
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Syed Atif Hasan Naqvi | -- | 3076 | 2022-05-19 22:37:14 | | | |
| 2 | Amina Yu | -10 word(s) | 3066 | 2022-05-20 04:50:20 | | |
Video Upload Options
Xanthomonas citri subsp. citri, a causative agent of the citrus canker (CC) disease, belongs to one of the essential groups of the bacterial phytopathogen family, Xanthomonadaceae. It has been a potential threat to the globally significant citrus fruit crop, which has remained under investigation for disease management and epidemiology since the 1980s. In Pakistan, the average yield of citrus is 11 t/ha, which is lower than other countries, including China, Brazil, and India, having average productions of 27, 26, and 22 tons/hectare, respectively. Citrus canker is one of the most devastating diseases, posing a significant threat to crop yield and fruit quality. To date, five distinct types (or forms) of the citrus canker have been recognized; the Asiatic (Canker A) form is most destructive and affects most citrus cultivars. Severe infection outcomes include dieback, defoliation, severely blemished fruit, premature fruit drop, and reduced fruit quality. The infection increases under humid, warm, cloudy climate, wind, and heavy rainfall.
1. Taxonomy of Citrus Canker Bacterium
2. Phylogenetically Distinct Groups of CC
3.1. Asiatic Citrus Canker Strains
3.2. South-American Canker Strains
3. Symptomatology

3.1. Leaf Lesions
3.2. Fruit Lesions
3.3. Twig Lesions
4. Disease Cycle and Epidemiology
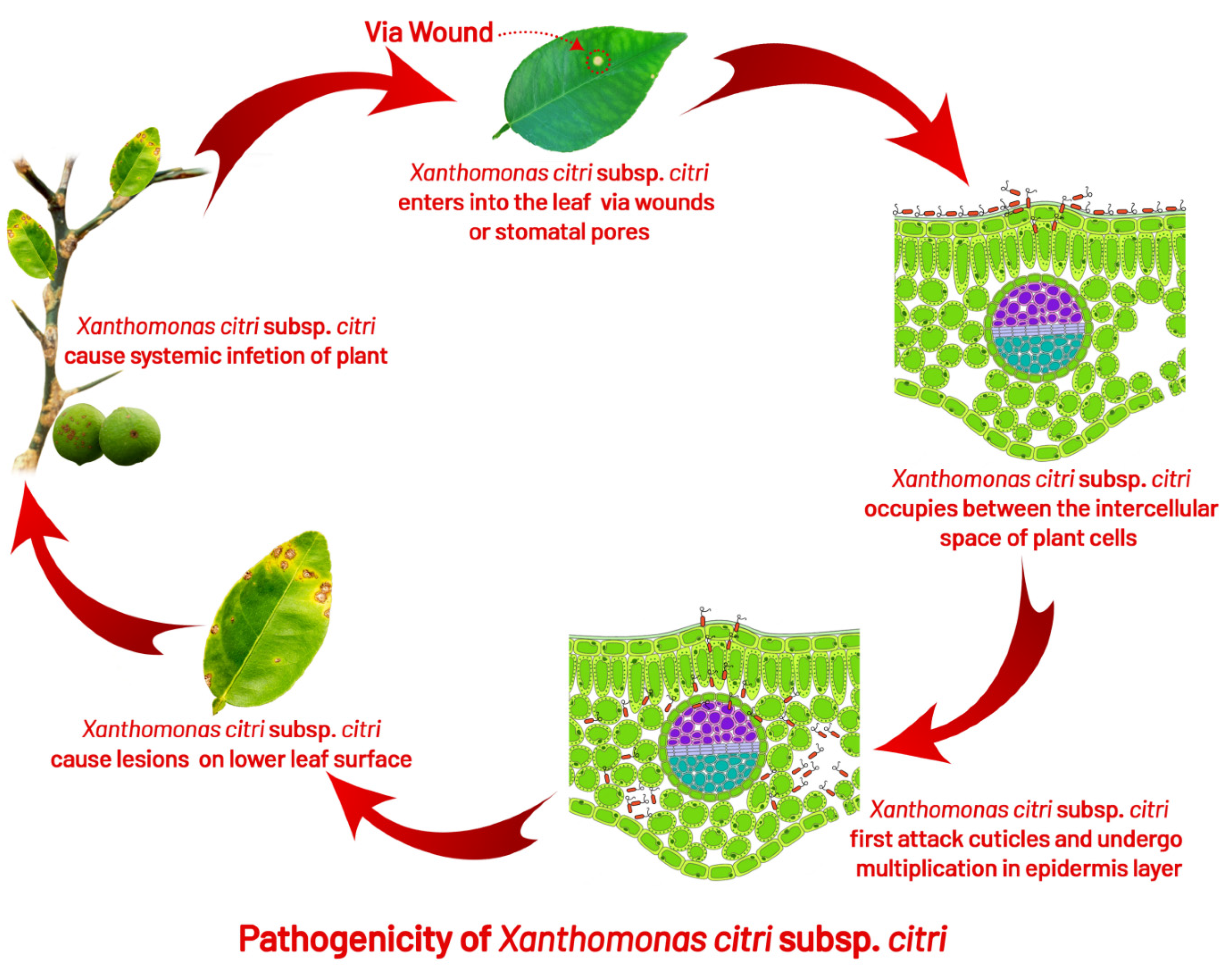
4.1. Infection
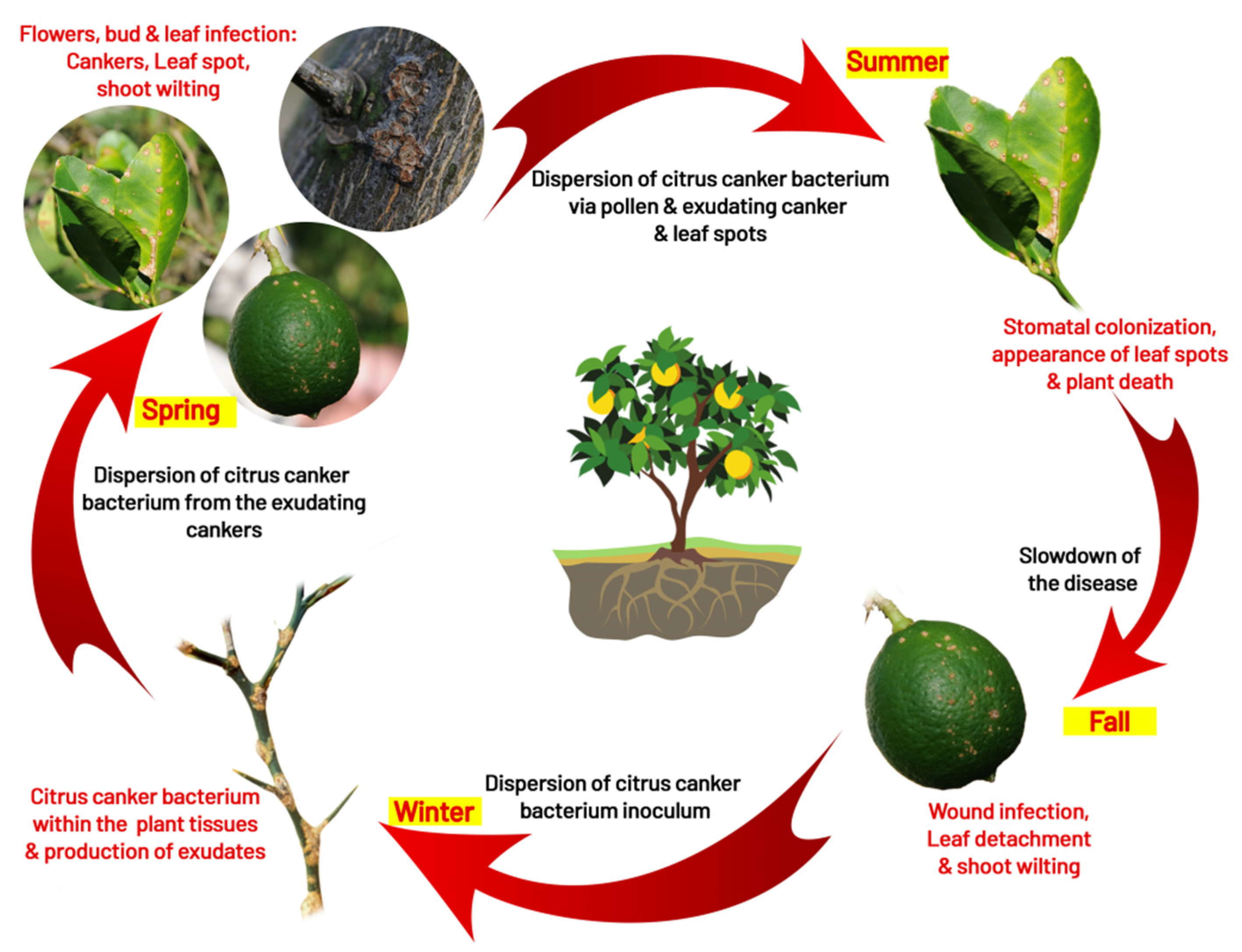
4.2. Survival
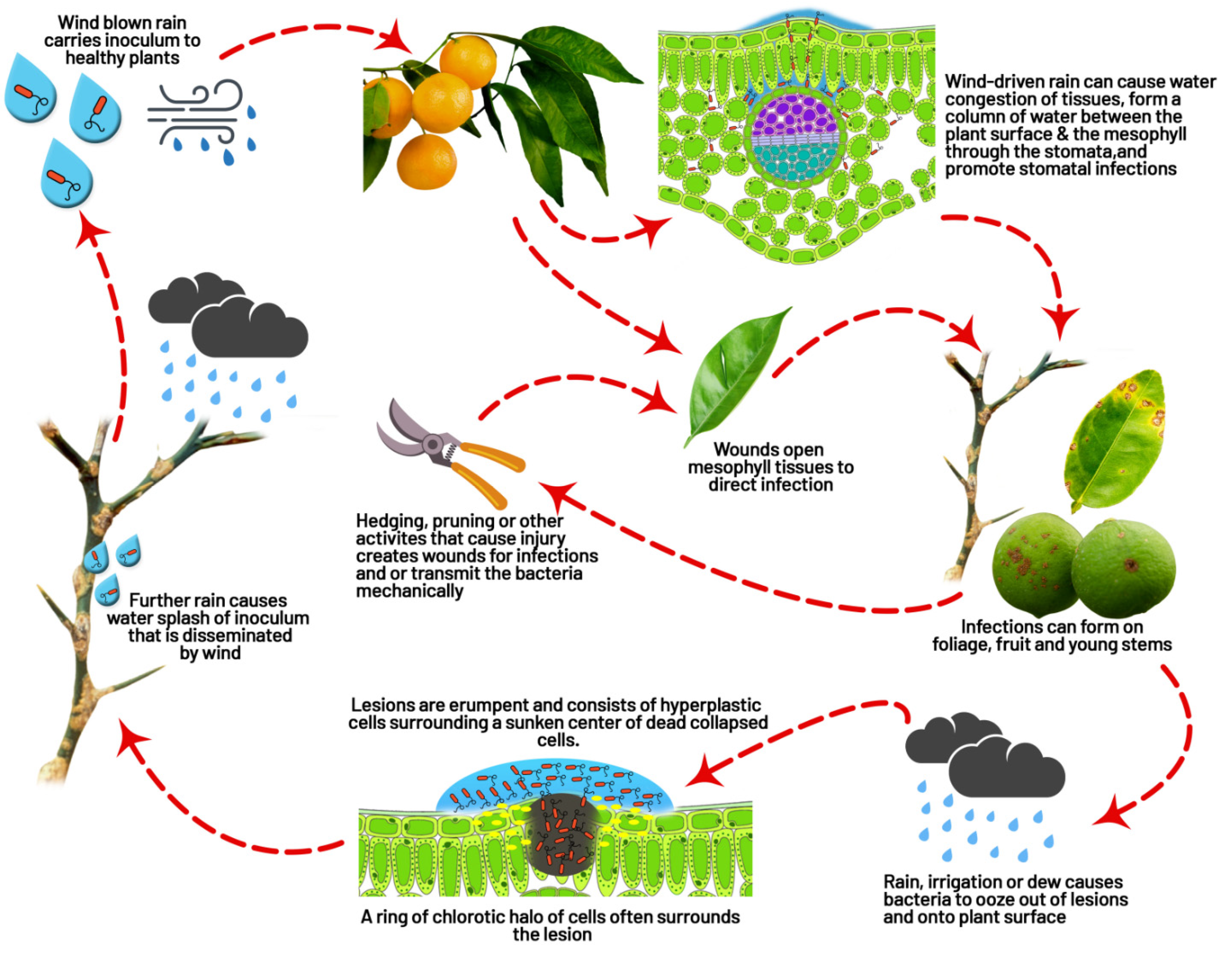
4.3. Dispersal
4.4. Role of Insect (Leaf Miner Interaction)
References
- Stevens, H.E. Citrus canker. A preliminary bulletin. Fla. Agric. Expt. Sta. Bull. 1914, 122, 113–118.
- Hasse, C.H. Pseudomonas citri, the cause of citrus canker—A preliminary report. J. Agric. Res. 1915, 4, 97–100.
- Ference, C.M.; Gochez, A.M.; Behlau, F.; Wang, N.; Graham, J.H.; Jones, J.B. Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol. Plant Pathol. 2018, 19, 1302–1318.
- Doidge, E.M. The Origin and Cause of Citrus Canker in South Africa. Union So. Afr. Dept. Agric. Sei. Bul. 1916, 8, 20.
- Dowson, W.J. On the systematic position and generic names of the gram negative bacterial plant pathogens. Zentr. Bakteriol. Parasitenk. Abt. II 1939, 100, 177–193.
- Sena-Velez, M.; Redondo, C.; Graham, J.H.; Cubero, J. Presence of extracellular DNA during biofilm formation by Xanthomonas citri subsp citri strains with different host range. PLoS ONE 2016, 11, e0156695.
- Bansal, K.; Kumar, S.; Patil, P.B. Phylogenomic insights into diversity and evolution of nonpathogenic Xanthomonas strains associated with citrus. mSphere 2020, 5, e00087-20.
- Patané, J.S.L.; Martins, J.; Rangel, L.T.; Belasque, J.; Digiampietri, L.A.; Facincani, A.P.; Ferreira, R.M.; Jaciani, F.J.; Zhang, Y.; Varani, A.M.; et al. Origin and diversification of Xanthomonas citri subsp. citri pathotypes revealed by inclusive phylogenomic, dating, and biogeographic analyses. BMC Genom. 2019, 20, 700.
- Young, J.M.; Dye, D.W.; Bradbury, J.F.; Panagopoulos, C.G.; Robbs, C.F. Proposed nomenclature and classification for plant pathogenic bacteria. N. Z. J. Agric. Res. 1978, 21, 153–177.
- Gabriel, D.W.; Kingsley, M.T.; Hunter, J.E.; Gottwald, T. Reinstatement of Xanthomonas citri (ex Hasse) and Xanthomonas phaseoli (ex Smith) to species and reclassification of all Xanthomonas campestris pv citri strains. Int. J. Syst. Bacteriol. 1989, 39, 14–22.
- Young, J.M.; Bradbury, J.F.; Gardan, L.; Gvozdyak, R.I.; Stead, D.E.; Takikawa, Y.; Vidaver, A.K. Comment on the reinstatement of Xanthomonas citri (Ex Hasse 1915) Gabriel et al. 1989 and X. phaseoli (Ex Smith 1897) Gabriel et al. 1989—Indication of the need for minimal standards for the genus Xanthomonas. Int. J. Syst. Bacteriol. 1991, 41, 172–177.
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 1995, 45, 472–489.
- Martins, P.M.M.; de Oliveira Andrade, M.; Benedetti, C.E.; de Souza, A.A. Xanthomonas citri subsp. citri: Host interaction and control strategies. Trop. Plant Pathol. 2020, 45, 213–236.
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv. malvacearum (ex Smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) Dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and ‘‘var. fuscans’’ of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov. Syst. Appl. Microbiol. 2005, 28, 494–518.
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Emended classification of Xanthomonad pathogens on citrus. Syst. Appl. Microbiol. 2006, 29, 690–695.
- Euzeby, J. List of new names and new combinations previously effectively, but no validly, published, list. Int. J. Syst. Evol. Microbiol. 2007, 57, 893–897.
- Constantin, E.C.; Cleenwerck, I.; Maes, M.; Baeyen, S.; Van Malderghem, C.; De Vos, P.; Cottyn, B. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016, 65, 792–806.
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157.
- Maloy, O.; Baudoin, A. Disease control principles. In Enclyclopedia of Plant Pathology; Maloy, O.C., Murray, T.D., Eds.; Wiley: New York, NY, USA, 2001; pp. 330–332.
- Izadiyan, M.; Taghavi, S.M.; Farahbakhsh, F. Characterization of Xanthomonas citri subsp. CITRI isolated from grapefruit in Iran. J. Plant Pathol. 2018, 100, 257–267.
- Civerolo, E. Bacterial canker disease of citrus . J. Rio Gd. Val. Hortic. Soc. 1984, 35, 811–818.
- Civerolo, E. Citrus bacterial canker disease in tropical regions. Colloques-Inra 1994, 66, 45.
- Stall, R.E.; Civerolo, E.L. Research relating to the recent outbreak of citrus canker in Florida. Annu. Rev. Phytopathol. 1991, 29, 399–420.
- Humphries, J. Bacteriology; John Murray Albermack Street: London, UK, 1974; p. 452.
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for the Identification of Plant Pathogenic Bacteria; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2001.
- Vernière, C.; Hartung, J.S.; Pruvost, O.P.; Civerolo, E.L.; Alvarez, A.M.; Maestri, P.; Luisetti, J. Characterization of phenotypically distinct strains of Xanthomonas axonopodispv. citri from Southwest Asia. Eur. J. Plant Pathol. 1998, 104, 477–487.
- Cubero, J.; Graham, J. Genetic relationship among worldwide strains of Xanthomonas causing canker in citrus species and design of new primers for their identification by PCR. Appl. Environ. Microbiol. 2002, 68, 1257–1264.
- Leite, R.; Minsavage, G.V.; Bonas, U.; Stall, R.E. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microbiol. 1994, 60, 1068–1077.
- Vauterin, L.; Yang, P.; Hoste, B.; Vancanneyt, M.; Civerolo, E.; Swings, J.; Kersters, K. Differentiation of Xanthomonas campestris pv. citri strains by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins, fatty acid analysis, and DNA-DNA hybridization. Int. J. Syst. Evol. Microbiol. 1991, 41, 535–542.
- Wu, M.K.; Gee, A.D.; Wesselink, P.; Moorer, W. Fluid transport and bacterial penetration along root canal fillings. Int. Endod. J. 1993, 26, 203–208.
- Egel, D.; Graham, J.; Stall, R. Genomic relatedness of Xanthomonas campestris strains causing diseases of citrus. Appl. Environ. Microbiol. 1991, 57, 2724–2730.
- Pruvost, O.; Hartung, J.; Civerolo, E.; Dubois, C.; Perrier, X. Plasmid DNA fingerprints distinguish pathotypes of Xanthomonas campestris pv. citri, the causal agent of citrus bacterial canker disease. Phytopathology 1992, 82, 485–490.
- Hartung, J.; Daniel, J.-F.; Pruvost, O. Detection of Xanthomonas campestris pv. citri by the polymerase chain reaction method. Appl. Environ. Microbiol. 1993, 59, 1143–1148.
- Zhang, M.; Meng, Q. Automatic citrus canker detection from leaf images captured in field. Pattern Recognit. Lett. 2011, 32, 2036–2046.
- Swarup, S.; De Feyter, R.; Brlansky, R.H.; Gabriel, D.W. A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of X. campestris to elicit cankerlike lesions on citrus. Phytopathology 1991, 81, 802–809.
- Swarup, S.; Yang, Y.; Kingsley, M.T.; Gabriel, D.W. An Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol. Plant Microbe. Interact. 1992, 5, 204–213.
- da Gama, M.A.S.; de Lima Ramos Mariano, R.; da Silva J’unior, W.J.; de Farias, A.R.G.; Barbosa, M.A.G.; da Silva Velloso Ferreira, M.A.; J’unior, C.R.L.C.; Santos, L.A.; de Souza, E.B. Taxonomic Repositioning of Xanthomonas campestris pv. viticola (Nayudu 1972) Dye 1978 as Xanthomonas citri pv. viticola (Nayudu 1972) Dye 1978 comb. nov. and Emendation of the Description of Xanthomonas citri pv. anacardii to Include Pigmented Isolates Pathogenic to Cashew Plant. Phytopathology 2018, 108, 1143–1153.
- Medina-Urrutia, V.M.; Stapleton, J.J. Control of Mexican lime bacteriosis with copper-based products. Proc. Fla. State Hortic. Soc. 1987, 99, 22–25.
- Stapleton, J.; Garza-Lopez, J. Epidemiology of a citrus leaf-spot disease in Colima, Mexico. Phytopathology 1988, 78, 440–443.
- Urrutia, M. Isolation, pathogenicity, and partial host range of Alternaria limicola, causal agent of mancha foliar de los citricos in Mexico. Plant Dis. 1994, 78, 879.
- Graham, J.H.; Gottwald, T. Research perspectives on eradication of citrus bacterial diseases in Florida. Plant Dis. 1991, 75, 1193–1200.
- International Standards for Phytosanitary Measures (ISPM) ISPM 27 Diagnostic Protocols, DP 6: Xanthomonas citri subsp. citri; IPPC, FAO: Rome, Italy, 2014.
- Timmer, L. Anthracnose diseases. In Compendium of Citrus Diseases, 2nd ed.; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; APS Press: St. Paul, MN, USA, 2000; pp. 21–22.
- Cernadas, R.A.; Benedetti, C.E. Role of auxin and gibberellin in citrus canker development and in the transcriptional control of cell-wall remodeling genes modulated by Xanthomonas axonopodis pv. citri. Plant Sci. 2009, 177, 190–195.
- Swings, J.; Van den Mooter, M.; Vauterin, L.; Hoste, B.; Gillis, M.; Mew, T.; Kersters, K. Reclassification of the Causal Agents of Bacterial Blight (Xanthomonas campestris pv. oryzae) and Bacterial Leaf Streak (Xanthomonas campestris pv. oryzicola) of Rice as Pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 1990, 40, 309–311.
- Mahaffee, W.F.; Kloepper, J.W. Temporal changes in the bacterial communities of soil, rhizosphere and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb. Ecol. 1997, 34, 210–223.
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994.
- Gottwald, T.R.; Sun, X.; Riley, T.; Graham, J.H.; Ferrandino, F.; Taylor, E.L. Geo-referenced spatiotemporal analysis of the urban citrus canker epidemic in Florida. Phytopathology 2002, 92, 361–377.
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodispv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15.
- Daungfu, O.; Youpensuk, S.; Lumyong, S. Endophytic Bacteria Isolated from Citrus Plants for Biological Control of Citrus Canker in Lime Plants. Trop. Life Sci. Res. 2019, 30, 73–88.
- Graham, J.; Gottwald, T.; Riley, T.; Achor, D. Penetration through leaf stomata and growth of strains of Xanthomonas campestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology 1992, 82, 1319–1325.
- Koizumi, M. Citrus Canker: The World Situation. Citrus Canker: An International Perspective; Timmer, L.W., Ed.; University of Florida: Lake Alfred, FL, USA, 1985; pp. 2–7.
- Loucks, K.W. Citrus Canker and Its Eradication in Florida; Department of Agriculture, Division of Plant Industry: St. Gainesville, FL, USA, 1934.
- Goto, M. Citrus canker. Plant Dis. Int. Importance 1992, 3, 170–208.
- Chagas, M.; Parra, J.R.; Namekata, T.; Hartung, J.S.; Yamamoto, P.T. Phyllocnistiscitrella Stainton (Lepidoptera: Gracillariidae) and its relationship with the citrus canker bacterium Xanthomonas axonopodispvcitri in Brazil. Neotrop. Entomol. 2001, 30, 55–59.
- Christiano, R.; Dalla Pria, M.; Jesus Junior, W.C.; Parra, J.R.P.; Amorim, L.; Bergamin Filho, A. Effect of citrus leaf-miner damage, mechanical damage and inoculum concentration on severity of symptoms of Asiatic citrus canker in Tahiti lime. Crop Prot. 2007, 26, 59–65.
- Hall, D.G.; Gottwald, T.R.; Bock, C.H. Exacerbation of citrus canker by citrus leafminerPhyllocnistiscitrella in Florida. Fla. Entomol. 2010, 93, 558–566.
- Das, A. Citrus canker—A review. J. Appl. Hortic. 2003, 5, 52–60.
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of eradicating citrus canker in Florida—Again. Plant Dis. 2001, 85, 340–356.
- Stall, R. Xanthomonas campestris pv. citri detection and identification by enzyme-linked immunosorbent assay. Plant Dis. 1982, 231, 231–236.
- Bergamin Filho, A.; Hughes, G. Citrus Canker Epidemiology-Methodologies and Approaches: A Moderated Discussion Session. In Proceedings of the International Citrus Canker Research Workshop, Ft. Pierce, FL, USA, 20–22 June 2022; pp. 24–25.
- Francis, M.; Redondo, A.; Burns, J.; Graham, J. Soil application of imidacloprid and related SAR-inducing compounds produces effective and persistent control of citrus canker. Eur. J. Plant Pathol. 2009, 124, 283–292.
- Dewdney, M.; Graham, J. Florida Citrus Pest Management Guide: Citrus Canker; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2012; p. 4.
- Duan, Y.P.; Castaneda, A.; Zhao, G.; Erdos, G.; Gabriel, D. Expression of a single, host-specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant-Microbe Interact. 1999, 12, 556–560.
- Gottwald, T.; Graham, J.; Schubert, T. An epidemiological analysis of the spread of citrus canker in urban Miami, Florida, and synergistic interaction with the Asian citrus leafminer. Fruits 1997, 6, 383–390.
- Graham, J.; Gottwald, T.; Riley, T.; Cubero, J.; Drouillard, D. Survival of Xanthomonas campestris pv. citri (Xcc) on various surfaces and chemical control of Asiatic Citrus Canker (ACC). In Proceedings of the International Citrus Canker Research Workshop, Ft. Pierce, FL, USA, 20–22 June 2000; p. 7.
- Rao, Y.; Hingorani, M. Survival of Xanthomonas citri (Hasse) Dowson in leaves and soil. Indian Phytopath 1963, 16, 362–364.
- Verniere, C.; Gottwald, T.; Pruvost, O. Disease development and symptom expression of Xanthomonas axonopodispv. citri in various citrus plant tissues. Phytopathology 2003, 93, 832–843.
- Goto, M.; Serizawa, S.; Morita, M. Studies on Citrus Canker Disease. III. Survival of Xanthomonas Citri (Hasse) Dowson in Soils and on the Surface of Weeds; Bulletin of the Faculty of Agriculture, Shizuoka University: Shizuoka, Japan, 1970; Volume 20, pp. 21–29.
- Goto, M. Survival of Xanthomonas citri in the bark tissues of citrus trees. Can. J. Bot. 1972, 50, 2629–2635.
- Leite, R., Jr.; Mohan, S. Evaluation of citrus cultivars for resistance to canker caused by Xanthomonas campestris pv. citri (Hasse) Dye in the State of Paraná, Brazil. Proc. Int. Soc. Citric. 1984, 1, 385–389.
- Graham, J.; Gottwald, T.; Civerolo, E.; McGuire, R. Population dynamics and survival of Xanthomonas campestris in soil in citrus nurseries in Maryland and Argentina. Plant Dis. 1989, 43, 423–427.
- Teper, D.; Pandey, S.S.; Wang, N. The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria. Microorganisms 2021, 9, 187.
- Luthra, J.C.; Sattar, A. Citrus canker and its control in Punjab. Punjab Fruit J. 1942, 6, 179–182.
- Stall, R.E.; Miller, J.; Marco, G.M.; de Echenique, B.C. Population dynamics of Xanthomonas citri causing cancrosis of citrus in Argentina. In Proceedings of the Florida State Horticultural Society; Florida State Horticultural Society: Alexandria, VA, USA, 1980; pp. 10–14.
- Traoré, Y.N.; Ngoc, L.B.T.; Vernière, C.; Pruvost, O. First report of Xanthomonas citripv. citri causing citrus canker in Mali. Plant Dis. 2008, 92, 977.
- Heppner, J.B. Citrus leafminer, Phyllocnistiscitrella, in Florida (Lepidoptera: Gracillariidae: Phyllocnistinae). Trop. Lepid. Res. 1993, 1, 49–64.
- Parsai, P.S. Citrus canker. In Proceedings of the Seminar on Diseases of Horticultural Plants, Simla, India, 10–15 June 1959; pp. 91–95.
- Bacon, C.W.; Hinton, D.M. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Control 2002, 23, 274–284.
- Knapp, J. Citrus Leafminer, Phyllocnistiscitrella Stainton: Current Status in Florida-1995; University of Florida: Gainesville, FL, USA, 1995.
- Peña, J.E.; Hunsberger, A.; Schaffer, B. Citrus leafminer (Lepidoptera: Gracillariidae) density: Effect on yield of ‘Tahiti’ lime. J. Econ. Entomol. 2000, 93, 374–379.




