Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hugo Albrecht | -- | 3539 | 2022-05-19 09:00:33 | | | |
| 2 | Camila Xu | -21 word(s) | 3518 | 2022-05-19 09:38:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Albrecht, H.; Khetan, R.; Dharmayanti, C.; Gillam, T.; Kübler, E.; Klingler-Hoffmann, M.; Ricciardelli, C.; Oehler, M.; Blencowe, A.; Garg, S. Using GPCRs to Target Ovarian Cancer with Nanomedicines. Encyclopedia. Available online: https://encyclopedia.pub/entry/23107 (accessed on 17 January 2026).
Albrecht H, Khetan R, Dharmayanti C, Gillam T, Kübler E, Klingler-Hoffmann M, et al. Using GPCRs to Target Ovarian Cancer with Nanomedicines. Encyclopedia. Available at: https://encyclopedia.pub/entry/23107. Accessed January 17, 2026.
Albrecht, Hugo, Riya Khetan, Cintya Dharmayanti, Todd Gillam, Eric Kübler, Manuela Klingler-Hoffmann, Carmela Ricciardelli, Martin Oehler, Anton Blencowe, Sanjay Garg. "Using GPCRs to Target Ovarian Cancer with Nanomedicines" Encyclopedia, https://encyclopedia.pub/entry/23107 (accessed January 17, 2026).
Albrecht, H., Khetan, R., Dharmayanti, C., Gillam, T., Kübler, E., Klingler-Hoffmann, M., Ricciardelli, C., Oehler, M., Blencowe, A., & Garg, S. (2022, May 19). Using GPCRs to Target Ovarian Cancer with Nanomedicines. In Encyclopedia. https://encyclopedia.pub/entry/23107
Albrecht, Hugo, et al. "Using GPCRs to Target Ovarian Cancer with Nanomedicines." Encyclopedia. Web. 19 May, 2022.
Copy Citation
G protein-coupled receptors (GPCRs) are the largest family of membrane receptors, and many are overexpressed in solid tumors, including ovarian cancer.
ovarian cancer
G protein-coupled receptor
GPCR
nanoparticle
drug delivery
active targeting
1. Introduction
The symptoms of ovarian cancer are non-specific, and due to a lack of effective screening strategies, it is generally not detected at an early stage when the tumor is still confined to the organ of origin. Resultingly, 70% of patients are diagnosed when their ovarian cancer has progressed to advanced stages (III or IV). The epidemiology of ovarian cancer is very complex and is affected by various factors, including the status of inherited and acquired somatic mutations, hormonal effects during menopause, environmental hazards, pelvic inflammatory disease, endometriosis, and polycystic ovarian syndrome [1]. Effective disease management is extremely challenging, with an overall 5-year survival rate of less than 50%, in comparison to 91% for breast cancer. Only 25% of women with advanced stage ovarian cancer will survive for longer than 5 years.
The current frontline treatment for ovarian cancer includes debulking surgery, followed by dual carboplatin plus paclitaxel chemotherapy to kill residual tumor tissue [2]. The drugs are generally administered intravenously, or according to some treatment protocols, intraperitoneally (IP) [3]. In some patients, good results have also been achieved via a neoadjuvant approach, providing chemotherapy prior to surgical intervention. Whilst approximately 80% of patients who receive the first-line standard treatment of carboplatin plus paclitaxel will respond, often with apparent complete remission, the majority of patients relapse, and about 20% of patients do not respond to the treatment at all, meaning they possess an innate form of chemotherapy resistance [4][5]. Moreover, the currently used treatments are associated with severe and dose-limiting adverse effects.
To achieve treatments with improved therapeutic indices, several specific molecular therapies have been introduced into the clinic. Poly ADP-ribose polymerase (PARP) inhibition is now used as a strategy against tumors with germline or somatic BRCA1/2 and other DNA repair gene mutations [6][7]. Olaparib was the first PARP inhibitor to gain FDA approval and was followed by others, such as Rucaparib and Niraparib [8]. Administration of these inhibitors can cause severe and dose-limiting hematologic adverse effects, such as thrombocytopenia, neutropenia, and anemia [8]. Grade 3/4 toxicities have been reported to force dose interruptions and reductions [9]. Apart from other mild side effects, increased frequencies of myeloblastic syndromes and acute myeloid leukemia have also been observed [10]. Another treatment strategy employs Bevacizumab as antibody-based, anti-angiogenic therapy, as maintenance therapy in the adjuvant setting, or in combination with other chemotherapy drugs such as liposomal doxorubicin or gemcitabine at recurrence [11]. An alternative anti-angiogenic is the kinase inhibitor Cediranib, an inhibitor of VEGFR-1, -2, -3, and c-kit. Additional novel therapies under investigation include vaccines [12], CAR-T immunotherapy, anti CA125 antibody therapy, and viral and small molecule immune checkpoint inhibitors [13][14][15][16][17][18].
2. Harnessing GPCRs to Target Ovarian Cancer Cells with Nanomedicines
Many tumors have been shown to aberrantly express GPCRs and the overwhelming complexity of an underlying signaling network has been discovered [19][20][21][22][23]. The clinical relevance of GPCR expression in the case of ovarian cancer is highlighted by its effects on cell growth, migration, metastasis, invasion, survival, metabolism, and secretion [24][25][26]. Many GPCRs are expressed in stromal, immune, and endothelial cells of ovarian cancer tissue where they play important roles in tumor growth via stimulation of angiogenesis and other mechanisms. In addition to this, β arrestin 2 expression has been associated with impaired prognosis, hence further boosting the role of GPCRs [27]. On one hand, small molecule-mediated modulation of GPCRs presents a potentially rewarding avenue towards novel anti-cancer solutions; whilst on the other hand, and in context with the presented research, the functional overexpression of these receptors lends itself to the targeting of cancer tissues with ligand decorated nanomedicines.
Ovarian cancers are genetically unstable, most often due to mutations in DNA repair genes (e.g., BRCA1/2) and in the tumor protein P53 gene [28]. The latter is prevalent in ~96% of serous ovarian cancer cells, driving chromosomal instability and leading to aberrant gene expression [29]. Considering that GPCRs are distributed throughout the genome, it is expected that some become frequently overexpressed when healthy cells turn into neoplastic cells. As shown in Figure 1, many GPCR loci are close to copy number alterations (CNAs) or on frequently amplified chromosome arms 1q, 3q, 6p, 7q, 8q, 12p, 20p, and 20q [30]. Although the detailed mechanisms are poorly understood, many GPCRs are very likely to be upregulated within amplicons or as a consequence of chromosomal translocations. Alternatively, the gene expression can be triggered indirectly through upregulated signaling pathways. Whilst some genetic loci may be hotspots for gene amplification and chromosomal translocations, others may be infrequent or at random.
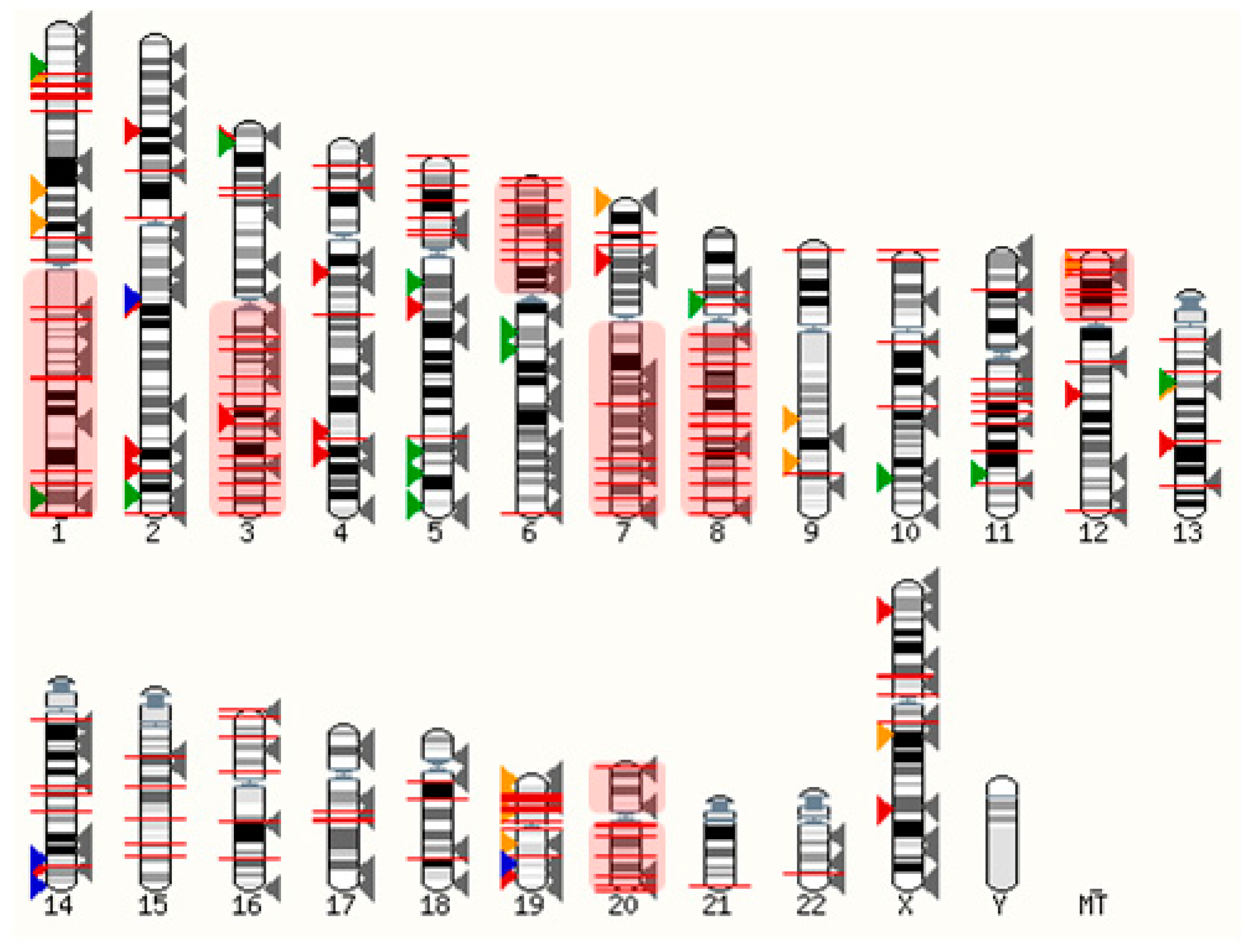
Figure 1. Distribution of GPCRs and copy number alterations (CNA on genome). All GPCRs (except olfactory receptors) are indicated with arrowheads. Overexpressed receptors are shown in color, according to Table 1. Red: Peptide and protein activated receptors; Orange: Lipid receptors; Green: Adrenergic receptors; Blue: Ionic receptors; Grey: Not frequently overexpressed in ovarian cancer (310 receptors). The red lines indicate 2902 CNAs with frequencies ranging from 5%–34% (from TCGA, Pan Cancer Atlas, 572 ovarian serous cystadenocarcinoma samples; accessed via cBioportal [31][32] on 3 March 2022). Frequently amplified chromosome arms 1q, 3q, 6p, 7q, 8q, 12p, 20p, 20q are shown in red shaded boxes. The human karyotype figure was generated using Ensembl 2021 [33].
The most studied GPCRs in the context of ovarian cancer are the somatostatin (SSTR1–5) [34][35][36][37], cholecystokinin (CCKAR/CCKBR) [38][39], gastrin-releasing peptide (GRPR) [40][41][42], luteinizing hormone-releasing hormone (LHRHR) [43][44] and neurotensin receptors (NTSR1/2) [45]. Surprisingly, GPCRs have remained largely untapped for targeted drug delivery in cancer tissues. A large body of research reveals many more receptors with the potential to be established as novel biomarkers or docking sites for ligand decorated and drug-loaded NPs (summarized in Table 1). Receptor-mediated internalization will bring NPs directly into endosomal compartments, whereby a pH-responsive drug release trigger can be used to drive NP disassembly, followed by endosomal membrane rupture and drug escape into the cytoplasm, ultimately inducing apoptotic cell death [46][47].
2.1. Ionic GPCRs
G-protein coupled receptor 4 (GPR4) is a type of GPCR that is activated by protons and is involved in cancer-related angiogenesis. GPR4 is found to be detected in a higher amount in the endothelium of vessels of EOCs compared to benign ovarian tumors [48]. Bai et al. reported that significant inhibition of invasion and cell growth can be induced in A2780 ovarian cancer cells with the knockdown of GPR4 and transcription factor 7 (TCF7) while promoting apoptosis [49]. Similarly, GPR68 also known as ovarian cancer G protein-coupled receptor 1 (OGR1) has also been identified as a proton sensing receptor [50][51]. The expression of OGR1 in human ovarian tumor HEY cells resulted in the inhibition of cell migration and proliferation [51]. Furthermore, G2A (or GPR132) is also known as a proton sensing receptor that regulates proliferation, immunity, and oncogenesis and exerts antitumorigenic properties via cell cycle arrest at the G2/M stage [52][53][54]. GPR4, GPR68, and GPR132 are recognized to be activated by lysophosphatidylcholine (LPC) and sphingosylphosphorylcholine (SPC), which induces growth inhibition [48][52][55]. Lastly, GPR39 is frequently overexpressed in ovarian cancer tissue and mediates Zn2+ induced signaling [56]. GPR39 was found to be an inhibitor of cell death, hence representing a potential therapeutic target for the treatment of ovarian cancer [57].
Most of these receptors do not have suitable ligands to functionalize NPs. However, specific antibodies could be applied to decorate NPs to induce specific binding to the receptor. In the case of proton sensing receptors, endosomal uptake will be triggered in the acidic tumor microenvironment.
2.2. Aminergic GPCRs
Aminergic GPCRs, a subset of class A rhodopsin-like GPCRs, are the targets for approximately 25% of the current clinically used drugs [58]. Ovarian cancer is known to be affected by receptor ligands produced by the immune and nervous systems. Receptors from the aminergic GPCR family are excellent drug targets as they are associated with memory, neurotransmission, mood and circadian cycle regulation, cognition, and vasoconstriction [59]. Ovarian cancer is known to be affected by receptor ligands produced by the immune and nervous systems. In line with this, histamine, acetylcholine, serotonin, dopamine, and adrenaline receptors are frequently expressed in ovarian cancer cells and have been linked to their functions including proliferation, survival, and migration [60][61].
Oppitz et al. reported that 23 out of 39 ovarian tumors tested, expressed adrenaline receptors, which was associated with reduced patient survival [62]. Dopamine receptor 2 (DRD2) is known to be overexpressed in ovarian cancer cells. Yong et al. studied the effect of a DRD2 antagonist, thioridazine and it was observed that it exhibited an anticancer effect in A2780 and SKOV3 cell lines as well as SKOV3 xenografts in nude mice by inducing apoptosis and oxidative stress [63]. Moreover, thioridazine interacted with extracellular-signal-regulated kinase (ERK) and AKT signaling pathways and inhibited tumor angiogenesis. Histamine activates the histamine receptor H1 (HRH1), which stimulates the growth of ovarian tumor cells in vitro and promotes the release of extracellular vesicles (EVs) that modulate different steps of the metastatic process. Pyrilamine, a selective HRH1 antagonist can block the cell proliferating effect of histamine on OVCAR3 cells, hence acting as a therapeutic drug target for the death of tumor cells [64].
2.3. Lipid GPCRs
Lipids can act as a signaling molecules, store energy, are involved in post-translational modifications, and, lastly, are a major constituent of cellular membranes [65][66][67]. These membrane lipids also play an important role in various tumorigenesis processes such as migration, proliferation, and inflammation [68][69][70]. They are known to directly interact with their targets or bind to extracellular or intracellular receptors. A significant number of lipid-activated GPCRs are known to be expressed in ovarian cancer tissue and their cognate ligands, such as lysophosphatidic acid (LPA), sphingosine-1-phosphate, platelet-activating factor (PAF; 1–0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) and various free fatty acids achieve high local concentrations. Here, researchers discuss four classes of lipid GPCRs: fatty acid, lysophospholipid, phospholipid, and steroid GPCRs.
2.3.1. Fatty Acid GPCRs
Fatty acids play a vital role in metabolic disorders and inflammation, hence contributing to tumorigenesis [71]. FFAR1 is a free fatty acid (FFA) receptor that has been found to be overexpressed in HGSOCs. FFA-mediated cancer cell growth has been demonstrated, and targeting this receptor is a potential future strategy [72]. Munkarah et al. demonstrated that the high concentration of GW1100, which is an FFAR1 antagonist was able to partially inhibit the proliferation and viability of cancer cell lines in the presence of serum [72]. Moreover, Hopkins et al. studied the function of FFARs in OVCAR3 and SKOV3 cell lines and examined if FFAR agonists affect their proliferation [73]. mRNA expression studies revealed that both the OVCAR3 and SKOV3 cell lines expressed FFAR1, and SKOV3 also expressed FFAR4 in small amounts. Furthermore, the FFAR1 agonist (GW9508) was able to inhibit the proliferation of both cell lines.
2.3.2. Lysophospholipid GPCRs
Lysophospholipids are known to play an essential role in cellular processes, including migration, proliferation, and immune responses [74][75][76]. The most studied lysophospholipids in cancer biology are LPA and sphingosine-1-phosphate (S1P) [77].
The ascitic fluid contains elevated LPA concentrations and ovarian cancer cells are known to produce high levels of LPA [78][79][80]. LPA has been shown to drive ovarian cancer cell migration and invasion [81], activate NF-kB [82] and AP-1 transcription factors [83], increase cyclooxygenase 2 production, and induce metabolic reprogramming of ovarian cancer cells inducing a glycolytic shift via hypoxia-inducible factor 1 activation [79]. Additional pathways to mediate or synergistically act in concert with LPA stimulation are EGFR and other RTKs, and the Hippo/YAP pathway [81][84]. LPA Receptors (LPARs) are widely expressed in normal ovaries but frequently overexpressed in benign tumors and ovarian cancer tissue [78][79]. Particularly, LPAR2 and LPAR3 have been shown to be frequently overexpressed in ovarian cancer cells and tissues [56][80]. Lysophosphatidylethanolamine has been described as an alternative ligand on some LPA receptors inducing a Ca2+ signal and boosting cell migration [85][86]. Overall, these accumulated findings make a very strong case for LPA blockage as a potential anti-cancer strategy.
Similarly, S1P plays a vital role in the regulation of angiogenesis, apoptosis, cell growth, and inflammation. S1P controls the invasiveness of epithelial ovarian cancer cells through a complex mechanism involving multiple GPCR pathways, which regulate ECM-proteolysis and attachment of cells [87]. All five of the known S1P receptors might be involved in this complex interplay, and targeting these receptors could be relevant for some anti-cancer strategies. Visentin et al. demonstrated that the S1P-specific monoclonal antibody LT1002 can neutralize S1P by decreasing the systemic level of IL-8, hence, reducing cell survival and proliferation of tumors in a mouse model [88]. Moreover, S1PR antagonists such as VPC44116, VPC23019, and VPC25239 are known to inhibit the invasion and migration of OVCAR3 cells [89]. Hence, the role of S1P in ovarian cancer needs to be further understood to be able to discover new therapeutic strategies for the management of the disease.
2.3.3. Phospholipid GPCRs
PAF binds to the PAF receptor (PTAFR) and is involved in inflammation and platelet aggregation [90]. PTAFR has been shown to activate the EGFR and ERK signaling pathways in ovarian cancer cells, therefore potentially contributing to cancer progression [91]. Yu et al. investigated the effect of WEB2086 (a PTAFR antagonist) in combination with AG1478 (an EGFR inhibitor) on CAOV3 and SKOV3 cell lines and it was observed that the combination significantly inhibited the invasion and proliferation by inducing apoptosis and arresting the cells at the G0/G1 phase [92]. Moreover, Gao et al. studied that PAF increases the stemness of SKOV3 and A2780 cell lines, and the application of Ginkgolide B, which is a PTAFR inhibitor successfully reduced tumor growth [93]. Therefore, targeting PTAFR could be a potential approach for the treatment of ovarian cancer.
2.3.4. Steroid GPCRs
Steroids are typically hydrophobic polycyclic signaling molecules that elicit cellular actions by binding to intracellular nuclear receptors [94]. Androgens, estrogens, mineralocorticoids, progestogens, and glucocorticoids are a few examples of steroid hormones that regulate cellular interactions with nuclear receptors. Estrogen, for instance, transmits signals via G protein-coupled estrogen receptor 1 (GPER1) to activate the EGFR and promote proliferation [95]. Increased membrane estrogen receptor expression has been observed in high-grade serous samples and correlated with impaired prognosis [27]. However, there are controversies in the study of GPER1 and its effect on ovarian cancer. Ignatov et al. reported that the expression of GPER1 was lower in ovarian cancer tissues when compared to benign ovarian tumors [96]. Moreover, the selective GPER1 agonist, G1, was able to suppress the proliferation of SKOV3 and OVCAR3 cell lines. Conversely, Liu et al. demonstrated that 17β-estradiol and G1 induced proliferation of OVCAR5 cell lines [97]. Limited information is available on the impact and role of GPER1 on ovarian cancer, and therefore further studies are required to confirm the tumor-suppressing or proliferating effect of GPER1 before using it as a drug target.
Table 1. GPCRs expressed in ovarian cancer.
| Receptor Protein Symbol 1 | Endogenous Agonists (Signaling 2) | Antagonists | References | |
|---|---|---|---|---|
| Ionic | GPR4 | Protons (Gs, Gi/o, Gq/11, G12/13) | GPR4 antagonist 3b, NE 52-QQ57 | [48] |
| GPR39 | Zn2+ (Gq/11) | - | [56] | |
| GPR68 | Protons (Gi/o, Gq/11) | Psychosine | [50][51][98] | |
| GPR132 | Protons (NA 3) | Lysophosphatidylcholine | [52][53] | |
| Aminergic | ADRA1B | Adrenaline, Noradrenaline (Gq/11) | AH 11110, L-765314, Rec 15/2615 | [60] |
| ADRB1 | Adrenaline, Noradrenaline (Gs) | Acebutolol, Atenolol, Betaxolol | ||
| ADRB2 | Adrenaline, Noradrenaline (Gs) | Sotalol, Propafenone, Nadolol | ||
| ADRB3 | Adrenaline, Noradrenaline (Gs) | L-748337, L-748328 | ||
| CHRM3 | Acetylcholine (Gq/11) | Tropicamide, Tolterodine, Oxybutynin | [60] | |
| DRD1 | Dopamine, 5-Hydroxytryptamine, Noradrenaline (Gs) | Ecopipam, SCH-23390, SKF-83566 | [60] | |
| DRD2 | Dopamine (Gi, Gi/o) | ML321, Raclopride, Domperidone | ||
| HRH1 | Histamine (Gq/11) | Astemizole, Triprolidine, Azelastine | [60][61] | |
| HTR1A | 5-Hydroxytryptamine (Gi/o) | Robalzotan, WAY-100635 | [60] | |
| HTR1B | 5-Hydroxytryptamine (Gi/o) | GR-55562 | ||
| HTR1D | 5-Hydroxytryptamine (Gi/o) | SB 714786 | ||
| HTR1E | 5-Hydroxytryptamine (Gi/o) | Rauwolscine, Fluspirilene, Metergoline | ||
| HTR2A | 5-Hydroxytryptamine (Gq/11) | Compund 3b, Ketanserin | ||
| HTR2B | 5-Hydroxytryptamine (Gq/11) | EGIS-7625, RS-127445, BF-1 | ||
| HTR4 | 5-Hydroxytryptamine (Gs) | RS 100235, GR 113808, SB 204070 | ||
| Lipid | FFAR1 (GPR40) | docosahexaenoic acid, α-linolenic acid, myristic acid, oleic acid, long chain carboxylic acids (Gq/11) | GW1100 | [72] |
| GPER1 | 17β-estradiol (Gi/o) | G15, G36 | [27] | |
| LPAR1 | LPA (Gi/o, Gq/11, G12/13) | AM095, ONO-7300243, AM966 | [56][78][79][80][81][82][83][84][85][86][99][100] | |
| LPAR2 | LPA, Farnesyl diphosphate, Farnesyl monophosphate (Gi/o, Gq/11, G12/13) | H2L5186303 | ||
| LPAR3 | LPA, Farnesyl diphosphate, Farnesyl monophosphate (Gi/o, Gq/11) | Dioctanoylglycerol pyrophosphate | ||
| LPAR4 | LPA, Farnesyl diphosphate (Gs, Gi/o, Gq/11, G12/13) | AM966, Farnesyl diphosphate, Farnesyl monophosphate | ||
| LPAR5 | LPA, Farnesyl diphosphate, Farnesyl monophosphate, n-arachidonoylglycine (Gq/11, G12/13) | TCLPA5, AS2717638 | ||
| LPAR6 | LPA (Gs, Gi/o, G12/13) | - | ||
| PTAFR | PAF, Methylcarbamyl PAF (Gi/o, Gq/11) | Rupatadine, Apafant, BN 50739 | [101] | |
| S1PR1 | S1P, Dihydrosphingosine 1-phosphate, Sphingosylphosphorylcholine (Gi/o) | NIBR-0213, W146 | [87] | |
| S1PR2 | S1P, Dihydrosphingosine 1-phosphate, Sphingosylphosphorylcholine (GS, Gq/11, G12/13) | JTE-013 | ||
| S1PR3 | S1P, Dihydrosphingosine 1-phosphate, Sphingosylphosphorylcholine (Gi/o, Gq/11, G12/13) | TY-52156 | ||
| S1PR4 | S1P, Dihydrosphingosine 1-phosphate, Sphingosylphosphorylcholine (Gi/o, G12/13) | CYM-50358 | ||
| S1PR5 | S1P, Dihydrosphingosine 1-phosphate, Sphingosylphosphorylcholine (Gi/o, G12/13) | - | ||
| Peptide- and protein-activated receptors | AGTR1 | Angiotensin II (Gq/11, Gi/o) | Iosartan, Olmesartan, Telmisartan | [82][102] |
| AGTR2 | Angiotensin II (Gi/o) | Olodanrigan, PD123319 | ||
| BDKRB2 | Bradykinin (Gs, Gi/o, Gq/11) | Anatibant, Icatibant, FR173657 | [60] | |
| CCKAR | CCK-8, -33, -39, -58 (Gq/11) | Dexloxiglumide, JNJ-17156516, Devazepide | [38][39] | |
| CCKBR | CCK-4, -8, -33, gastrin-17 (Gq/11) | Lorglumide, GW-5823, tetronothiodin | ||
| CXCR1 | Interleukin 8 (Gi/o) | Navarixin, AZD5069 | [60] | |
| CXCR2 | Interleukin 8 (Gi/o) | SX-517, Elubirixin, SB 225002 | [103] | |
| CXCR4 | CXCL12 (Gi/o) | Mavorixafor, T134, Plerixafor | [104] | |
| EDNRA | Endothelin-1, -2 (Gq/11) | Macitentan, Ambrisentan, BQ123 | [105][106][107][108] | |
| EDNRB | Endothelin-1, -2, -3 (Gs, Gi/o, Gq/11) | K-8794, IRL 2500, BQ788 | ||
| F2R (PAR1) | Protease activated/Thrombin (Gq/11) | RWJ-56110, SCH-79797, Vorapaxar | [109] | |
| F2RL1 (PAR2) | Protease activated/Serine proteases (Gq/11) | GB88, I-191, AZ8838 | [110] | |
| FPR2 | n-formyl-methionyl peptides (FMLP) (Gi/o) | WRWWWW, t-BOC-FLFLF | [111] | |
| FSHR | Follicle-stimulating Hormone (Gs) | FSH deglycosylated α/β | [27][112][113] | |
| GHRHR | Growth Hormone-releasing Hormone (Gs) | - | [114] | |
| GNRHR | Type 1 gonadotropin-releasing Hormone (Gq/11) | Abarelix, Degarelix, Elagolix | [115] | |
| GRPR | GRP-(14–27), GRP-(18–27), Neuromedin B and C, (Gq/11) | Bantag-1, PD 168368, AM-37 | [40][41][42] | |
| LGR5 (GPR49) | R-spondin-1, -2, -3, -4 (Wnt) | - | [116][117] | |
| LHCGR (LHRHR) | Luteinizing hormone, Chorionic gonadotropin (Gs) | Deglycosylated chorionic gonadotropin | [27][43][44] | |
| NTSR1 | Neurotensin, Large neuromedin n (Gq/11) | Meclinertant, SR142948A | [45][118] | |
| NTSR2 | Neurotensin (Gq/11) | - | ||
| OXTR | Oxytocin, Vasopressin (Gq/11) | Retosiban, SSR126768A, L-372662 | [56] | |
| PTH2R | Parathyroid Hormone (Gs) | PTHrP-(7–34), TIP39-(7–39) | [56] | |
| RXFP1 | Relaxin-1, -2, -3 (Gs, Gi/o) | B-R13/17K H2 relaxin | [119] | |
| SSTR1 | Cortistatin-14, Somatostatin-14, -28 (Gi/o) | BIM 23454, SRA880 | [34][35][36][37] | |
| SSTR2 | Cortistatin-14, -17, Somatostatin 14, -28 (Gi/o) | BIM 23454, [D-Tyr8]CYN 154806, BIM 23627 | ||
| SSTR3 | Somatostatin-28, -14, Cortistatin-17 (Gi/o) | ACQ090, MK-4256 | ||
| SSTR4 | Somatostatin-28, -14, Cortistatin-17 (Gi/o) | PRL-2915, [L-Tyr8]CYN 154806, BIM 23454 | ||
| SSTR5 | Somatostatin-14, -28, Cortistatin-14, -17 (Gi/o) | S5A1, BIM 23056 |
1 According to the European Bioinformatics Institute (EMBL-EBI), the National Center for Biotechnology Information (NCBI), the Protein Information Resource (PIR) and the Swiss Institute for Bioinformatics (SIB); 2 guidetopharmacology.org; 3 not applicable, coupling unknown.
2.4. Peptide- and Protein-Activated GPCRs
Endogenous protein and peptide ligands are known to activate approximately 118 GPCRs in the human body [120]. Some examples of protein-activated GPCRs and their effects on ovarian carcinoma are discussed below.
Endothelin-1 (ET-1) is secreted by endothelial cells, and it acts as a potent vasoconstrictor through activation of the endothelin receptors A and B (EDNRA/B) on smooth muscle cells. Moreover, endothelin receptors are involved in the regulation of cell survival, mitogenesis, angiogenesis, invasion, and epithelial-to-mesenchymal transition (EMT) in malignancies [121]. These receptors stimulate autocrine growth of ovarian carcinoma in vitro and in vivo in response to ET-1 secretion and drive ovarian tumor progression, metastasis, and drug resistance. Various cancer-promoting pathways have been linked to endothelin receptor activation, including β catenin and EGFR signaling. In line with this, the dual EDNRA/B inhibitor macitentan blocked metastatic progression of ovarian cancer cells [105][106][107][108], and zibotentan, a specific EDNRA inhibitor showed synergistic effects on apoptosis and inhibition of ovarian cancer cell invasion, when used in combination with EGFR inhibitors [122][123].
Various protease-activated receptors (PARs) have been implicated in tumor progression. For example, PAR-2 (F2RL1) is overexpressed in some ovarian cancer tissues mainly inducing cell migration, and PAR-1 (F2R) has been found to be abundant in invasive carcinomas, but not in the healthy ovarian epithelium, driving FAK signaling and promoting cancer malignancy [109][110]. F2R activation leads to the expression and secretion of pro-angiogenic chemokines, such as CCL2 (MCP-1), CXCL1 (GRO-α), and CXCL8 (IL-8).
Some chemokine receptors are known to stimulate angiogenesis using paracrine interaction between chemokine receptors expressed on endothelial cells, and chemokines released from ovarian tumor cells [124]. Noteworthy, CXCL8 is known to trigger the CXCR1 and CXCR2 receptors on endothelial cells, inducing cell migration and proliferation [125]. Specific inhibitors have been shown to block CXCL8 mediated cell migration and to synergistically enhance DOX activity [103]. Another chemokine receptor, CXCR4, is overexpressed in human ovarian cancer and its ligand CXCL12 has been shown to be present in ascitic fluid collected from patients with ovarian carcinoma [126][127][128].
Several inflammation-associated receptors are known to exert relevant effects on ovarian cancer cells: bradykinin and several chemokines can trigger intracellular Ca2+ signals in ovarian cancer cells [60], and relaxin production is induced by inflammation, activating prooncogenic pathways via the LGR7 (RXFP1) receptor. Furthermore, relaxin/RXFP1 inhibition reduced ovarian cancer cell viability and reversed cisplatin resistance [119]. The expression of another immune modulatory receptor, the formyl-peptide receptor-2 (FPR2), has been detected in EOC tissues and it plays a role in cell migration and invasion [111]. FPR2 detection is correlated with poor prognosis and could be a valuable prognostic marker.
The multifunctional scaffold protein β-arrestin 2 regulates the signal transduction and internalization of activated GPCRs including the luteinizing hormone/choriogonadotropin receptor (LHCGR) and FSHR [129]. High expression levels of β-arrestin 2 were associated with FSHR and LHCGR expression and correlated with impaired prognosis [27]. FSHR elevated gene expression has been frequently detected in patient-derived tumor samples, and it has been suggested as a potential cancer biomarker [112][113].
Several receptors have been found to be upregulated in ovarian cancer tissues, including growth hormone releasing hormone receptor (GHRHR) [130], GRPR [131], leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) [116][117], oxytocin receptor (OXTR) and parathyroid hormone 2 receptor (PTH2R) [56]. The angiotensin receptors (AGTR1/2) mediated NF-kB transcription factor activation in ovarian cancer cells, indicating functional expression [82].
In summary, it appears that the peptide and protein-activated GPCRs harbor a massive potential for the specific targeting of nanomedicines against ovarian carcinoma.
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32.
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203.
- Ceelen, W.; Braet, H.; van Ramshorst, G.; Willaert, W.; Remaut, K. Intraperitoneal chemotherapy for peritoneal metastases: An expert opinion. Expert Opin. Drug Deliv. 2020, 17, 511–522.
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239.
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1–19.
- Harter, P.; Hauke, J.; Heitz, F.; Reuss, A.; Kommoss, S.; Marme, F.; Heimbach, A.; Prieske, K.; Richters, L.; Burges, A.; et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS ONE 2017, 12, e0186043.
- Manchana, T.; Phoolcharoen, N.; Tantbirojn, P. BRCA mutation in high grade epithelial ovarian cancers. Gynecol. Oncol. Rep. 2019, 29, 102–105.
- Franzese, E.; Centonze, S.; Diana, A.; Carlino, F.; Guerrera, L.P.; Di Napoli, M.; De Vita, F.; Pignata, S.; Ciardiello, F.; Orditura, M. PARP inhibitors in ovarian cancer. Cancer Treat. Rev. 2019, 73, 1–9.
- Zhou, J.X.; Feng, L.J.; Zhang, X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2017, 11, 3009–3017.
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28.
- McClung, E.C.; Wenham, R.M. Profile of bevacizumab in the treatment of platinum-resistant ovarian cancer: Current perspectives. Int. J. Womens Health 2016, 8, 59–75.
- Chow, S.; Berek, J.S.; Dorigo, O. Development of Therapeutic Vaccines for Ovarian Cancer. Vaccines 2020, 8, 657.
- Charbonneau, B.; Goode, E.L.; Kalli, K.R.; Knutson, K.L.; Derycke, M.S. The immune system in the pathogenesis of ovarian cancer. Crit. Rev. Immunol. 2013, 33, 137–164.
- Chatterjee, J.; Dai, W.; Aziz, N.H.A.; Teo, P.Y.; Wahba, J.; Phelps, D.L.; Maine, C.J.; Whilding, L.M.; Dina, R.; Trevisan, G.; et al. Clinical Use of Programmed Cell Death-1 and Its Ligand Expression as Discriminatory and Predictive Markers in Ovarian Cancer. Clin. Cancer Res. 2017, 23, 3453–3460.
- Gordon, A.N.; Schultes, B.C.; Gallion, H.; Edwards, R.; Whiteside, T.L.; Cermak, J.M.; Nicodemus, C.F. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol. Oncol. 2004, 94, 340–351.
- Senzer, N.; Barve, M.; Kuhn, J.; Melnyk, A.; Beitsch, P.; Lazar, M.; Lifshitz, S.; Magee, M.; Oh, J.; Mill, S.W.; et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol. Ther. 2012, 20, 679–686.
- Yan, W.; Hu, H.; Tang, B. Advances Of Chimeric Antigen Receptor T Cell Therapy In Ovarian Cancer. Onco Targets Ther. 2019, 12, 8015–8022.
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213.
- Bhola, N.E.; Grandis, J.R. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front. Biosci. 2008, 13, 1857–1865.
- Almutairi, F.; Lee, J.K.; Rada, B. Regulator of G protein signaling 10: Structure, expression and functions in cellular physiology and diseases. Cell Signal. 2020, 75, 109765.
- Hayes, M.P.; Roman, D.L. Regulator of G Protein Signaling 17 as a Negative Modulator of GPCR Signaling in Multiple Human Cancers. AAPS J. 2016, 18, 550–559.
- Bodle, C.R.; Mackie, D.I.; Roman, D.L. RGS17: An emerging therapeutic target for lung and prostate cancers. Future Med. Chem. 2013, 5, 995–1007.
- Xu, H.; Wang, H.; Li, G.; Jin, X.; Chen, B. The Immune-Related Gene ELF3 is a Novel Biomarker for the Prognosis of Ovarian Cancer. Int. J. Gen. Med. 2021, 14, 5537–5548.
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94.
- Lappano, R.; Maggiolini, M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 2011, 10, 47–60.
- O’Hayre, M.; Degese, M.S.; Gutkind, J.S. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr. Opin. Cell Biol. 2014, 27, 126–135.
- Czogalla, B.; Partenheimer, A.; Jeschke, U.; von Schonfeldt, V.; Mayr, D.; Mahner, S.; Burges, A.; Simoni, M.; Melli, B.; Benevelli, R.; et al. beta-arrestin 2 Is a Prognostic Factor for Survival of Ovarian Cancer Patients Upregulating Cell Proliferation. Front. Endocrinol. 2020, 11, 554733.
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474.
- Nakayama, K.; Nakayama, N.; Jinawath, N.; Salani, R.; Kurman, R.J.; Shih Ie, M.; Wang, T.L. Amplicon profiles in ovarian serous carcinomas. Int. J. Cancer 2007, 120, 2613–2617.
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615.
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404.
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1.
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891.
- Barragan, F.; Carrion-Salip, D.; Gomez-Pinto, I.; Gonzalez-Canto, A.; Sadler, P.J.; de Llorens, R.; Moreno, V.; Gonzalez, C.; Massaguer, A.; Marchan, V. Somatostatin subtype-2 receptor-targeted metal-based anticancer complexes. Bioconjugate Chem. 2012, 23, 1838–1855.
- de Jong, M.; Breeman, W.A.; Kwekkeboom, D.J.; Valkema, R.; Krenning, E.P. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 2009, 42, 873–880.
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9.
- Okarvi, S.M. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat. Rev. 2008, 34, 13–26.
- Accardo, A.; Morisco, A.; Tesauro, D.; Pedone, C.; Morelli, G. Naposomes: A new class of peptide-derivatized, target-selective multimodal nanoparticles for imaging and therapeutic applications. Ther. Deliv. 2011, 2, 235–257.
- Aloj, L.; Aurilio, M.; Rinaldi, V.; D’Ambrosio, L.; Tesauro, D.; Peitl, P.K.; Maina, T.; Mansi, R.; von Guggenberg, E.; Joosten, L.; et al. Comparison of the binding and internalization properties of 12 DOTA-coupled and (1)(1)(1)In-labelled CCK2/gastrin receptor binding peptides: A collaborative project under COST Action BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1417–1425.
- Accardo, A.; Mansi, R.; Morisco, A.; Mangiapia, G.; Paduano, L.; Tesauro, D.; Radulescu, A.; Aurilio, M.; Aloj, L.; Arra, C.; et al. Peptide modified nanocarriers for selective targeting of bombesin receptors. Mol. Biosyst. 2010, 6, 878–887.
- Parry, J.J.; Kelly, T.S.; Andrews, R.; Rogers, B.E. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin(7-14) analogues containing different amino acid linker moieties. Bioconjugate Chem. 2007, 18, 1110–1117.
- Smith, C.J.; Volkert, W.A.; Hoffman, T.J. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl. Med. Biol. 2005, 32, 733–740.
- He, Y.; Zhang, L.; Song, C. Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes. Int. J. Nanomed. 2010, 5, 697–705.
- Nagy, A.; Schally, A.V. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol. Reprod. 2005, 73, 851–859.
- Falciani, C.; Brunetti, J.; Lelli, B.; Accardo, A.; Tesauro, D.; Morelli, G.; Bracci, L. Nanoparticles exposing neurotensin tumor-specific drivers. J. Pept. Sci. 2013, 19, 198–204.
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019, 30, 263–272.
- Allen, J.K.; Brock, D.J.; Kondow-McConaghy, H.M.; Pellois, J.P. Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs. Biomolecules 2018, 8, 50.
- Ren, J.; Jin, W.; Gao, Y.E.; Zhang, Y.; Zhang, X.; Zhao, D.; Ma, H.; Li, Z.; Wang, J.; Xiao, L.; et al. Relations between GPR4 expression, microvascular density (MVD) and clinical pathological characteristics of patients with epithelial ovarian carcinoma (EOC). Curr. Pharm. Des. 2014, 20, 1904–1916.
- Bai, Z.; Wu, Y.; Yan, Y.; Bai, S.; Kang, H.; Ma, W.; Zhang, J.; Gao, Y.; Hui, B.; Ma, H.; et al. Downregulation of GPR4 and TCF7 Promotes Apoptosis and Inhibits Growth and Invasion of Ovarian Cancer Cells. Anticancer. Agents Med. Chem. 2021, 21, 1544–1550.
- Wiley, S.Z.; Sriram, K.; Salmeron, C.; Insel, P.A. GPR68: An Emerging Drug Target in Cancer. Int. J. Mol. Sci. 2019, 20, 559.
- Ren, J.; Zhang, L. Effects of ovarian cancer G protein coupled receptor 1 on the proliferation, migration, and adhesion of human ovarian cancer cells. Chin. Med. J. 2011, 124, 1327–1332.
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Bio.l Chem. 2004, 279, 42484–42491.
- Radu, C.G.; Nijagal, A.; McLaughlin, J.; Wang, L.; Witte, O.N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1632–1637.
- Weng, Z.; Fluckiger, A.C.; Nisitani, S.; Wahl, M.I.; Le, L.Q.; Hunter, C.A.; Fernal, A.A.; Le Beau, M.M.; Witte, O.N. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 1998, 95, 12334–12339.
- Xu, Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim. Biophys. Acta 2002, 1582, 81–88.
- Albrecht, H.; Kubler, E. Systematic Meta-Analysis Identifies Co-Expressed Kinases and GPCRs in Ovarian Cancer Tissues Revealing a Potential for Targeted Kinase Inhibitor Delivery. Pharmaceutics 2019, 11, 454.
- Dittmer, S.; Sahin, M.; Pantlen, A.; Saxena, A.; Toutzaris, D.; Pina, A.L.; Geerts, A.; Golz, S.; Methner, A. The constitutively active orphan G-protein-coupled receptor GPR39 protects from cell death by increasing secretion of pigment epithelium-derived growth factor. J. Biol. Chem. 2008, 283, 7074–7081.
- Rask-Andersen, M.; Almen, M.S.; Schioth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590.
- Vass, M.; Podlewska, S.; de Esch, I.J.P.; Bojarski, A.J.; Leurs, R.; Kooistra, A.J.; de Graaf, C. Aminergic GPCR-Ligand Interactions: A Chemical and Structural Map of Receptor Mutation Data. J. Med. Chem. 2019, 62, 3784–3839.
- Predescu, D.V.; Cretoiu, S.M.; Cretoiu, D.; Pavelescu, L.A.; Suciu, N.; Radu, B.M.; Voinea, S.C. G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. Int. J. Mol. Sci. 2019, 20, 5568.
- Wang, M.; Wei, X.; Shi, L.; Chen, B.; Zhao, G.; Yang, H. Integrative genomic analyses of the histamine H1 receptor and its role in cancer prediction. Int. J. Mol. Med. 2014, 33, 1019–1026.
- Oppitz, M.; Mobus, V.; Brock, S.; Drews, U. Muscarinic receptors in cell lines from ovarian carcinoma: Negative correlation with survival of patients. Gynecol. Oncol. 2002, 85, 159–164.
- Yong, M.; Yu, T.; Tian, S.; Liu, S.; Xu, J.; Hu, J.; Hu, L. DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol. Lett. 2017, 14, 8171–8177.
- Popper, L.; Batra, S. Muscarinic acetylcholine and histamine-receptor mediated calcium mobilization and cell-growth in human ovarian-cancer cells. Int. J. Oncol. 1994, 4, 453–459.
- Czech, M.P.; Tencerova, M.; Pedersen, D.J.; Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013, 56, 949–964.
- Resh, M.D. Covalent lipid modifications of proteins. Curr. Biol. 2013, 23, R431–R435.
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124.
- Houben, A.J.; Moolenaar, W.H. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011, 30, 557–565.
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503.
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193.
- Kebede, M.A.; Alquier, T.; Latour, M.G.; Poitout, V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes. Metab. 2009, 11 (Suppl. S4), 10–20.
- Munkarah, A.; Mert, I.; Chhina, J.; Hamid, S.; Poisson, L.; Hensley-Alford, S.; Giri, S.; Rattan, R. Targeting of free fatty acid receptor 1 in EOC: A novel strategy to restrict the adipocyte-EOC dependence. Gynecol. Oncol. 2016, 141, 72–79.
- Hopkins, M.M.; Meier, K.E. Free fatty acid receptor (FFAR) agonists inhibit proliferation of human ovarian cancer cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 122, 24–29.
- Bian, D.; Su, S.; Mahanivong, C.; Cheng, R.K.; Han, Q.; Pan, Z.K.; Sun, P.; Huang, S. Lysophosphatidic Acid Stimulates Ovarian Cancer Cell Migration via a Ras-MEK Kinase 1 Pathway. Cancer Res. 2004, 64, 4209–4217.
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009, 15, 539–550.
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591.
- Kihara, Y.; Mizuno, H.; Chun, J. Lysophospholipid receptors in drug discovery. Exp. Cell Res. 2015, 333, 171–177.
- Cui, R.; Bai, H.; Cao, G.; Zhang, Z. The Role of Lysophosphatidic Acid Receptors in Ovarian Cancer: A Minireview. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 265–272.
- Ha, J.H.; Radhakrishnan, R.; Jayaraman, M.; Yan, M.; Ward, J.D.; Fung, K.M.; Moxley, K.; Sood, A.K.; Isidoro, C.; Mukherjee, P.; et al. LPA Induces Metabolic Reprogramming in Ovarian Cancer via a Pseudohypoxic Response. Cancer Res. 2018, 78, 1923–1934.
- Mills, G.B.; Eder, A.; Fang, X.; Hasegawa, Y.; Mao, M.; Lu, Y.; Tanyi, J.; Tabassam, F.H.; Wiener, J.; Lapushin, R.; et al. Critical role of lysophospholipids in the pathophysiology, diagnosis, and management of ovarian cancer. Cancer Treat. Res. 2002, 107, 259–283.
- Cai, H.; Xu, Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun. Signal. 2013, 11, 31.
- Sun, J. CARMA3: A novel scaffold protein in regulation of NF-kappaB activation and diseases. World J. Biol. Chem. 2010, 1, 353–361.
- Oyesanya, R.A.; Lee, Z.P.; Wu, J.; Chen, J.; Song, Y.; Mukherjee, A.; Dent, P.; Kordula, T.; Zhou, H.; Fang, X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. FASEB J. 2008, 22, 2639–2651.
- Oyesanya, R.A.; Greenbaum, S.; Dang, D.; Lee, Z.; Mukherjee, A.; Wu, J.; Dent, P.; Fang, X. Differential requirement of the epidermal growth factor receptor for G protein-mediated activation of transcription factors by lysophosphatidic acid. Mol. Cancer 2010, 9, 8.
- Lee, J.M.; Park, S.J.; Im, D.S. Calcium Signaling of Lysophosphatidylethanolamine through LPA1 in Human SH-SY5Y Neuroblastoma Cells. Biomol. Ther. 2017, 25, 194–201.
- Park, S.J.; Lee, K.P.; Im, D.S. Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells. Biomol. Ther. 2014, 22, 129–135.
- Devine, K.M.; Smicun, Y.; Hope, J.M.; Fishman, D.A. S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecol. Oncol. 2008, 110, 237–245.
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238.
- Park, K.S.; Kim, M.K.; Lee, H.Y.; Kim, S.D.; Lee, S.Y.; Kim, J.M.; Ryu, S.H.; Bae, Y.S. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem. Biophys. Res. Commun. 2007, 356, 239–244.
- Honda, Z.; Nakamura, M.; Miki, I.; Minami, M.; Watanabe, T.; Seyama, Y.; Okado, H.; Toh, H.; Ito, K.; Miyamoto, T.; et al. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature 1991, 349, 342–346.
- Wang, Z.; Zhao, S.; shi, J.; Meng, F.; Yuan, J.; Zhong, Z. Folate-mediated targeted PLK1 inhibition therapy for ovarian cancer: A comparative study of molecular inhibitors and siRNA therapeutics. Acta Biomater. 2022, 138, 443–452.
- Yu, Y.; Zhang, M.; Zhang, X.; Cai, Q.; Hong, S.; Jiang, W.; Xu, C. Synergistic effects of combined platelet-activating factor receptor and epidermal growth factor receptor targeting in ovarian cancer cells. J. Hematol. Oncol. 2014, 7, 39.
- Gao, T.; Zhao, R.; Yao, L.; Xu, C.; Cong, Q.; Jiang, W. Platelet-activating factor induces the stemness of ovarian cancer cells via the PAF/PAFR signaling pathway. Am. J. Transl. Res. 2020, 12, 7249–7261.
- Weigel, N.L.; Moore, N.L. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl. Recept. Signal. 2007, 5, e005.
- Filardo, E.J.; Thomas, P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012, 153, 2953–2962.
- Ignatov, T.; Modl, S.; Thulig, M.; Weissenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013, 6, 51.
- Liu, H.; Yan, Y.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. A novel estrogen receptor GPER mediates proliferation induced by 17beta-estradiol and selective GPER agonist G-1 in estrogen receptor alpha (ERalpha)-negative ovarian cancer cells. Cell Biol. Int. 2014, 38, 631–638.
- Sato, K.; Mogi, C.; Mighell, A.J.; Okajima, F. A missense mutation of Leu74Pro of OGR1 found in familial amelogenesis imperfecta actually causes the loss of the pH-sensing mechanism. Biochem. Biophys. Res. Commun. 2020, 526, 920–926.
- Xu, Y. Targeting Lysophosphatidic Acid in Cancer: The Issues in Moving from Bench to Bedside. Cancers 2019, 11, 1523.
- Han, S.G.; Baek, S.I.; Son, T.J.; Lee, H.; Kim, N.H.; Yu, Y.G. Preparation of functional human lysophosphatidic acid receptor 2 using a P9( *) expression system and an amphipathic polymer and investigation of its in vitro binding preference to Galpha proteins. Biochem. Biophys. Res. Commun. 2017, 487, 103–108.
- Yu, Y.; Zhang, M.; Zhang, X.; Cai, Q.; Zhu, Z.; Jiang, W.; Xu, C. Transactivation of epidermal growth factor receptor through platelet-activating factor/receptor in ovarian cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 85.
- Park, Y.A.; Choi, C.H.; Do, I.G.; Song, S.Y.; Lee, J.K.; Cho, Y.J.; Choi, J.J.; Jeon, H.K.; Ryu, J.Y.; Lee, Y.Y.; et al. Dual targeting of angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian carcinoma. Gynecol. Oncol. 2014, 135, 108–117.
- Xue, D.; Chen, W.; Neamati, N. Discovery, structure-activity relationship study and biological evaluation of 2-thioureidothiophene-3-carboxylates as a novel class of C-X-C chemokine receptor 2 (CXCR2) antagonists. Eur. J. Med. Chem. 2020, 204, 112387.
- Xu, D.; Li, R.; Wu, J.; Jiang, L.; Zhong, H.A. Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway. Curr. Top. Med. Chem. 2016, 16, 1441–1451.
- Ju, M.S.; Ahn, H.M.; Han, S.G.; Ko, S.; Na, J.H.; Jo, M.; Lim, C.S.; Ko, B.J.; Yu, Y.G.; Lee, W.K.; et al. A human antibody against human endothelin receptor type A that exhibits antitumor potency. Exp. Mol. Med. 2021, 53, 1437–1448.
- Rosano, L.; Cianfrocca, R.; Bagnato, A. Methods to Investigate beta-Arrestin-1/beta-Catenin Signaling in Ovarian Cancer Cells. Methods Mol. Biol. 2019, 1957, 393–406.
- Tocci, P.; Rosano, L.; Bagnato, A. Targeting Endothelin-1 Receptor/beta-Arrestin-1 Axis in Ovarian Cancer: From Basic Research to a Therapeutic Approach. Front. Endocrinol. 2019, 10, 609.
- Vacca, F.; Bagnato, A.; Catt, K.J.; Tecce, R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000, 60, 5310–5317.
- Grisaru-Granovsky, S.; Salah, Z.; Maoz, M.; Pruss, D.; Beller, U.; Bar-Shavit, R. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int. J. Cancer 2005, 113, 372–378.
- Jiang, Y.; Lim, J.; Wu, K.C.; Xu, W.; Suen, J.Y.; Fairlie, D.P. PAR2 induces ovarian cancer cell motility by merging three signalling pathways to transactivate EGFR. Br. J. Pharmacol. 2021, 178, 913–932.
- Xie, X.; Yang, M.; Ding, Y.; Yu, L.; Chen, J. Formyl peptide receptor 2 expression predicts poor prognosis and promotes invasion and metastasis in epithelial ovarian cancer. Oncol. Rep. 2017, 38, 3297–3308.
- Crepin, R.; Veggiani, G.; Djender, S.; Beugnet, A.; Planeix, F.; Pichon, C.; Moutel, S.; Amigorena, S.; Perez, F.; Ghinea, N.; et al. Whole-cell biopanning with a synthetic phage display library of nanobodies enabled the recovery of follicle-stimulating hormone receptor inhibitors. Biochem. Biophys. Res. Commun. 2017, 493, 1567–1572.
- Heublein, S.; Vrekoussis, T.; Mayr, D.; Friese, K.; Lenhard, M.; Jeschke, U.; Dian, D. Her-2/neu expression is a negative prognosticator in ovarian cancer cases that do not express the follicle stimulating hormone receptor (FSHR). J. Ovarian Res. 2013, 6, 6.
- Matsoukas, M.T.; Spyroulias, G.A. Dynamic properties of the growth hormone releasing hormone receptor (GHRHR) and molecular determinants of GHRH binding. Mol. Biosyst. 2017, 13, 1313–1322.
- Tzoupis, H.; Nteli, A.; Platts, J.; Mantzourani, E.; Tselios, T. Refinement of the gonadotropin releasing hormone receptor I homology model by applying molecular dynamics. J. Mol. Graph. Model. 2019, 89, 147–155.
- Carmon, K.S.; Gong, X.; Yi, J.; Wu, L.; Thomas, A.; Moore, C.M.; Masuho, I.; Timson, D.J.; Martemyanov, K.A.; Liu, Q.J. LGR5 receptor promotes cell-cell adhesion in stem cells and colon cancer cells via the IQGAP1-Rac1 pathway. J. Biol. Chem. 2017, 292, 14989–15001.
- McClanahan, T.; Koseoglu, S.; Smith, K.; Grein, J.; Gustafson, E.; Black, S.; Kirschmeier, P.; Samatar, A.A. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol. Ther. 2006, 5, 419–426.
- Falciani, C.; Fabbrini, M.; Pini, A.; Lozzi, L.; Lelli, B.; Pileri, S.; Brunetti, J.; Bindi, S.; Scali, S.; Bracci, L. Synthesis and biological activity of stable branched neurotensin peptides for tumor targeting. Mol. Cancer Ther. 2007, 6, 2441–2448.
- Burston, H.E.; Kent, O.A.; Communal, L.; Udaskin, M.L.; Sun, R.X.; Brown, K.R.; Jung, E.; Francis, K.E.; La Rose, J.; Lowitz, J.; et al. Inhibition of relaxin autocrine signaling confers therapeutic vulnerability in ovarian cancer. J. Clin. Investig. 2021, 131, e142677.
- Wu, F.; Song, G.; de Graaf, C.; Stevens, R.C. Structure and Function of Peptide-Binding G Protein-Coupled Receptors. J. Mol. Biol. 2017, 429, 2726–2745.
- Bagnato, A.; Rosano, L. The endothelin axis in cancer. Int. J. Biochem. Cell Biol. 2008, 40, 1443–1451.
- Rosano, L.; Cianfrocca, R.; Spinella, F.; Di Castro, V.; Nicotra, M.R.; Lucidi, A.; Ferrandina, G.; Natali, P.G.; Bagnato, A. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin. Cancer Res. 2011, 17, 2350–2360.
- Bagnato, A.; Loizidou, M.; Pflug, B.R.; Curwen, J.; Growcott, J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br. J. Pharmacol. 2011, 163, 220–233.
- Muralidhar, G.G.; Barbolina, M.V. Chemokine receptors in epithelial ovarian cancer. Int. J. Mol. Sci. 2013, 15, 361–376.
- Agarwal, A.; Tressel, S.L.; Kaimal, R.; Balla, M.; Lam, F.H.; Covic, L.; Kuliopulos, A. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: Implications for antiangiogenic therapy. Cancer Res. 2010, 70, 5880–5890.
- Barbolina, M.V.; Kim, M.; Liu, Y.; Shepard, J.; Belmadani, A.; Miller, R.J.; Shea, L.D.; Stack, M.S. Microenvironmental regulation of chemokine (C-X-C-motif) receptor 4 in ovarian carcinoma. Mol. Cancer Res. 2010, 8, 653–664.
- Guo, L.; Cui, Z.M.; Zhang, J.; Huang, Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin. J. Cancer 2011, 30, 336–343.
- Scotton, C.J.; Wilson, J.L.; Milliken, D.; Stamp, G.; Balkwill, F.R. Epithelial cancer cell migration: A role for chemokine receptors? Cancer Res. 2001, 61, 4961–4965.
- Shenoy, S.K.; Lefkowitz, R.J. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011, 32, 521–533.
- Kahan, Z.; Arencibia, J.M.; Csernus, V.J.; Groot, K.; Kineman, R.D.; Robinson, W.R.; Schally, A.V. Expression of growth hormone-releasing hormone (GHRH) messenger ribonucleic acid and the presence of biologically active GHRH in human breast, endometrial, and ovarian cancers. J. Clin. Endocrinol. Metab. 1999, 84, 582–589.
- Sun, B.; Schally, A.V.; Halmos, G. The presence of receptors for bombesin/GRP and mRNA for three receptor subtypes in human ovarian epithelial cancers. Regul. Pept. 2000, 90, 77–84.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
851
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




