Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Hidalgo Molina | -- | 3106 | 2022-05-18 20:18:38 | | | |
| 2 | Beatrix Zheng | -5 word(s) | 3101 | 2022-05-19 05:01:57 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 3101 | 2022-05-20 08:41:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hidalgo Molina, A.; Vázquez-Gandullo, E.; , .; Morales González, M.; Arnedillo-Munoz, A. Inspiratory Muscle Training in Patients with Pulmonary Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/23086 (accessed on 06 March 2026).
Hidalgo Molina A, Vázquez-Gandullo E, , Morales González M, Arnedillo-Munoz A. Inspiratory Muscle Training in Patients with Pulmonary Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/23086. Accessed March 06, 2026.
Hidalgo Molina, Antonio, Eva Vázquez-Gandullo, , Maria Morales González, Aurelio Arnedillo-Munoz. "Inspiratory Muscle Training in Patients with Pulmonary Disease" Encyclopedia, https://encyclopedia.pub/entry/23086 (accessed March 06, 2026).
Hidalgo Molina, A., Vázquez-Gandullo, E., , ., Morales González, M., & Arnedillo-Munoz, A. (2022, May 18). Inspiratory Muscle Training in Patients with Pulmonary Disease. In Encyclopedia. https://encyclopedia.pub/entry/23086
Hidalgo Molina, Antonio, et al. "Inspiratory Muscle Training in Patients with Pulmonary Disease." Encyclopedia. Web. 18 May, 2022.
Copy Citation
Chronic Obstructive Pulmonary Disease (COPD) is a complex and heterogeneous disease, with pulmonary and extrapulmonary manifestations, which leads to the need to personalize the assessment and treatment of these patients. The latest updates of national and international guidelines for the management of COPD reveal the importance of respiratory rehabilitation (RR) and its role in improving symptoms, quality of life, and psychosocial sphere of patients. Within RR, the inspiratory muscle training (IMT) has received special interest, showing benefits in maximum inspiratory pressure, perception of well-being, and health status in patients with chronic heart disease, respiratory diseases, and dyspnea during exercise.
inspiratory muscle training
inspiratory restriction device
chronic obstructive pulmonary disease
respiratory rehabilitation
quality of life
1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a preventable and treatable disease, characterized by the presence of persistent respiratory symptoms and airflow obstruction [1]. Currently, this disease is one of the first causes of worldwide morbimortality [2]. The best known risk factor is exposure to tobacco smoke, although other factors such as environmental pollution or exposure to biomass combustion, together with individual predisposition, have also been correlated [1]. It is a complex and heterogeneous disease, with pulmonary and extrapulmonary manifestations, which leads to the need for a comprehensive approach to it [3][4]. The variability between individuals and within the same individual is ostensible, the cardinal symptom being dyspnea, frequently accompanied by cough and expectoration [5]. These symptoms are related not only to bronchial obstruction, but also to deconditioning, which leads to a decrease in physical activity. The air trapping and dynamic hyperinflation present in these patients are associated with an overload at the muscular level, which ends up conditioning a vicious circle that is difficult to break [6].
The guidelines for the management of COPD highlight the importance of respiratory rehabilitation (RR) and its role in improving symptoms, quality of life, and in the psychosocial sphere. Understanding this intervention is akin to a global evaluation of health status, followed by therapies adjusted to individual needs, including exercise training, education, and behavioral therapy [1][7][8]. RR is postulated as being the most cost-effective treatment strategy [8]. The implementation of respiratory rehabilitation in patients with COPD is widely accepted, demonstrating a reduction in hospital admissions and mortality in patients with frequent exacerbations [9]. Inspiratory muscle training (IMT) has received special interest as part of RR. In the 1970s and 1980s, Rochester [10] and Chen [11] highlighted inspiratory muscle weakness in COPD patients and the potential benefits of targeted training. Posterior studies have confirmed the efficacy of implementing IMT as part of a RR program in a certain profile of patients with COPD, showing improvements in maximum inspiratory pressure, perception of well-being, and health status in patients with chronic heart disease, other respiratory diseases, and dyspnea during exercise [12][13][14].
2. Inspiratory Muscle Training Programs Based on the Use of Mechanical Devices
Mechanical devices have shown to improve muscle strength and endurance in patients with COPD, leading to an improvement in the quality of life of these patients [13][15]. However, it has not been demonstrated that there is any additional benefit to an isolated pulmonary rehabilitation program without inspiratory muscle training [8][15][16]. In fact, its implementation in RR programs in COPD patients in general is not recommended by the British Thoracic Society (BTS) or by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR), although the latter recommends adding IMT for patients with inspiratory muscle weakness, defined as a maximal inspiratory pressure of less than 60 cmH2O [17][18]. This variety of training usually employs small devices that are easily manipulated by the patient. Interest in this subject has been progressing over the last half century, reflecting the increase in the number of publications, which may be related to technological advances. These studies use simple devices, which generally increase inspiratory resistance by gradually decreasing the inspiratory orifice, showing a benefit for inspiratory muscle training in patients with COPD. As a main limitation of these studies, the sample size included is small [19][20][21]. Dekhuijzen et al. [22] compared a respiratory rehabilitation program to which they associated IMT with a flow resistance device, versus RR, observing an improvement in physical exercise capacity measured by the distance covered in a 6MWT and the maximal oxygen consumption (VO2max), in those patients in whom they followed a muscular inspiratory training program associated with the RR program.
Some IMT devices are currently available and are described below (Figure 1).
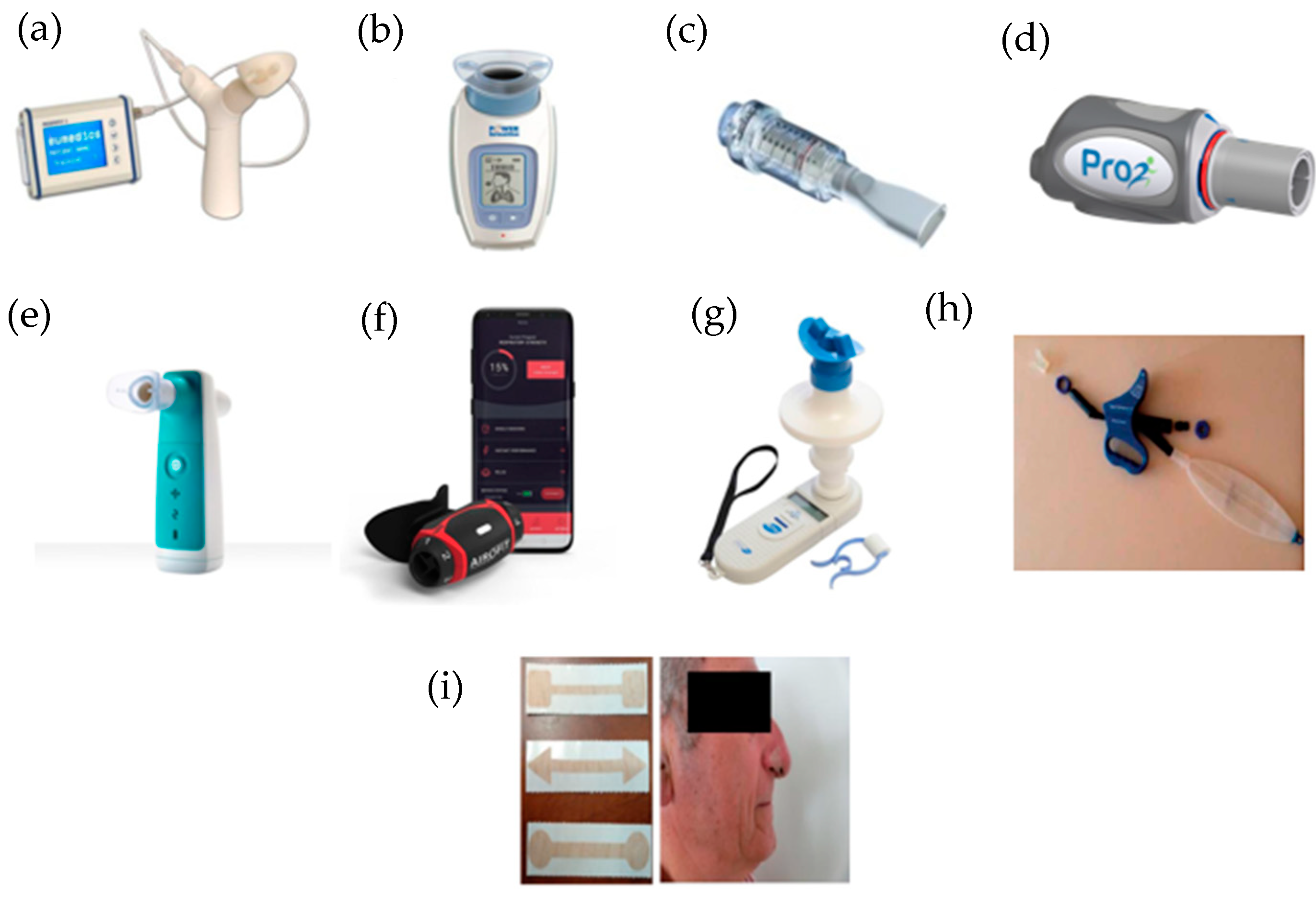
Figure 1. IMT devices used in respiratory rehabilitation. (a) Respifit STM; (b) PowerBreathe®; (c) Threshold IMT®; (d) PrO2Fit TM®; (e) Aerosure Medic®; (f) AeroFit IMT®; (g) MicroRPM; (h) SpiroTiger®; (i) FeelBreathe®.
2.1. Respifit STM
Respifit STM is a threshold type device for IMT that is portable, small, and easy to use. It features a “Y” shape, mouthpiece, nasal closure clip, and a display that shows results and facilitates therapy monitoring. The mode of use consists of initially performing normal inhalations and exhalations, after which the subject should inhale and exhale slowly and deeply, without hyperventilating. The subject then makes slow turns of the head to one side and inhales and exhales, and then to the other side, repeating breaths, for one minute. The next minute, he performs the same procedure but with up and down movements of the head. Finally, the subject will lean forward to touch their toes while breathing in and out. Finally, the subject will return to normal inhalation and exhalation.
This device has been studied by Petrovic et al. [12], aiming to analyze the effects of IMT on exercise capacity, dyspnea, and inspiratory fraction during exercise in patients with COPD. A total of 20 patients were included and divided into two groups. Patients in the treatment group performed an inspiratory muscle training program using the Respifit STM device daily for 8 weeks, with the other group serving as a control. Assessment of exercise capacity was measured by cardiopulmonary exercise test prior to the start of training and one week after the end of training. Improvements in respiratory muscle function, exercise capacity and quality of life were observed, as well as a decrease in dyspnea.
2.2. PowerBreathe®
PowerBreathe® is an electronic resistive loading device that is small and light with a mouthpiece at the top and a display on which different parameters such as maximal inspiratory pressure (cmH2O), maximum inspiratory flow (l/s), training load (cmH2O), power (watts), average inhaled volume (l), and T-index (training intensity index) can be observed. Inside the device there is a quick response valve with electronic control to generate inhalation resistance.
The patient should inhale and exhale through the mouthpiece 30 times. This regimen is recommended to be repeated twice a day. When inhaling, the patient will notice a resistance that varies in relation to the volume of air. The training resistance is maximal at the beginning of the inhalation (RV—residual volume) and gradually decreases to values close to zero at the end of the inhalation (TLC—total lung capacity). This resistance is designed to match the length-tension relationship of the inspiratory muscles, providing a constant relative training intensity at all lung volumes.
This training method ensures optimal stimulation throughout the entire range of motion of the inspiratory muscles. The training load is introduced gradually over the first five breaths of a training session. The first two breaths are performed without load. During these breaths, inhaled volume and flow are measured and used to establish an appropriate training load. The training load is then gradually introduced during breaths three and four times until the full load is reached in the following breaths.
The training load is adjustable and should be set at a level appropriate for the patient to effectively train the inspiratory muscles. Research has shown that inspiratory muscle training loads should exceed 30% of the maximal inspiratory muscle pressure (force) to be effective.
Magadle et al. [23], analyzed the effect of adding IMT in patients with severe COPD (FEV1 < 50%) without respiratory failure, who were already included in a rehabilitation program, assessing lung function, inspiratory muscle strength, perception of dyspnea, exercise performance, and quality of life. All patients participated in a rehabilitation program for 12 weeks, after which they were randomized into two groups: one group was given IMT with PowerBreathe® and the other was given a sham inspiratory training device (control group). Statistically significant differences were observed in inspiratory strength and in the perception of dyspnea, however, there were no differences between the two groups with respect to FEV1 or 6MWT.
The following research also evaluates the added effect of IMT together with full body resistance training. For this purpose, it divides the patients into an experimental group (IMT using the PowerBreathe® device together with full body training) and a control group (full body training alone). After the training period an improvement in the Berg Balance Scale (BBS) was demonstrated and statistically significant differences were also found in inspiratory muscle strength, increasing up to 37% in the experimental group. However, no differences were found between both groups in the 6MWT [24].
Following the same line, Charususin et al. [25] conducted a study with the aim of evaluating the added benefits of IMT to a rehabilitation program in COPD patients with inspiratory muscle weakness. Although a statistically significant improvement in inspiratory muscle strength and endurance was established, this was not reflected in the 6MWT, where no differences were found.
An advantage of the PowerBreathe® device is that it allows unsupervised training. Langer et al. [26] compared the efficacy of a mechanical loading threshold device versus an electronic device for home use, the PowerBreathe®. They note that the use of the electronic device with a home training program requires less time investment for the health services, and there is an improvement in inspiratory muscle capacity. In addition, study participants in the electronic device group tolerated higher training intensities and achieved significantly greater improvements in inspiratory muscle function [27].
2.3. Threshold IMT®
Threshold IMT® is a small and light threshold type device that is cylindrical in shape, with a nozzle at one end. This device incorporates a unidirectional valve independent of the flow, which ensures constant resistance and allows pressure adjustment (in cmH2O). This valve provides a resistance to the air flow, which forces the subject to make a greater effort to overcome the pressure [28]. The subject must repeat inspiration and expiration manoeuvres through the mouthpiece, perceiving a variable resistance only in inspiration. A use of about 10–15 min twice a day is recommended.
According to the existing literature, the benefits derived from IMT with mechanical devices are greater in COPD patients with a maximal inspiratory pressure of less than 60 cmH2O (13), but there are studies that aim to demonstrate the efficacy of the use of these devices in patients with a maximal inspiratory pressure above 60 cmH2O. Beaumont et al. [29], with the IMT Threshold IMT® device, found no statistically significant differences in terms of improvement in dyspnea or functional parameters. No benefits were observed in a study in which two groups were compared: training with cycloergometer plus IMT with Threshold IMTR; versus training with cycloergometer alone. It was observed that combined training improves inspiratory strength versus isolated cycloergometer training; however, no differences were observed with regard to improved physical performance or dyspnea. Another interesting aspect of this research is that an analysis was performed in a subgroup of patients with inspiratory muscle weakness, defined as a maximal inspiratory pressure of less than 60 cmH2O, without noticing the benefits of combined training in this subgroup [30].
2.4. PrO2Fit TM®
PrO2Fit TM® is a small, lightweight, and portable resistive load device with a nozzle on the end. It is connected to a mobile application, which provides the user with a graphic representation of the effort made. For its use, the patient must repeatedly inhale and exhale through the mouthpiece, following the indications of the mobile application.
This device allows the researchers to evaluate the inspiratory musculature by means of an incremental respiratory endurance test, known as TIRE (Test of Incremental Respiratory Endurance). In this way, the maximum inspiratory pressure is measured over time, obtaining the maximum sustained inspiratory pressure.
This device was developed for inspiratory muscle training in athletes and is of interest in patients with respiratory pathology; however, there are few studies that evaluate its efficacy in patients with COPD. One such study, by Formiga et al. [31], evaluates the reliability of such a device for measuring inspiratory muscle strength and endurance, but is not designed to test its efficacy as an inspiratory muscle training device. Although not focused on COPD patients, McCreery et al. [32] evaluated the effect of inspiratory muscle training using the PrO2 Fit device in patients with bronchiectasis, concluding that there is an increase in inspiratory muscle strength and endurance in these patients.
The protocol of a randomized clinical trial comparing a home training program based on an incremental breathing endurance test (TIRE), using the PrO2 device, versus a traditional inspiratory muscle training program using the Threshold IMTR has recently been carried out. The results of this research are not yet published [33].
2.5. Aerosure Medic®
Resistive charging device designed to provide resistance on inspiration. It consists of a rectangular device with a mouthpiece and a charger. It has two modes, one for muscle training in which the patient must repeatedly inhale and exhale through the mouthpiece and a second mode with a mucolytic effect on the airway.
In the study carried out by Daynes et al. [34], such a device is evaluated. Patients were included in a training program with Aerosure Medic®, to be used 3 times a day for a total of 8 weeks. A statistically significant improvement in dyspnea and in maximal inspiratory pressure (PImax) and maximal expiratory pressure (PImax) was observed, with the improvement in PImax being greater in the subgroup of patients with inspiratory muscle weakness, also defined in this research as a PImax of less than 60 cmH2O.
2.6. AeroFit IMT®
AeroFit IMT® is a resistive loading device for IMT, currently under study. It consists of a small, portable, lightweight oral pressure manometer with a rubber rimmed mouthpiece. It contains resistance wheels that provide adjustable airflow. This resistance causes fatigue in the respiratory muscles, which is then compensated by increased muscle mass, making these muscles stronger, faster, and more efficient.
The device is connected via an app to the cell phone where the subject can choose the desired training program and observe the progress achieved through training.
It has been studied in athletes, demonstrating an increase in maximal respiratory strength without reporting dyspnea or respiratory fatigue after use. These results suggest its future application in patients with respiratory diseases such as COPD within an IMT. A clinical trial is currently under development in which the ability of AeroFit IMTR to improve inspiratory muscle strength in COPD patients is being evaluated. The results are not yet published [35].
2.7. MicroRPM
MicroRPM is a resistive loading device that is small, portable, lightweight, and noninvasive, containing a mouth-pressure manometer with a rubber flanged mouthpiece. It displays the test results in a device monitor, uses software and calculates the maximal inspiratory pressure and maximal expiratory pressure values (in cmH2O), from the one-second average maximum pressure. The MicroRPM needs a different adapter to be adjusted for inhalation and exhalation, without the need for special preparation in terms of cleaning and disinfecting the device by simply adapting the respective inhaler and exhaler adapter and the removable mouthpiece. Stavrou et al. did not find differences between two devices (MicroPRM vs. AirOFit PRO™); the comparison showed no differences in the maximal inspiratory pressure and maximal expiratory pressure variables, but statistically significant differences were observed between the devices in the parameter ease of use and information during the trials [35].
2.8. SpiroTiger®
This is a voluntary isocapnic hyperpnea device. It consists of a tube that connects to a breathing bag with a mouthpiece at a 90° angle. Between these components there is a side port with an opening that allows inspiration and expiration into the ambient air. It also has a monitor that shows and assists the patient by providing auditory and visual feedback. When the patient exhales, the bag fills with air, with higher concentrations of carbon dioxide. When the bag is full, the side valve opens and allows the remaining exhaled air to escape. Once expiration is complete, the valve closes and gives way to inspiration. During inspiration, the bag is first emptied completely and then the side valve is opened.
A review conducted in 2015 evaluated five articles analyzing the SpiroTiger® device, one of them in patients with COPD and the rest in patients with cystic fibrosis. Finally, they conclude that this device presents an improvement in physical condition, measured in the different studies using the 6MWT and in VO2max, as well as an improvement in the quality of life. However, the improvement in FEV1 is not conclusive, as it is present in only two of the studies evaluated, and therefore more studies are required [36].
There is a study evaluating the efficacy of SpiroTiger® training for four weeks in patients with COPD. Maximum inspiratory pressure, 6MWT distance, and quality of life were measured using the St George Respiratory Questionnaire for subjects with COPD, and an increase in maximum inspiratory pressure, 6MWT distance, and quality of life were observed [37].
2.9. FeelBreathe®
So far, the aforementioned devices must be used in a static position and breathing through the mouth, which is not considered physiological. Recently a device for inspiratory muscle training called FeelBreathe® has been designed. It is a nasal ventilatory flow restriction device consisting of a strip of hypoallergenic material that is placed and adhered under the nostrils, producing resistance to flow. Depending on the size and/or porosity of the device material, the inspiratory process is more or less difficult.
The advantage of this device with respect to those mentioned above is, on the one hand, the fact that it is a nasal device, so breathing is more physiological and, on the other hand, it allows for use in dynamic situations, so the researchers can use it while the patient is exercising.
This device has been shown to be effective in improving ventilatory and cardiac efficiency in healthy patients, and in patients with COPD it has also demonstrated less dynamic hyperinflation with better ventilatory efficiency [33]. This ability of the device to be used in motion allows it to be implemented in pulmonary rehabilitation programs by using it during different exercises, and hence the hypothesis that the FeelBreathe® device brings added benefits to respiratory rehabilitation. Gonzalez-Montesinos et al. studied this circumstance and observed that patients who have used FeelBreathe® together with a pulmonary rehabilitation program show an improvement in quality of life, dyspnea, exercise capacity, and inspiratory muscle strength. However, the main limitation of these studies is the small number of patients, so more studies are needed to reach a definitive conclusion on the addition of FeelBreathe® in a pulmonary rehabilitation program [38][39][40].
References
- Global Strategy for Diagnosis, Management, and Prevention of COPD. 2022. Available online: https://goldcopd.org/2022-gold-reports/ (accessed on 1 February 2022).
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940.
- Agustí, A.; Sobradillo, P.; Celli, B. Addressing the complexity of chronic obstructive pulmonary disease: From phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am. J. Respir. Crit. Care Med. 2011, 183, 1129–1137.
- Agusti, A. The path to personalised medicine in copd. Thorax 2014, 69, 857–864.
- Miravitlles, M.; Izquierdo, J.L.; Esquinas, C.; Pérez, M.; Calle, M.; López-Campos, J.L.; González-Moro, J.M.R.; Casanova, C.; Esteban, C.; de Lucas, P. The variability of respiratory symptoms and associated factors in COPD. Respir. Med. 2017, 129, 165–172.
- Anzueto, A.; Miravitlles, M. Pathophysiology of dyspnea in COPD. Postgrad. Med. 2017, 129, 366–374.
- Cosío, B.G.; Hernández, C.; Chiner, E.; Gimeno-Santos, E.; Pleguezuelos, E.; Seijas, N.; Rigau, D.; López-Campos, J.L.; Soler-Cataluña, J.J.; Calle, M.; et al. Spanish COPD Guidelines (GesEPOC 2021): Non-pharmacological Treatment Update. Arch. Bronconeumol. 2022, 58, 69–81.
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zu Wallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64.
- Puhan, M.A.; Gimeno-Santos, E.; Scharplatz, M.; Troosters, T.; Walters, E.H.; Steurer, J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2016, 12, CD00530510.
- Larson, J.L.; Covey, M.K.; Corbridge, S. Inspiratory muscle strength in chronic obstructive pulmonary disease. AACN Clin. Issues 2002, 13, 320–332.
- Chen, H.I.; Dukes, R.; Martin, B.J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1985, 131, 251–255.
- Petrovic, M.; Reiter, M.; Zipko, H.; Pohl, W.; Wanke, T. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int. J. COPD 2012, 7, 797–805.
- Gosselink, R.; De Vos, J.; Van Den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur. Respir. J. 2011, 37, 416–425.
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008, 102, 1715–1729.
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2178–2188.
- Camillo, C.A.; Osadnik, C.R.; van Remoortel, H.; Burtin, C.; Janssens, W.; Troosters, T. Effect of “add-on” interventions on exercise training in individuals with COPD: A systematic review. ERJ Open Res. 2016, 2, 00078–2015.
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.-C.; et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013, 68 (Suppl. 2), ii1–ii30.
- Güell Rous, M.R.; Díaz Lobato, S.; Rodríguez Trigo, G.; Morante Vélez, F.; San Miguel, M.; Cejudo, P.; Ortega Ruiz, F.; Muñoz, Á.; Galdiz Iturri, J.B.; Garcia, A.; et al. Rehabilitación respiratoria. Arch. Bronconeumol. 2014, 50, 332–344.
- Sonne, L.J.; Davis, J.A. Increased exercise performance in patients with severe COPD following inspiratory resistive training. Chest 1982, 81, 436–439.
- Andersen, J.B.; Falk, P. Clinical experience with inspiratory resistive breathing training. Disabil. Rehabil. 1984, 6, 183–185.
- Belman, M.J.; Shadmehr, R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. J. Appl. Physiol. 1988, 65, 2726–2735.
- Dekhuijzen, P.N.R.; Beek, M.M.L.; Folgering, H.T.M.; van Herwaarden, C.L.A. Psychological changes during pulmonary rehabilitation and target-flow inspiratory muscle training in COPD patients with a ventilatory limitation during exercise. Int. J. Rehabil. Res. 1990, 13, 109–118.
- Magadle, R.; McConnell, A.K.; Beckerman, M.; Weiner, P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir. Med. 2007, 101, 1500–1505.
- Tounsi, B.; Acheche, A.; Lelard, T.; Tabka, Z.; Trabelsi, Y.; Ahmaidi, S. Effects of specific inspiratory muscle training combined with whole-body endurance training program on balance in COPD patients: Randomized controlled trial. PLoS ONE 2021, 16, e0257595.
- Charususin, N.; Gosselink, R.; Decramer, M.; Demeyer, H.; McConnell, A.; Saey, D.; Maltais, F.; Derom, E.; Vermeersch, S.; Heijdra, Y.F.; et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018, 73, 942–950.
- Langer, D.; Charususin, N.; Jácome, C.; Hoffman, M.; McConnell, A.; Decramer, M.; Gosselink, R. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys. Ther. 2015, 95, 1264–1273.
- Ferraro, F.V.; Gavin, J.P.; Wainwright, T.; McConnell, A. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: A randomized, double-blind, placebo-controlled study. Physiol. Rep. 2019, 7, 1–12.
- Nield, M.A. Inspiratory muscle training protocol using a pressure threshold device: Effect on dyspnea in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 1999, 80, 100–102.
- Beaumont, M.; Mialon, P.; Ber-Moy, C.; Lochon, C.; Péran, L.; Pichon, R.; Gut-Gobert, C.; Leroyer, C.; Morelot-Panzini, C.; Couturaud, F. Inspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron. Respir. Dis. 2015, 12, 305–312.
- Wang, K.; Zeng, G.Q.; Li, R.; Luo, Y.W.; Wang, M.; Hu, Y.H.; Xu, W.-H.; Zhou, L.-Q.; Chen, R.-C.; Chen, X. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: A comprehensive assessment in stable COPD patients. Int. J. COPD 2017, 12, 2655–2668.
- Formiga, M.F.; Roach, K.E.; Vital, I.; Urdaneta, G.; Balestrini, K.; Calderon-Candelario, R.A.; Campos, M.; Cahalin, L.P. Reliability and validity of the test of incremental respiratory endurance measures of inspiratory muscle performance in COPD. Int. J. COPD 2018, 13, 1569–1576.
- McCreery, J.L.; Mackintosh, K.A.; Mills-Bennett, R.; McNarry, M.A. The effect of a high-intensity pro2fit inspiratory muscle training intervention on physiological and psychological health in adults with bronchiectasis: A mixed-methods study. Int. J. Environ. Res. Public Health 2021, 18, 3051.
- Formiga, M.F.; Dosbaba, F.; Hartman, M.; Batalik, L.; Plutinsky, M.; Brat, K.; Ludka, O.; Cahalin, L.P. Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in copd: Protocol for a randomized controlled trial. Int. J. COPD 2020, 15, 2147–2155.
- Daynes, E.; Greening, N.J.; Harvey-Dunstan, T.C.; Singh, S.J. High-frequency airway oscillating device for respiratory muscle training in subjects with copd. Respir. Care 2018, 63, 584.
- Stavrou, V.T.; Tourlakopoulos, K.N.; Daniil, Z.; Gourgoulianis, K.I. Respiratory Muscle Strength: New Technology for Easy Assessment. Cureus 2021, 13, e14803.
- Włodarczyk, O.M.; Barinow-Wojewódzki, A. The impact of resistance respiratory muscle training with a SpiroTiger® device on lung function, exercise performance, and health-related quality of life in respiratory diseases. Kardiochirurgia Torakochirurgia Pol. 2015, 12, 386–390.
- Bernardi, E.; Pomidori, L.; Bassal, F.; Contoli, M.; Cogo, A. Respiratory muscle training with normocapnic hyperpnea improves ventilatory pattern and thoracoabdominal coordination, and reduces oxygen desaturation during endurance exercise testing in COPD patients. Int. J. COPD 2015, 10, 1899–1906.
- Gonzalez-Montesinos, J.L.; Arnedillo, A.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; García, P.A.; Castro-Piñero, J.; Ponce-González, J.G. A new nasal restriction device called feelbreathe® improves breathing patterns in chronic obstructive pulmonary disease patients during exercise. Int. J. Environ. Res. Public Health 2020, 17, 4876.
- Arnedillo, A.; Gonzalez-Montesinos, J.L.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; España-Domínguez, C.; Ponce-González, J.G.; Marin-Galindo, A.; Arnedillo, A. Effects of a rehabilitation programme with a nasal inspiratory restriction device on exercise capacity and quality of life in COPD. Int. J. Environ. Res. Public Health 2020, 17, 3669.
- Gonzalez-Montesinos, J.L.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; Ponce-Gonzalez, J.G.; Marin-Galindo, A.; Arnedillo, A. Effects of a rehabilitation programme using a nasal inspiratory restriction device in copd. Int. J. Environ. Res. Public Health 2021, 18, 4207.
More
Information
Subjects:
Respiratory System
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
3 times
(View History)
Update Date:
20 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





