1. Introduction
During in utero development, the various organs form via developmental programing. Subsequently, in humans, the organs contain the necessary cells and cell types to yield an integrated group of cells that will contribute to a functioning system via cell–cell communication, an organ-specific extracellular matrix (ECM), and further differentiation of the associated parenchymal cells, endothelial cells of the microvasculature, endogenous neural elements, and endogenous pluripotent cells known as “mesenchymal stem cells or medicinal signaling cells”. Thus, fetal development lays the groundwork for the organization of an organ and the functional integration of its component cells. Therefore, within a specific organ, the endothelial cells of the microvasculature, the various parenchymal cells, the MSC/pericytes, the innervation where appropriate, and the ECM must function internally as a functional unit and externally as a unit functionally integrated into the context of the systems biology of the host.
A critical component of this system is the endothelial cells of the microvasculature. As the microvasculature is the key interface between the host and specific parenchymal cells and pericytes within an organ, they play a unique role in regulation. As reviewed recently by Augustin and Koh [
33], the endothelial cells of the microvasculature undergo organotypic differentiation to accommodate specific organ systems. How this develops and is maintained is still undefined for the most part, but it exemplifies the complexity of the cell–cell and cell-matrix interactions that contribute to a functionally integrated organ system.
With the likely exception of the liver [
34], overt damage to a human organ or removal of part of it will not lead to tissue regeneration, while some species such as planarians [
35], some amphibians [
36] and fish [
37] have retained such abilities. Thus, in humans, this natural regenerative process is quite limited [
38].
Once the template for an organ or a tissue is developed during fetal progression, the organs and tissues of a human continue to mature and grow in utero, particularly during the third trimester. Thus, at the time of full-term birth, the human is able to function in a mostly coordinated and integrated manner with the assistance of the mother via lactation. In this context, an organ such as a liver, kidney, heart, lung, or centers of the brain is/are set to continue to grow and mature in the postnatal environment.
In contrast, some tissues of the musculoskeletal system, such as ligaments and tendons designed to function in a mechanically active environment, exist as cellular tissues devoid of much ECM at the time of birth (i.e., “cell-rich and matrix-poor”). Subsequently, the tissues will progressively lay down a more organized ECM between the cells yielding a tissue that is hypocellular at skeletal maturity but is rich in ECM. This process is driven in part by the demands of the biomechanical environment and the presence of anabolic factors. Removing the mechanical stimuli from such an in vivo growing ligament stops growth and maturation [
39]. In this case, the tissue is no longer responsive to any in vivo anabolic stimuli.
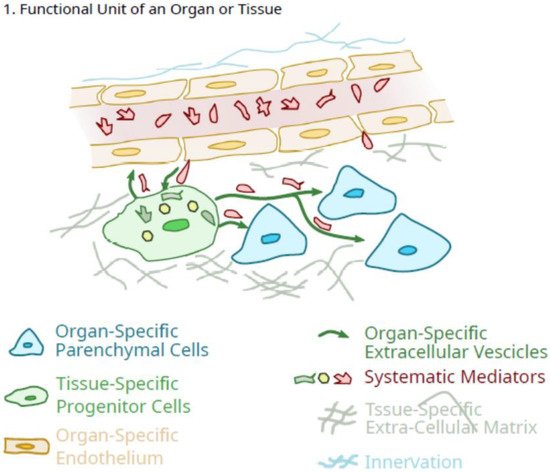
Thus, as the growth and maturation of organs in the early postnatal period (0–3 years of age) occur in a regulated manner consistent with an integrated systems biology approach, the dependence on anabolic signals is likely more common. Whether much of this growth occurs as a continuum or a series of regulated “steps” (i.e., growth “spurts”), or both may depend on the specific tissue, but at skeletal maturity, the multicellular organs are comprised of different cell types, function as integrated units. However, it is clear that many organs exist as a “functional unit” consisting of various cell types [endothelial cells, pericytes (some of which exhibit pluripotency properties and can be called mesenchymal stem cells or medicinal signaling cells or pluripotent mesenchymal regulatory cells; 2, 32), organ-specific cells, neural elements] (Figure 1), and continue to expand during the growth and maturation phase of the lifespan after birth via controlled and coordinated proliferation of various cells with orderly deposition of ECM, controlled neovascularization and accompanying innervation, as well as endogenous MSC/pericytes.
Figure 1. Schematic of the role of tissue-specific pluripotent cells in the integrated function unit of an organ.
As outlined in
Figure 1, as a result of developmental programs individual organs/tissues can be defined as a “functional unit” consisting of one or more organ-specific cells that define the function of the organ (i.e., osteoclasts and osteoblasts and osteocytes in bone; liver parenchymal cells, etc.). Different organs also appear to contain other what could be considered “regulatory” cell types, such as organ-specific differentiated endothelial cells [
33,
40], neural elements that can influence both the microvasculature in various situations [
41,
42] as well as other organ-specific cells (i.e., parenchymal cells in the liver; osteoblasts and osteocytes in bone as examples), and pericytes which often exhibit the characteristics of what have been called mesenchymal stem cells [
43].
However, not all of the pericytes may be what has been called MSC, but their heterogeneity may also represent other cell types [
44]. The role of the neural elements is not clear as following transplantation of an organ such as a liver, the nerves have been transected and there does not appear to be an overt loss of function [
45]. Analogous to the endothelial cells, such organ-localized pluripotent regulatory cells could take advantage of the lineage “plasticity” and ability to differentiate in vitro towards different lineages [
2,
46], and differentiate locally to enhance their “signalling” role to optimize the contents of their extracellular vesicles and their secretions to maintain the integrity of the specific “functional units” that constitute an organ system. Such differentiation could be directed by signals from the endogenous organ-specific cells or in part, from the cells of the microvasculature. The latter could be influenced by the resident pericytes to maintain differentiation as outlined in the proposed scheme in
Figure 1. In this proposed scheme, the tissue-specific pericytes would be ideally located to facilitate regulation in response to systemic mediators in the vasculature and translate them into a more organ-specific response pattern. Thus, the tissue-specific pericytes function as facilitators, amplifiers, and regulators for fine-tuning response patterns. There may also “cross-talk” between the regulatory pericytes and the endothelial cells of the microvasculature [
47]. In the context of such a role, there would be more to it than maintenance of integrity, but also controlled expansion during the growth and maturation stage of life (0 to ~10/12 years of age) and then further sex-specific maturation following the onset of puberty. Such regulation by these tissue-specific regulatory cells could also be influenced in females by the conditions of pregnancy in an organ-specific manner.
The last element in the functional unit of an organ that should be mentioned is the extracellular matrix (ECM). As discussed recently by Yang et al. [
48], it is likely that each organ system has a unique ECM that contains specific components or perhaps even unique splice variants of common molecules. This ECM can arise from the pericytes [
49] or the parenchymal cells of the tissue. Specificity may be manifested via the glycolytic linkages in glycoproteins or on cells [
7,
50]. Such an organ-specific ECM could contribute to the localization of the cells in the organ via cell receptors as has been reviewed recently by Hart [
51], as a source of biologically active molecules bound to the elements of the ECM, such as in bone [
52], as an example, and contribute to the morphology of specific organs [
53]. Furthermore, the ECM in an organ is likely dynamic and can be altered by injury or during the aging process [
54,
55], factors that could impact organ integrity and functioning.
Growth and maturation of tissue and organs progress at very individualized rates until the next transition in maturation occurs, that of the onset of puberty. While some sex-dependent aspects of growth and maturation occur prior to puberty, the onset of puberty is associated with another burst of growth and sexual development/maturation in both males and females. However, the onset of puberty, particularly in females, sets the stage for subsequent pregnancies and the altered regulation of many tissues and organs to accommodate the necessary adaptations leading to a successful pregnancy. As the onset of puberty is also accompanied by a time of growth as well, this requires the successful coordination and integration of both aspects of growth and the adaptations associated with puberty. Thus, in the time frame from the onset of puberty to skeletal maturity, there must be retention of integrated organ function that occurred during pre-pubertal growth and maturation alongside continued growth but with an altered maturation goal after puberty onset. In this context, the endogenous pericytes in each organ system or tissue must continue to serve unique functions that are integrated with the associated cell types and regulatory elements. With increasing commitment, many, if not most, likely develop epigenetic [
56] or carbohydrate [
7] signatures to reflect this commitment. Of note, puberty itself is accompanied by epigenetic alterations to many cells [
57,
58,
59,
60], so this could also include cells such as those that are labelled MSC/pericytes in different tissues.
For females, the next opportunity for regulatory impact on organ systems and tissues is pregnancy and lactation, potentially multiple times throughout most of evolutionary history. Obviously, many systems have to adapt to the conditions of pregnancy, including organs and systems such as the cardiovascular system, kidneys, lungs, the MSK system and others, with the adaptations somewhat dynamic throughout the nine-month pregnancy as the fetus matures, gains weight and puts different stresses on the maternal systems. After birth and during lactation, there are other adaptations required. However, many of the systems affected by pregnancy return to near normal conditions [but not all of the MSK systems may return to pre-pregnancy conditions]. Therefore, the regulation of these systems must be at least in part reversible. In the return to the post-pregnancy environment, functional integrity is maintained. The interactions between cells in an organ system must be resilient against loss of biological integrity, including the role of the MSC in the various organs.
With aging, the functioning of a number of systems and organs can decline in different populations, and again males and females are affected differently. Some of this decline in function may relate to genetics and epigenetic changes occurring during life [
61,
62,
63]. In females, this relates to the onset of menopause at age ~45–50. Interestingly, the average lifespan for much of evolutionary history was likely <30 years age, so the number of females who actually went through menopause until the relatively recent past was only a subset of females who reached 50 years of age.
Of note, menopause appears to be a process that can take years and thus is not an acute event. Therefore, the changes occurring in a variety of tissues and organs following the decline in ovarian function and systemic levels of sex hormones and development of secondary effects is not an abrupt change but rather a relatively slow process that in different subsets of females can result in conditions such as osteoporosis, obesity, increased cardiovascular risk, and risk for cognitive decline and the onset of dementia [
64]. With regard to osteoporosis, some females lose bone integrity in a somewhat wide range of rates, with some losing much more bone than others [
65,
66] and many similarly aged females not losing much at all. Therefore, there is likely some genetic basis for the rate of bone loss after menopause in those at risk for osteoporosis development. This is likely true for osteoporosis, dementia and obesity, while that for cardiovascular disease risk is still not clear. Thus, this focus on specific targets in these post-menopausal conditions tends to shape the research effort and may contribute to the lack of progress in some areas [
64].
2. Potential Role of Pluripotent Organ-Specific Pericytes in Growth and Maturation, as Well as Senescence and Decline in Organ and Tissue Integrity
As mentioned above, progenitor cells have been found in just about every tissue and organ system that has been examined [
5], and these cells are usually found as pericytes in close proximity to the microvasculature as well [
43,
67,
68]. Thus, these tissue-localized MSC are well situated to respond to biological signals traveling through the blood, as well as signals from the tissue-specific endothelial cells that are in direct contact with the bloodborne signals. Furthermore, signals from the MSC-like pericytes could engage in crosstalk with the endothelial cells for mutual benefit [
47]. In addition, they are also in a position to be influenced by neural elements, directly or indirectly via neural influences on the organ-associated endothelial cells and or the tissue-specific pericytes. Interestingly, MSC have been reported to express neuropeptide receptors [
69] such as CGRP [
70] and neuropeptide Y [
71,
72].
Furthermore, when MSC are isolated from tissues or organs of young animals and adult or older animals, the younger cells exhibit characteristics that differ from the older MSC both in vitro and in vivo. This can be manifested at the level of the extracellular matrix the MSC provides [
49], cell proliferation [
73], susceptibility to oxidative stress [
74], lineage differentiation [
75], and functionality in vivo [
76,
77] and in vitro [
73]. Thus, MSC from younger animals appear to be well suited to assist in the growth and maturation of tissues and organs when they are associated with specific tissues, such as pericytes. This concept is outlined in
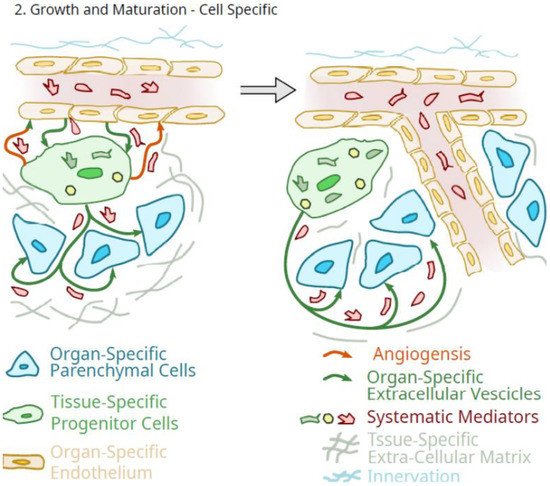
Figure 2.
Figure 2. Schematic of the potential role of tissue-specific pluripotent cells in the regulated growth of an organ.
A role for the tissue-specific pericytes in the growth and maturation of organs and tissues, as outlined in Figure 2, would be one of amplification of circulating systemic molecules that mediate such growth and maturation. In such a role as an organ-specific amplification component of the organ that is differentiated to optimize the delivery of specifically tailored extracellular vesicles and secretions, systemic growth mediator effects would be greatly amplified by these cells. However, as outlined in Figure 2, such a scheme does not imply that such systemic mediators of growth could not also directly impact the organ-specific parenchymal cells.
Therefore, some differentiation potential is also required of progenitor pericytes as they integrate into the functionality of an organ at the level of the parenchymal cells. It should also not be forgotten that the progenitor pericytes could also function to maintain the differentiation of the organ-specific endothelial cells of the microvasculature [
78]. Thus, in this role, MSC could serve as sentinels and gatekeepers of regulatory signals and functions [
79], but critically, during growth and maturation when the orderly growth of both the organ-specific parenchymal cells and the microvasculature is required. Of note, there appears to be an onset of MSC senescence in bone in late puberty in mice [
80], potentially indicating that this could be due to the completion of a role during growth and maturation.
Being integrated into the unique environment of a tissue or organ at an early age, as well as being positioned in close proximity to the microvascular component, and capable of secreting relevant molecules as well as regulated “shedding” exosomes or extracellular vesicles containing relevant molecules that can migrate to target cells in a paracrine manner, make the cells currently called MSC ideal candidates to “translate” generalized anabolic factors in the bloodstream during growth and development into signals that are more organ- or tissue-relevant or impactful to a coordinated growth and maturation of each tissue or organ to retain function during periods of transition as in growth and development. Thus, these cells that have been called MSC may more appropriately be called Pluripotent Mesenchymal Regulatory Cells (PMRC) [
32], a term that captures both the differentiation and signaling functions and abilities of MSC when becoming tissue-specific pericytes rather than using two different terms as suggested by Caplan [
21,
22]. In such a role, the pluripotent aspects of their capabilities may have benefits in maintaining tissue integrity via their self-renewal potential, as well as their ability to replace cells that have died via apoptosis or via errors in replication, and their ability to secrete tissue-specific mediators and release extracellular vesicles. Thus, the ability of these pluripotent cells to differentiate in order to optimize their regulatory functions in a tissue/organ-specific manner may be a critical feature of the cells. Additionally, an injection of BM-derived [
81,
82] or adipose tissue-derived [
83,
84] MSC into the knees of patients with osteoarthritis can often relieve the pain and inflammation in such joints; resident tissue-specific pericytes could also potentially function to dampen low levels of endogenous inflammation arising in such an environment to avoid evoking an inappropriate systemic inflammatory response and an elevated risk for loss of organ function due to fibrosis or cell death. Such immunomodulatory cells in bone marrow or adipose tissue may be influenced by donor age [
73]. However, care must be taken when interpreting some characteristics of these cells, as some properties may be influenced by passaging the cells in vitro [
85], possibly due to epigenetic drift [
86].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23105496