem bark cigarettes are smoked to relieve sinusitis pain [25]. Indochina, the young shoots and leaves are crushed, mixed with alcohol, and applied in baths to treat scabies, as well as to the back for lumbago and to relieve rheumatic pains [25]. Currently, dried leaves and their extracts have been commercially available in Thailand as herbal tea due to their blood glucose-lowering potential [26]. In Yogyakarta, Indonesia, the leaves are used as a galactagogue to maintain, induce, and augment maternal milk production [27]. It is used orally as anti-fertility for men [28][29]. In Peninsular Malaysia, it is cultivated in gardens for its young shoots, which can be eaten raw. Its leaf decoction is used to counteract fever, and the sap expressed from leaves is used for dysentery [30]. In Dayak Pesaguan tribes, P. indica leaves are utilized to eliminate bad body odor, increase appetite, and overcome digestive disorders [31].
4. Biological Activities
4.1. Anti-Inflammatory Activity
4.2. Anti-Obesity and Anti-Hyperlipidemic Activities
The leaf H2O extract (concentrations of 750 to 1000 µg/mL) markedly reduced the accumulation of lipids, suppressed adipogenesis, and modified protein, carbohydrate, glycogen, and nucleic acid concentrations in the 3T3-L1 adipocytes. Additionally, it possessed lipase inhibitory potential (concentration 250 to 1000 µg/mL) [32]. In another study, the leaf extract was found to prohibit pancreatic lipase with % inhibition ranging from 11.7% to 18.4% at a concentration ranging from 625 to 1000 ppm compared to orlistat (% inhibition from 26.6 to 36.6%) and epicatechin (% inhibition from 10.7 to 18.6%) at the same concentrations [33]. Therefore, it could be further developed into an herbal supplement for managing obesity or overweight [32]. P. indica tea (herb, 400 and 600 mg/kg, orally) ameliorated hyperglycemia, dyslipidemia, and obesity in high-fat diet-induced (HFD) mice. Moreover, it significantly lowered TG, TC, LDL-C, and perigonadal fat weight in HFD-treated mice; however, it increased HDL-C. This was related to its total phenolic and flavonoid contents (TFC), caffeoylquinic acid derivatives, betacaryophyllene, and gamma-gurjunene [34].
4.3. Antidiabetic Activity
Nopparat et al. reported that the pretreatment with the leaf EtOH extract (dose 100 mg/kg for 2 weeks) effectively alleviated β-cell injury produced by cytokine in STZ (streptozotocin) mice as it minimized the levels of inflammatory markers IFN-γ (interferon-γ), TNF-α (tumor necrosis factor-α), and IL-1β (interlukin1-β), along with prohibiting caspases; 3, 8, and 9, pSTAT1 phosphorylation (signal transducer and activator of transcription 1), NF-κBp65 (nuclear factor-κBp65), and iNOS. Further, it protected β-cells by boosting cell proliferation and suppressing apoptosis. The blood glucose-lowering potential of the leaf extract was attributed to the antioxidant capacities of the extract’s constituents, particularly resveratrol derivative and quercetin [11].
α-Glucosidase inhibitory assay-directed fractionation of the leaves’ EtOAc fraction yielded caffeoylquinic acid derivatives, 38 and 40–43, which were isolated using SiO2, RP-18 CC, and HPLC and elucidated by MS and NMR analyses. Compound 42 had the highest α-glucosidase inhibitory effectiveness among the separated caffeoylquinic acid derivatives (IC50 2.0 µM) compared to acarbose (IC50 0.5 µM), while the other compounds displayed moderate to weak activity compared to the activity of acarbose. It was found that increasing numbers of caffeoyl groups attached to the quinic acid moiety and methyl esterification of the carboxylic group in quinic acid promoted the α-glucosidase inhibitory capacity [35].
4.4. Insecticidal and Herbicidal Activity
4.5. Cytotoxicity Activity
4.6. Venom Neutralizing Activity
4.7. Hepatoprotective and Neurological Activities
Some reported findings validated the ethnomedicinal uses of P. indica for managing diabetic liver injury. P. indica leaf EtOH extract (doses 50 and 100 mg/kg for 2 weeks) ameliorated hyperglycemia-induced liver damage in STZ mice through the modulation of inflammatory responses and oxidative stress by inhibiting IL-6, TNF-α, TGF-β1, NF-κB p65, and PKC (protein kinase C), resulting in a reduction in hepatic apoptosis and improvement of hepatic architecture [44].
Another study reported that the root extract (doses 50–100 mg/kg, p.o.) remarkably prolonged pentobarbital sleep and reduced locomotor activity in isolated mice but not in group-housed mice. However, at high doses (dose 400 mg/kg, p.o.), it reduced locomotor activity and prolonged pentobarbital sleep in group-housed mice in comparison to diazepam (doses 0.1 and 0.5 mg/kg), which noticeably prolonged pentobarbital sleep in both isolated and group-housed mice. The effect of root extract and diazepam on pentobarbital sleep was significantly attenuated by flumazenil (1 mg/kg, i.v.). It suppressed the isolated mice’s aggressive behavior induced by social isolation. It was suggested that the GABAergic system was partly implicated in pentobarbital-caused sleep [21].
4.8. Antifertility Activity
4.9. Wound-Healing Activity
In Thai traditional medicines, P. indica leaf was used as stringent to heal wounds and ulcers [47]. Fibroblasts represent the major type of connective tissue cells that produce an extracellular matrix accountable for maintaining tissue structural integrity [48][49]. They have a substantial function in the wound-healing proliferative phase, causing deposition of extracellular matrix [50]. Over proliferation of fibroblast during wound healing leads to the production of abnormally large amounts of collagens and extracellular matrix, resulting in scar formation and functional impairment that may further trigger permanent fibrosis [51]. The EtOH extract of leaves that was rich in flavonoids (19.44 mg/gram) exhibited remarkable antioxidant potential (IC50 21.53 μg/mL) and had no effect on fibroblast 3T3 BALB C viability (IC50 311.776 μg/mL) in the prestoblue cytotoxicity test. This supported the use of leaf extract as a nutrient to accelerate wound healing in the oral cavity injury [47].
4.10. Anti-Hemorrhoidal Activity
The leaves of P. indica are traditionally utilized for treating hemorrhoids. This was supported by a study performed by Senvorasinh et al. They reported that the oral administration of hot P. indica tea H2O extract remarkably attenuated (dose 50 mg/kg/day) the rectal damage and hemorrhoids induced by croton oil in rats, as evident by no noticeable reduction in rectum and spleen weights. Conversely, it had no effect on gastrointestinal movement in mice, indicating it did not reduce constipation [52].
4.11. Antimicrobial Activity and Pharmaceutical Preparations
The leaf extract prohibited the growth of Streptococcus mutans, the causative organism of dental caries (MIC 10%), in the disk diffusion assay [53][54]. Further, the leaf extract toothpaste prevented dental caries in rats based on Rontgen examination results. Additionally, it reduced the virulence of mouth bacteria that initiated dental caries. Therefore, P. indica herbal toothpaste could treat caries in rat teeth [55]. P. indica leaf EtOH extract had inhibitory effects on C. albicans (MIC 100 mg/mL) [56]. Further, Alvionida et al. prepared different gel formulae using HPMC (hydroxypropyl methylcellulose) and carbopol 940. It was found that the gel formula with 1% carbopol 940 and 1.5% HPMC possessed antibacterial potential toward P. aeruginosa and S. aureus in the cup-plate diffusion assay [57]. Komala et al. stated that the deodorant rolls with 3 to 5% P. indica leaf extracts exhibited antibacterial potential towards S. epidermidis without causing skin irritation in both women and men. The 3% extract deodorant roll was most effective against S. epidermidis than the other formulae for removing the bad body odor [58], which proved its traditional use to eliminate bad odor. The leaf extract was evaluated for antibacterial potential (concentration 2.5 to 6.5%) towards E. faecalis, P. gingivalis, F. nucleatum, and S. mutans, which are accountable for root canal infections, periodontal disease, and caries. It showed significant growth inhibition towards E. faecalis (inhibition zone diameter (IZD) 12.6 mm), followed by S. mutans (IZD 12.2 mm) and P. gingivalis (IZD 12.2 mm) at a concentration of 6.5%, compared to chlorhexidine (IZDs 10.9, 11.4, and 10.6 mm, conc 2%) [59]. It also prohibited biofilm formation and decreased adhesion of E. faecalis and F. nucleatum in the micro-titter plate and auto-aggregation assays, respectively [60]. Hence, it could be utilized as an alternative to root canal sterilization dressing [60]. It had activity versus S. epidermidis (IZD 21.73 mm) and P. aeruginosa (IZD 21.44 mm) at 1 mg/mL [61]. Sittiwet suggested the possible urinary tract infection treatment potential of the aerial parts aqueous extract through its inhibitory effect on E. coli and Klebsiella pneumoniae [62]. Further, the root MeOH extract of P. indica (doses 0.5 and 1.0 mg/kg body weight) remarkably alleviated S. typhimurium caused typhoid fever in mice in vivo [63]. Fresh stems, roots, and twigs prohibited the growth of S. aureus, P. fluorescens, B. cereus, S. typhimurium, and E. coli, whereas fresh samples had more potent inhibitory potential than dried samples [64].
4.12. Antioxidant Activity
P. indica fresh leaves are used in many kinds of foods, including soups, salads, and side dishes. Additionally, leaf extracts and dried leaves are commonly consumed as food supplements and tea in Thailand. Several studies confirmed the significant antioxidant potential of various extracts of P. indica that is highly correlated to its high total phenols and flavonoids contents.
References
- Abraham, J.; Thomas, T.D. Recent Advances in Asteraceae Tissue Culture. In Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Anis, M., Ahmad, N., Eds.; Springer: Singapore, 2016.
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009.
- Nikolic, M.; Stevovic, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789.
- Anderberg, A.A. Asteraceae. In Cladistics & Classification; Bremer, K., Ed.; Timber Press: Portland, OR, USA, 1994; pp. 292–303.
- Aggarwal, P. A review on phytochemical and biological investigation of plant genus Pluchea. Indo Am. J. Pharm. Res. 2013, 3, 3373–3392.
- Wang, J.; Pei, Y.H.; Lin, W.H.; Deng, Z.W.; Qiao, L. Chemical constituents from the stems and leaves of marine mangrove plant Pluchea indica (L.) Less. Shenyang Yaoke Daxue Xuebao 2008, 25, 960–963.
- Rodsom, T. A Study on the Diuretic Effects of Pluchea Indica in Healthy Subjects and Patients; Mahidol University: Bangkok, Thailand, 1993.
- Maharani, S.C.; Julianto, I.; Widhiati, S. The role of beluntas (Pluchea indica L.) leaf extract in preventing the occurrence of fibroblasts hyperproliferation: An in vitro preliminary study. Dermatol. Rep. 2019, 11, 8019.
- Widyawati, P.S.; Wijaya, C.H.; Hardjosworo, P.S.; Sajuthi, D. Volatile compounds of Pluchea indica less and Ocimum basillicum linn essential oil and potency as antioxidant. HAYATI J. Biosci. 2013, 20, 117–126.
- Ruan, J.; Yan, J.; Zheng, D.; Sun, F.; Wang, J.; Han, L.; Zhang, Y.; Wang, T. Comprehensive Chemical Profiling in the Ethanol Extract of Pluchea indica Aerial Parts by Liquid Chromatography/Mass Spectrometry Analysis of Its Silica Gel Column Chromatography Fractions. Molecules 2019, 24, 2784.
- Nopparat, J.; Nualla–Ong, A.; Phongdara, A. Ethanolic extracts of Pluchea indica (L.) leaf pretreatment attenuates cytokine–induced β–cell apoptosis in multiple low–dose streptozotocin–induced diabetic mice. PLoS ONE 2019, 14, e0212133.
- Chewchida, S.; Vongsak, B. Simultaneous HPTLC quantification of three caffeoylquinic acids in Pluchea indica leaves and their commercial products in Thailand. Rev. Bras. Farmacogn. 2019, 29, 179–181.
- Pramanik, K.C.; Biswas, R.; Mitra, A.; Bandyopadhyay, D.; Mishra, M.; Chatterjee, T.K. Tissue culture of the plant Pluchea indica (L.) and evaluation of diuretic potential of its leaves. Adv. Tradit. Med. 2007, 7, 197–204.
- Shannon, M.; Grieve, C. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38.
- Andarwulan, N.; Batari, R.; Sandrasari, D.A.; Bolling, B.; Wijaya, C.H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010, 121, 1231–1235.
- Andarwulan, N.; Kurniasih, D.; Apriady, R.A.; Rahmat, H.; Roto, A.V.; Bolling, B. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J. Funct. Foods 2012, 4, 339–347.
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors: Dehradun, India, 1999; pp. 1344–1345.
- Ahem, S.A.; Kamel, E.M. Phenolic constituents and biological activity of the genus Pluchea. Pharma Chem. 2014, 5, 109–114.
- Sen, T.; Basu, A.; Ray, R.N.; Chaudhuri, A.K.N. Hepatoprotective effects of Pluchea indica (L.) extract in experimental acute liver damage in rodents. Phytother. Res. 1993, 7, 352–355.
- Sen, T.; Ghosh, T.K.; Chaudhuri, A.K. Studies on the mechanism of anti-inflammatory and anti-ulcer activity of Pluchea indica–probable involvement of 5–lipooxygenase pathway. Life Sci. 1993, 52, 737–743.
- Thongpraditchote, S.; Matsumoto, K.; Temsiririrkkul, R.; Tohda, M.; Murakami, Y.; Watanabe, H. Neuropharmacological Actions of Pluchea indica L. Root Extract in Socially Isolated Mice. Biol. Pharm. Bull. 1996, 19, 379–383.
- Locher, C.; Burch, M.; Mower, H.; Berestecky, J.; Davis, H.; Van Poel, B.; Lasure, A.; Berghe, D.; Vlietinck, A. Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants. J. Ethnopharmacol. 1995, 49, 23–32.
- Sen, T.; Chaudhuri, A. Antiinflammatory evaluation of a Pluchea indica root extract. J. Ethnopharmacol. 1991, 33, 135–141.
- Roslida, A.H.; Erazuliana, A.K.; Zuraini, A. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Pluchea indica (L.) Less leaf. Pharmacologyonline 2008, 2, 349–360.
- Bandaranayake, W. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452.
- Widyawati, P.S.; Budianta, T.D.W.; Gunawan, D.I.; Wongso, R.S. Evaluation antidiabetic activity of various leaf extracts of Pluchea indica L. Int. J. Pharmacog. Phytochem. Res. 2015, 7, 597–603.
- Sumanth, M.; Narasimharaju, K. Evaluation of galactagogue activity of lactovedic: A polyherbal formulation. Int. J. Green Pharm. 2011, 5, 61–66.
- Susetyarini, E. Jumlah sel spermiogenesis tikus putih yang diberi tanin daun Beluntas (Pluchea indica) sebagai sumber belajar. Proc. Biol. Educ. Conf. 2015, 12, 462–466.
- Susetyarini, E. The level of glutamic acid in the semen of male white rat (Ratus norwegicus) after being treated with tannin of Pluchea indica. Procedia Chem. 2015, 14, 152–156.
- Noridayu, A.R.; Hii, Y.F.; Faridah, A.; Khozirah, S.; Lajis, N. Antioxidant and antiacetylcholinesterase activities of Pluchea indica Less. Int. Food Res. J. 2011, 18, 925–929.
- Due, R.; Symaswisna, M.R. Ethnobotany of dayak pesaguan medicinal plant and its implementation in the making of biodiversity flash cards. Biol. Study Fac. Teach. Train. Educ. Untan Pontianak 2013, 3, 1–15.
- Sirichaiwetchakoon, K.; Lowe, G.M.; Thumanu, K.; Eumkeb, G. The Effect of Pluchea indica (L.). Tea on Adipogenesis in 3T3-L1 Adipocytes and Lipase Activity. Evid. Based Complement. Altern. Med. 2018, 2018, 4108787–4108813.
- Abdul Rahman, H.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Abdul Hamid, A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017, 20, 2616–2629.
- Sirichaiwetchakoon, K.; Churproong, S.; Kupittayanant, S.; Eumkeb, G. The Effect of Pluchea indica (L.). Tea on Blood Glucose and Lipid Profile in People with Prediabetes: A Randomized Clinical Trial. J. Altern. Complement. Med. 2021, 27, 669–677.
- Arsiningtyas, I.S.; Gunawan–Puteri, M.D.; Kato, E.; Kawabata, J. Identification of α–glucosidase inhibitors from the leaves of Pluchea indica (L.) Less., a traditional Indonesian herb: Promotion of natural product use. Nat. Prod. Res. 2014, 28, 1350–1353.
- Rahayu, Y.S. The Using of Fenolic Compounds of Pluchea indica (L.). Leaves Extracts as A Bioinsecticide and Bioherbicide; In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; Volume 953, p. 012206.
- Kao, C. Antiproliferation and Apoptosis Induction of Ethanolic Extracts of Pluchea indica Root on Human Nasopharyngeal Carcinoma Cell Lines. Ph.D. Thesis, National Sun Yat–Sen University, Kaohsiung City, Taiwan, 2015.
- Kao, C.-L.; Cho, J.; Lee, Y.-Z.; Cheng, Y.-B.; Chien, C.-Y.; Hwang, C.-F.; Hong, Y.-R.; Tseng, C.-N.; Cho, C.-L. Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells. Molecules 2015, 20, 11508–11523.
- Ko, H.J. Effect of Crude Ethanol Extracts of Pluchea Indica on Human Leukemia K562 Cells. Master’s Thesis, National Sun Yat–Sen University, Kaohsiung City, Taiwan, 2015.
- Cho, J.; Cho, C.-L.; Kao, C.-L.; Chen, C.-M.; Tseng, C.-N.; Lee, Y.-Z.; Liao, L.-J.; Hong, Y.-R. Crude aqueous extracts of Pluchea indica (L.) inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death. BMC Complement. Altern. Med. 2012, 12, 265.
- Cho, C.-L.; Lee, Y.-Z.; Tseng, C.-N.; Cho, J.; Cheng, Y.-B.; Wang, K.; Chen, H.-J.; Chiou, S.-J.; Chou, C.-H.; Hong, Y.-R. Hexane fraction of Pluchea indica root extract inhibits proliferation and induces autophagy in human glioblastoma cells. Biomed. Rep. 2017, 7, 416–422.
- Alam, M.I.; Auddy, B.; Gomes, A. Viper venom neutralization by Indian medicinal plant (Hemidesmus indicus and Pluchea indica) root extracts. Phytother. Res. 1996, 10, 58–61.
- Gomes, A.; Saha, A.; Chatterjee, I.; Chakravarty, A.K. Viper and cobra venom neutralization by beta–sitosterol and stigmasterol isolated from the root extract of Pluchea indica L. (Asteraceae). Phytomedicine 2007, 14, 637–643.
- Nopparat, J.; Nualla–Ong, A.; Phongdara, A. Treatment with Pluchea indica (L.) leaf ethanol extract alleviates liver injury in multiple low-dose streptozotocin-induced diabetic BALB/c mice. Exp. Therap. Med. 2020, 20, 1385–1396.
- Amalina, N.; Suyatmi, S.; Suparyanti, E.L. Effect of Beluntas (Pluchea indica) leaf extract on mice spermatogenesis. Biofarmasi 2010, 8, 47–51.
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2015, 4, e979623.
- Sugiaman, V.K.; Nisyah, N.Q.; Anisa, N.; Pranata, N. Pluchea indica extract as a potential source of nutrition for accelerate wound healing. Sys. Rev. Pharm. 2021, 12, 570–573.
- Buranasukhon, W.; Athikomkulchai, S.; Tadtong, S.; Chittasupho, C. Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm. Biol. 2017, 55, 1767–1774.
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43.
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885.
- Ichim, T.E.; O’Heeron, P.; Kesari, S. Fibroblasts as a practical alternative to mesenchymal stem cells. J. Transl. Med. 2018, 16, 212.
- Senvorasinh, K.; Phunikhom, K.; Sattayasai, J. Anti-Hemorrhoidal activity of Pluchea indica leaves aqueous extract in croton oil–induced hemorrhoids in experimental animals. Srinagarind Med. J. 2019, 34, 590–594.
- Nurrohman, E.; Pantiwati, Y.; Susetyarini, E.; Umami, E.K. Extract of Beluntas (Pluchea indica) as an Antibacterial Towards Streptococcus mutans ATCC 25175 Causes of Dental Carries. BIO-EDU J. Pendidik. Biol. 2021, 6, 9–17.
- Fatimatuzzahr, N.; Rahayu, F.; Ningsih, N.S.; Darsono, A.; Salasia, S.I.O. Anticariogenic effect of Beluntas (Pluchea indica) Leaces extract as growth inhibitor of Streptococcus mutans which caused dental caries. J. Sain Vet. 2016, 34, 182–193.
- Fatimatuzzahr, N.; Rahayu, F.; Ningsih, N.S.; Darsono, A.; Salasia, S.I.O. Rontgen Result of Caries Molar in White Rat (Rattus novergicus) with Treatment of Herbal Toothpaste (Pluchea Indica Leaves Extract). In Proceedings of the 4th Asian Academic Society International Conference (AASIC), Globalizing Asia: Integrating Science, Technology and Humanities for Future Growth and Development, Nakhon Pathom, Thailand, 12–13 May 2016; pp. 183–187.
- Demolsky, W.L.; Sugiaman, V.K.; Pranata, N. Antifungal activity of Beluntas “Indian Camphorweed” (Pluchea indica) ethanol extract on Candida albicans in vitro using different solvent concentrations. Eur. J. Dent. 2021.
- Sulistyani, N.; Alvionida, F.; Sugihartini, N. Composition of carbopol 940 and HPMC affects antibacterial activity of beluntas (Pluchea indica (L.)) leaves extract gel. Pharmaciana 2021, 11, 427–438.
- Komala, O.; Wiendarlina, I.Y.; Rizqiyana, N. Antibacterial activity roll on deodorant with Pluchea indica (L.) leaf extract against Staphylococcus epidermidis (Evans 1916) in vitro. IOP Conf. Ser. Earth Environ. Sci. 2019, 293, 012031.
- Sylvana, D.; Amir, M.; Purnamasari, C.B.; Iskandar, A.; Asfirizal, V. Antibacterial activity of ethanol extract of Beluntas leaves on Streptococcus mutans, Porphyromonas gingivalis, and Enterococcus faecalis. Padjadjaran J. Dent. 2021, 33, 191–198.
- Pargaputri, A.F.; Munadziroh, E.; Indrawati, R. The Effect of Pluchea indica L. Leaves Extract Againts Biofilm of Enterococcus faecalis and Fusobacterium nucleatum In Vitro. Denta 2017, 11, 51.
- Simanjuntak, N.; Yuniarni, U.; Prayugo, D. Antibacterial activity of Pluchea indica and Piper beetle ethanol extract on Staphylococcus epidermidis and Pseudomonas aeruginosa. Pharmacol. Clin. Pharm. Res. 2016, 1, 62–68.
- Sittiwet, C. In vitro Antimicrobial Activity of Pluchea indica Aqueous Extract: The Potential for Urinary Tract Infection Treatment. J. Pharmacol. Toxicol. 2009, 4, 87–90.
- Pramanik, K.C.; Chatterjee, T.K. In vitro and in vivo antibacterial activities of root extract of tissue cultured Pluchea indica (L.) Less. Orient. Pharm. Exp. Med. 2008, 8, 295–301.
- Srimoon, R.; Ngiewthaisong, S. Antioxidant and antibacterial activities of Indian marsh fleabane (Pluchea indica (L.). KKU Res J. 2015, 20, 144–154.




