
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David A. Hart | -- | 2872 | 2022-05-24 22:34:04 | | | |
| 2 | Beatrix Zheng | + 4 word(s) | 2876 | 2022-05-25 05:43:35 | | |
Video Upload Options
Cells fitting the description of Mesenchymal Stem/Signaling Cells (MSC) have been isolated from a large number of adult tissues. The original characteristics of MSC as defined by Caplan’s group were adherence to plastic, expression of a subset of cell surface antigens, and the cells could be induced in vitro to differentiate towards different cell lineages, including chondrocytes, bone cells, and adipocytes. It has been noted that MSC from most tissues or fluids are very heterogeneous, and some sources appear to have unique features, including unique lectin-binding phenotypes. Interestingly, MSC from bone marrow appear to preferentially respond to osteogenic stimuli, while MSC from synovium respond well to chondrogenic stimuli. Thus, different locations may reflect the needs of different environments.
1. Introduction
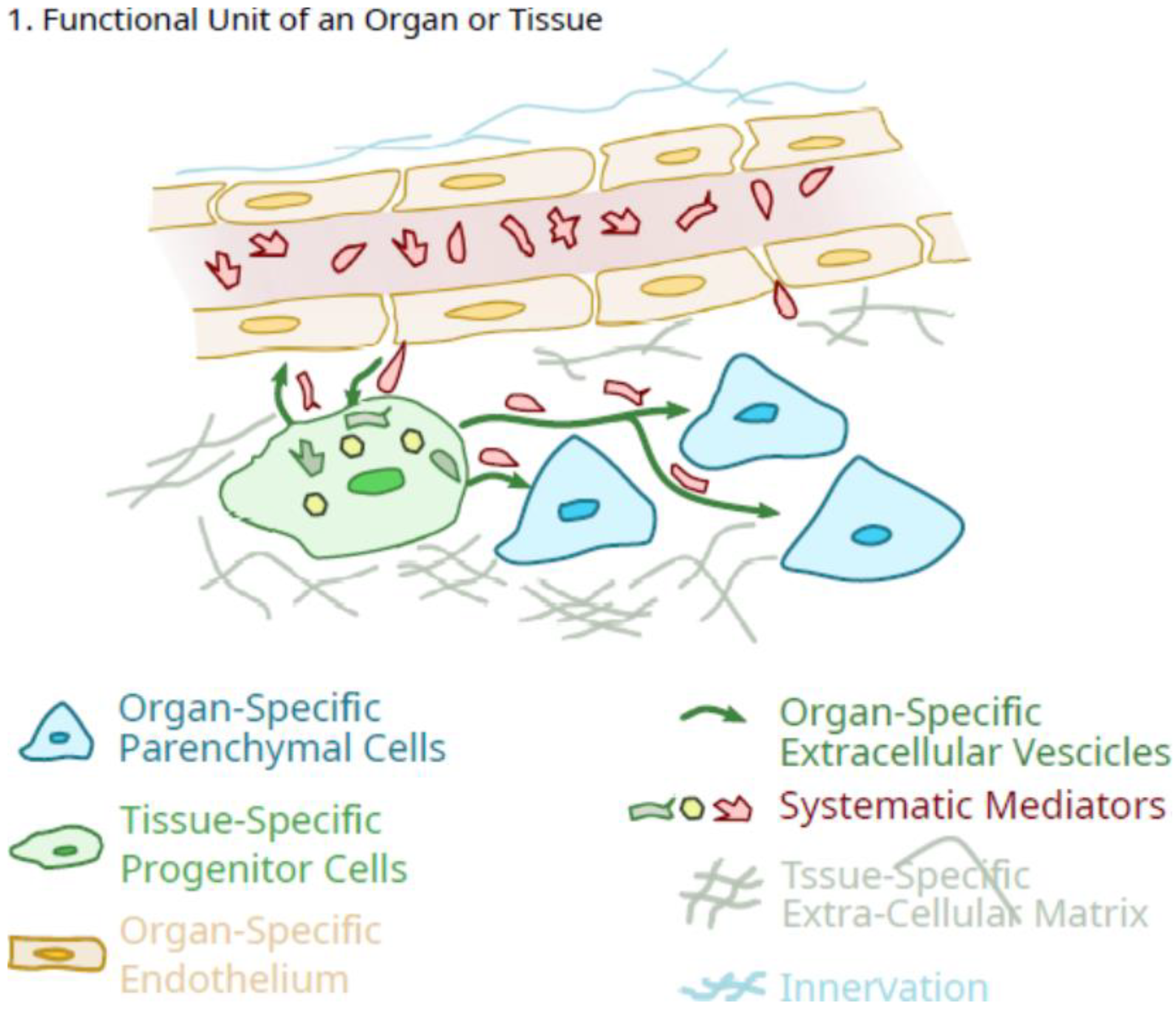
2. Potential Role of Pluripotent Organ-Specific Pericytes in Growth and Maturation, as Well as Senescence and Decline in Organ and Tissue Integrity
References
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379.
- Nguyen-Lefebvre, A.T.; Selzner, N.; Wrana, J.L.; Bhat, M. The hippo pathway: A master regulator of liver metabolism, regeneration, and disease. FASEB J. 2021, 35, e21570.
- Lobo, D.; Beane, W.S.; Levin, M. Modeling Planarian Regeneration: A Primer for Reverse-Engineering the Worm. PLOS Comput. Biol. 2012, 8, e1002481.
- Yamamoto, S.; Kashimoto, R.; Furukawa, S.; Sakamoto, H.; Satoh, A. Nerve-mediated FGF-signaling in the early phase of various organ regeneration. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 529–539.
- Kawakami, A. Stem cells in tissue regeneration in fish. Dev. Growth Differ. 2010, 52, 77–87.
- Maden, M. The evolution of regeneration–where does that leave mammals? Int. J. Dev. Biol. 2018, 62, 369–372.
- Hart, D.A.; Natsu-ume, T.; Sciore, P.; Tasevski, V.; Frank, C.B.; Shrive, N.G. Mechanobiology: Similarities and differences between in vivo and in vitro analysis at the functional and molecular levels. Recent Res. Dev. Biophys. Biochem. 2002, 2, 153–177.
- Gifre-Renom, L.; Daems, M.; Luttun, A.; Jones, E.A.V. Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. Int. J. Mol. Sci. 2022, 23, 1477.
- McDougall, J.; Giles, R.W.; Bray, R.C.; Hart, D.A. Pregnancy-induced changes in rabbit medial collateral ligament vasoregulation. Am. J. Physiol. 1998, 275, R1380–R1385.
- McDougall, J.J.; Bray, R.C.; Hart, D.A. Late gestational changes in sympathomimetic sensitivity in primigravid rabbit ligaments. Can. J. Physiol. Pharmacol. 2000, 78, 528–534.
- Yianni, V.; Sharpe, P.T. Perivascular-Derived Mesenchymal Stem Cells. J. Dent. Res. 2019, 98, 1066–1072.
- Sbierski-Kind, J.; Mroz, N.; Molofsky, A.B. Perivascular stromal cells: Directors of tissue immune niches. Immunol. Rev. 2021, 302, 10–31.
- Colle, I.; Van Vlierberghe, H.; Troisi, R.; De Hemptinne, B. Transplanted liver: Consequences of denervation for liver functions. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 924–931.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22.
- Ando, W.; Heard, B.J.; Chung, M.; Nakamura, N.; Frank, C.B.; Hart, D.A. Ovine synovial membrane-derived mesenchymal progenitor cells retain the phenotype of the original tissue that was exposed to an in vivo inflammation: Evidence for a suppressed chondrogenic differentiation potential of the cells. Inflam. Res. 2012, 61, 599–608.
- Cucu, I.; Nicolescu, M.I. A Synopsis of Signaling Crosstalk of Pericytes and Endothelial Cells in Salivary Gland. Dent. J. 2021, 9, 144.
- Yang, J.; Dang, H.; Xu, Y. Recent advancement of decellularization extracellular matrix for tissue engineering and biomedical application. Artif. Organs 2022, 46, 549–567.
- Carvalho, M.S.; Alves, L.; Bogalho, I.; Cabral, J.M.S.; da Silva, C.L. Impact of Donor Age on the Osteogenic Supportive Capacity of Mesenchymal Stromal Cell-Derived Extracellular Matrix. Front. Cell Del. Biol. 2021, 9, 747521.
- Talaei-Khozani, T.; Aleahmad, F.; Bazrafshan, A.; Aliabadi, E.; Vojdani, Z. Lectin Profile Variation in Mesenchymal Stem Cells Derived from Different Sources. Cell Tissues Organs 2019, 208, 101–112.
- Porter, G.A.; Palade, G.E.; Milici, A.J. Differential bonding of the lectins Griffonia simplicifolia I and Lycopersicon esculentum to microvascular endothelium: Organ-specific localization and partial glycoprotein characterization. Eur. J. Cell Biol. 1990, 5, 85–95.
- Hart, D.A. What Molecular Recognition Systems Do Mesenchymal Stem Cells/Medicinal Signaling Cells (MSC) Use to Facilitate Cell-Cell and Cell Matrix Interactions? A Review of Evidence and Options. Int. J. Mol. Sci. 2021, 22, 8637.
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-beta (TGF-B)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 2002, 277, 21352–21360.
- E Scott, J. The first and second ’laws’ of chemical morphology, exemplified in mammalian extracellular matrices. Eur. J. Histochem. 2002, 46, 111–124.
- Ewald, C.Y. The Matrisome during Aging and Longevity: A Systems-Level Approach Defining Matreotypes Promoting Healthy Aging. Gerontology 2020, 66, 266–274.
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661.
- Yianni, V.; Sharpe, P.T. Epigenetic mechanisms driving lineage commitment in mesenchymal stem cells. Bone 2020, 134, 115309.
- Huh, S.J.; Clement, K.; Jee, D.; Merlini, A.; Choudhury, S.; Maruyama, R.; Yoo, R.; Chytil, A.; Boyle, P.; Ran, F.A.; et al. Age- and Pregnancy-Associated DNA Methylation Changes in Mammary Epithelial Cells. Stem Cell Rep. 2015, 4, 297–311.
- Shalev, D.; Melamed, P. The role of the hypothalamus and pituitary epigenomes in central activation of the reproductive axis at puberty. Mol. Cell. Endocrinol. 2020, 518, 111031.
- Vazquez, M.J.; Daza-Dueñas, S.; Tena-Sempere, M. Emerging Roles of Epigenetics in the Control of Reproductive Function: Focus on Central Neuroendocrine Mechanisms. J. Endocr. Soc. 2021, 5, bvab152.
- Manotas, M.C.; González, D.M.; Céspedes, C.; Forero, C.; Moreno, A.P.R. Genetic and Epigenetic Control of Puberty. Sex. Dev. 2022, 16, 1–10.
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49.
- Ryan, C.P. “Epigenetic clocks”: Theory and applications in human biology. Am. J. Hum. Biol. 2021, 33, e23488.
- Li, S.; Ye, Z.; Mather, K.A.; Nguyen, T.L.; Dite, G.S.; Armstrong, N.J.; Wong, E.M.; Thalamuthu, A.; Giles, G.G.; Craig, J.M.; et al. Early life affects late-life health through determining DNA methylation across the lifespan: A twin study. EBioMedicine 2022, 77, 103927.
- Hart, D.A. Sex Differences in Biological Systems and the Conundrum of Menopause: Potential Commonalities in Post-Menopausal Disease Mechanisms. Int. J. Mol. Sci. 2022, 23, 4119.
- Hart, D.A.; Zernicke, R.F. Optimal Human Functioning Requires Exercise Across the Lifespan: Mobility in a 1g Environment Is Intrinsic to the Integrity of Multiple Biological Systems. Front. Physiol. 2020, 11, 156.
- Hart, D.A. Learning From Human Responses to Deconditioning Environments: Improved Understanding of the “Use It or Lose It” Principle. Front. Sports Act. Living 2021, 3, 685845.
- McLeod, C.; Mauck, R. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cells Mater. 2017, 34, 217–231.
- James, A.W.; Péault, B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J. Orthop. Res. 2019, 37, 1221–1228.
- Lee, L.L.; Chintalgattu, V. Pericytes in the Heart. Adv. Exp. Med. Biol. 2019, 1122, 187–210.
- Grassel, S.G. The role of peripheral nerve fibres and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485.
- Zhang, Y.; Yang, J.; Zhang, P.; Liu, T.; Xu, J.; Fan, Z.; Shen, Y.; Li, W.; Zhang, H. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci. Rep. 2016, 6, 27724.
- Dong, P.; Gu, X.; Zhu, G.; Li, M.; Ma, B.; Zi, Y. Melatonin Induces Osteoblastic Differentiation of Mesenchymal Stem Cells and Promotes Fracture Healing in a Rat Model of Femoral Fracture via Neuropeptide Y/Neuropeptide Y Receptor Y1 Signaling. Pharmacology 2018, 102, 272–280.
- Wu, J.-Q.; Jiang, N.; Yu, B. Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders. World J. Stem Cells 2020, 12, 986–1000.
- Wu, L.W.; Wang, Y.-L.; Christensen, J.M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D.S.; Lee, W.A.; Brandacher, G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl. Immunol. 2014, 30, 122–127.
- Wang, X.-Q.; Shao, Y.; Ma, C.-Y.; Chen, W.; Sun, L.; Liu, W.; Zhang, D.-Y.; Fu, B.-C.; Liu, K.-Y.; Jia, Z.-B.; et al. Decreased SIRT3 in aged human mesenchymal stromal/stem cells increases cellular susceptibility to oxidative stress. J. Cell. Mol. Med. 2014, 18, 2298–2310.
- Peffers, M.J.; Collins, J.; Loughlin, J.; Proctor, C.; Clegg, P.D. A proteomic analysis of chondrogenic, osteogenic and tenogenic constructs from aging mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 133.
- Fan, M.; Chen, W.; Liu, W.; Du, G.-Q.; Jiang, S.-L.; Tian, W.-C.; Sun, L.; Li, R.-K.; Tian, H. The Effect of Age on the Efficacy of Human Mesenchymal Stem Cell Transplantation after a Myocardial Infarction. Rejuvenation Res. 2010, 13, 429–438.
- Tashiro, J.; Elliot, S.J.; Gerth, D.J.; Xia, X.; Pereira-Simon, S.; Choi, R.; Catanuto, P.; Shahzeidi, S.; Toonkel, R.L.; Shah, R.H.; et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl. Res. 2015, 166, 554–567.
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359.
- Caplan, A.I. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159.
- Li, C.; Chai, Y.; Wang, L.; Gao, B.; Chen, H.; Gao, P.; Zhou, F.-Q.; Luo, X.; Crane, J.L.; Yu, B.; et al. Programmed cell senescence in skeleton during late puberty. Nat. Commun. 2017, 8, 1312.
- Hart, D.A. Perspective: Is it time to rename MSC with a name that reflects their in vivo functions and in vitro abilities?-Possibly “Pluripotent mesenchymal regulatory cells (PMRC)”. J. Biomed. Sci. Eng. 2021, 14, 317–324.
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451.
- Caplan, A.I. There Is No “Stem Cell Mess”. Tissue Eng. Part B 2019, 25, 291–293.
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147.
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 3635465211014500.
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280.
- Kim, K.-I.; Kim, M.-S.; Kim, J.-H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2022, 3635465211053893.
- Zhuang, Y.; Li, D.; Fu, J.; Shi, Q.; Lu, Y.; Ju, X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: Immunoreulatory ability is enhanced in aged cells. Mol. Med. Rep. 2015, 11, 166–174.
- Franzen, J.; Georgomanolis, T.; Selich, A.; Kuo, C.-C.; Stöger, R.; Brant, L.; Mulabdić, M.S.; Fernandez-Rebollo, E.; Grezella, C.; Ostrowska, A.; et al. DNA methylation changes during long-term in vitro cell culture are caused by epigenetic drift. Commun. Biol. 2021, 4, 598.




