Chronic Obstructive Pulmonary Disease (COPD) is a complex and heterogeneous disease, with pulmonary and extrapulmonary manifestations, which leads to the need to personalize the assessment and treatment of these patients. The latest updates of national and international guidelines for the management of COPD reveal the importance of respiratory rehabilitation (RR) and its role in improving symptoms, quality of life, and psychosocial sphere of patients. Within RR, the inspiratory muscle training (IMT) has received special interest, showing benefits in maximum inspiratory pressure, perception of well-being, and health status in patients with chronic heart disease, respiratory diseases, and dyspnea during exercise.
- inspiratory muscle training
- inspiratory restriction device
- chronic obstructive pulmonary disease
- respiratory rehabilitation
- quality of life
1. Introduction
2. Inspiratory Muscle Training Programs Based on the Use of Mechanical Devices
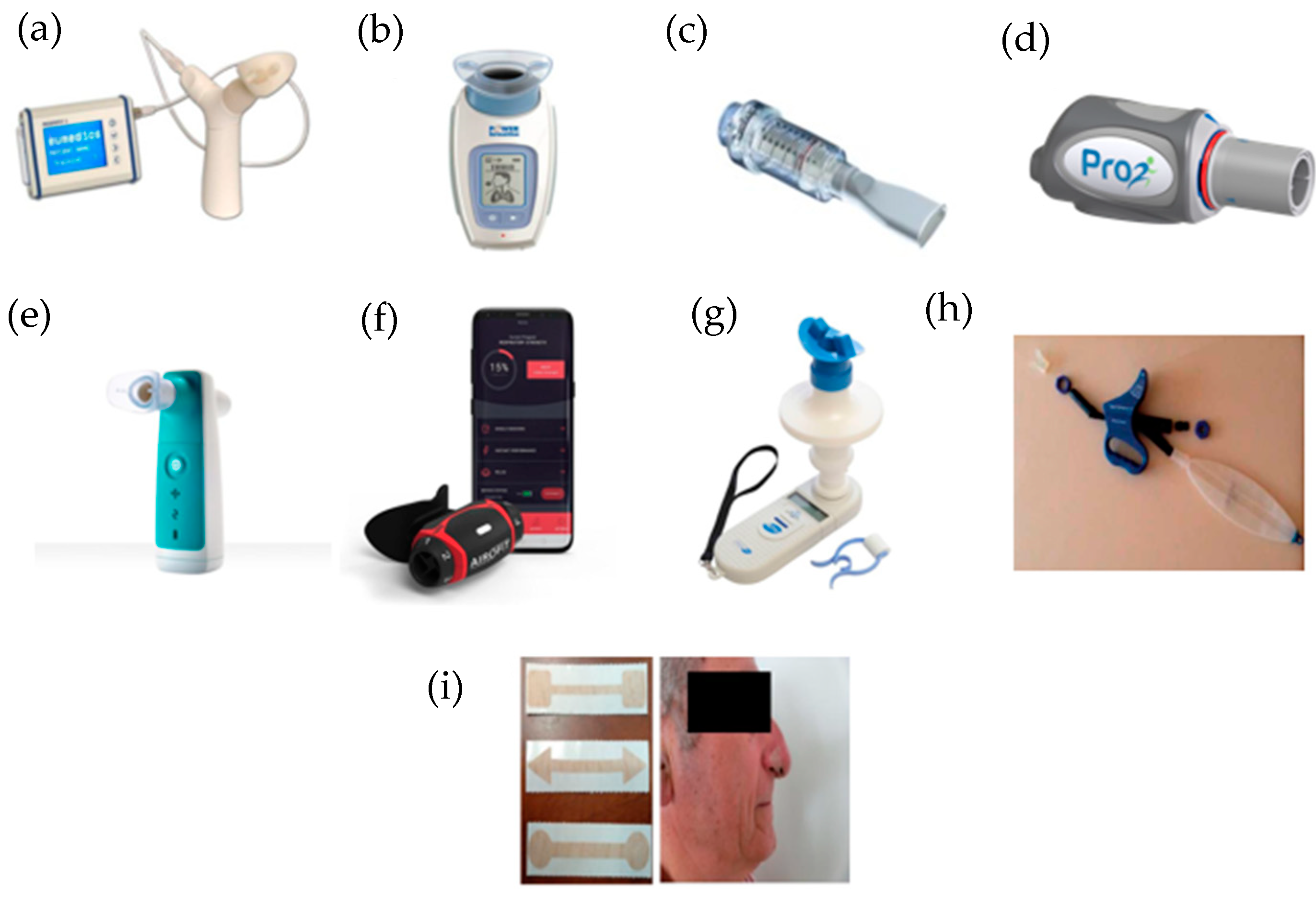
2.1. Respifit STM
2.2. PowerBreathe®
2.3. Threshold IMT®
2.4. PrO2Fit TM®
2.5. Aerosure Medic®
2.6. AeroFit IMT®
2.7. MicroRPM
2.8. SpiroTiger®
2.9. FeelBreathe®
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19095564
References
- Global Strategy for Diagnosis, Management, and Prevention of COPD. 2022. Available online: https://goldcopd.org/2022-gold-reports/ (accessed on 1 February 2022).
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940.
- Agustí, A.; Sobradillo, P.; Celli, B. Addressing the complexity of chronic obstructive pulmonary disease: From phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am. J. Respir. Crit. Care Med. 2011, 183, 1129–1137.
- Agusti, A. The path to personalised medicine in copd. Thorax 2014, 69, 857–864.
- Miravitlles, M.; Izquierdo, J.L.; Esquinas, C.; Pérez, M.; Calle, M.; López-Campos, J.L.; González-Moro, J.M.R.; Casanova, C.; Esteban, C.; de Lucas, P. The variability of respiratory symptoms and associated factors in COPD. Respir. Med. 2017, 129, 165–172.
- Anzueto, A.; Miravitlles, M. Pathophysiology of dyspnea in COPD. Postgrad. Med. 2017, 129, 366–374.
- Cosío, B.G.; Hernández, C.; Chiner, E.; Gimeno-Santos, E.; Pleguezuelos, E.; Seijas, N.; Rigau, D.; López-Campos, J.L.; Soler-Cataluña, J.J.; Calle, M.; et al. Spanish COPD Guidelines (GesEPOC 2021): Non-pharmacological Treatment Update. Arch. Bronconeumol. 2022, 58, 69–81.
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zu Wallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64.
- Puhan, M.A.; Gimeno-Santos, E.; Scharplatz, M.; Troosters, T.; Walters, E.H.; Steurer, J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2016, 12, CD00530510.
- Larson, J.L.; Covey, M.K.; Corbridge, S. Inspiratory muscle strength in chronic obstructive pulmonary disease. AACN Clin. Issues 2002, 13, 320–332.
- Chen, H.I.; Dukes, R.; Martin, B.J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1985, 131, 251–255.
- Petrovic, M.; Reiter, M.; Zipko, H.; Pohl, W.; Wanke, T. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int. J. COPD 2012, 7, 797–805.
- Gosselink, R.; De Vos, J.; Van Den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur. Respir. J. 2011, 37, 416–425.
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008, 102, 1715–1729.
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2178–2188.
- Camillo, C.A.; Osadnik, C.R.; van Remoortel, H.; Burtin, C.; Janssens, W.; Troosters, T. Effect of “add-on” interventions on exercise training in individuals with COPD: A systematic review. ERJ Open Res. 2016, 2, 00078–2015.
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.-C.; et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013, 68 (Suppl. 2), ii1–ii30.
- Güell Rous, M.R.; Díaz Lobato, S.; Rodríguez Trigo, G.; Morante Vélez, F.; San Miguel, M.; Cejudo, P.; Ortega Ruiz, F.; Muñoz, Á.; Galdiz Iturri, J.B.; Garcia, A.; et al. Rehabilitación respiratoria. Arch. Bronconeumol. 2014, 50, 332–344.
- Sonne, L.J.; Davis, J.A. Increased exercise performance in patients with severe COPD following inspiratory resistive training. Chest 1982, 81, 436–439.
- Andersen, J.B.; Falk, P. Clinical experience with inspiratory resistive breathing training. Disabil. Rehabil. 1984, 6, 183–185.
- Belman, M.J.; Shadmehr, R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. J. Appl. Physiol. 1988, 65, 2726–2735.
- Dekhuijzen, P.N.R.; Beek, M.M.L.; Folgering, H.T.M.; van Herwaarden, C.L.A. Psychological changes during pulmonary rehabilitation and target-flow inspiratory muscle training in COPD patients with a ventilatory limitation during exercise. Int. J. Rehabil. Res. 1990, 13, 109–118.
- Magadle, R.; McConnell, A.K.; Beckerman, M.; Weiner, P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir. Med. 2007, 101, 1500–1505.
- Tounsi, B.; Acheche, A.; Lelard, T.; Tabka, Z.; Trabelsi, Y.; Ahmaidi, S. Effects of specific inspiratory muscle training combined with whole-body endurance training program on balance in COPD patients: Randomized controlled trial. PLoS ONE 2021, 16, e0257595.
- Charususin, N.; Gosselink, R.; Decramer, M.; Demeyer, H.; McConnell, A.; Saey, D.; Maltais, F.; Derom, E.; Vermeersch, S.; Heijdra, Y.F.; et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018, 73, 942–950.
- Langer, D.; Charususin, N.; Jácome, C.; Hoffman, M.; McConnell, A.; Decramer, M.; Gosselink, R. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys. Ther. 2015, 95, 1264–1273.
- Ferraro, F.V.; Gavin, J.P.; Wainwright, T.; McConnell, A. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: A randomized, double-blind, placebo-controlled study. Physiol. Rep. 2019, 7, 1–12.
- Nield, M.A. Inspiratory muscle training protocol using a pressure threshold device: Effect on dyspnea in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 1999, 80, 100–102.
- Beaumont, M.; Mialon, P.; Ber-Moy, C.; Lochon, C.; Péran, L.; Pichon, R.; Gut-Gobert, C.; Leroyer, C.; Morelot-Panzini, C.; Couturaud, F. Inspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron. Respir. Dis. 2015, 12, 305–312.
- Wang, K.; Zeng, G.Q.; Li, R.; Luo, Y.W.; Wang, M.; Hu, Y.H.; Xu, W.-H.; Zhou, L.-Q.; Chen, R.-C.; Chen, X. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: A comprehensive assessment in stable COPD patients. Int. J. COPD 2017, 12, 2655–2668.
- Formiga, M.F.; Roach, K.E.; Vital, I.; Urdaneta, G.; Balestrini, K.; Calderon-Candelario, R.A.; Campos, M.; Cahalin, L.P. Reliability and validity of the test of incremental respiratory endurance measures of inspiratory muscle performance in COPD. Int. J. COPD 2018, 13, 1569–1576.
- McCreery, J.L.; Mackintosh, K.A.; Mills-Bennett, R.; McNarry, M.A. The effect of a high-intensity pro2fit inspiratory muscle training intervention on physiological and psychological health in adults with bronchiectasis: A mixed-methods study. Int. J. Environ. Res. Public Health 2021, 18, 3051.
- Formiga, M.F.; Dosbaba, F.; Hartman, M.; Batalik, L.; Plutinsky, M.; Brat, K.; Ludka, O.; Cahalin, L.P. Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in copd: Protocol for a randomized controlled trial. Int. J. COPD 2020, 15, 2147–2155.
- Daynes, E.; Greening, N.J.; Harvey-Dunstan, T.C.; Singh, S.J. High-frequency airway oscillating device for respiratory muscle training in subjects with copd. Respir. Care 2018, 63, 584.
- Stavrou, V.T.; Tourlakopoulos, K.N.; Daniil, Z.; Gourgoulianis, K.I. Respiratory Muscle Strength: New Technology for Easy Assessment. Cureus 2021, 13, e14803.
- Włodarczyk, O.M.; Barinow-Wojewódzki, A. The impact of resistance respiratory muscle training with a SpiroTiger® device on lung function, exercise performance, and health-related quality of life in respiratory diseases. Kardiochirurgia Torakochirurgia Pol. 2015, 12, 386–390.
- Bernardi, E.; Pomidori, L.; Bassal, F.; Contoli, M.; Cogo, A. Respiratory muscle training with normocapnic hyperpnea improves ventilatory pattern and thoracoabdominal coordination, and reduces oxygen desaturation during endurance exercise testing in COPD patients. Int. J. COPD 2015, 10, 1899–1906.
- Gonzalez-Montesinos, J.L.; Arnedillo, A.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; García, P.A.; Castro-Piñero, J.; Ponce-González, J.G. A new nasal restriction device called feelbreathe® improves breathing patterns in chronic obstructive pulmonary disease patients during exercise. Int. J. Environ. Res. Public Health 2020, 17, 4876.
- Arnedillo, A.; Gonzalez-Montesinos, J.L.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; España-Domínguez, C.; Ponce-González, J.G.; Marin-Galindo, A.; Arnedillo, A. Effects of a rehabilitation programme with a nasal inspiratory restriction device on exercise capacity and quality of life in COPD. Int. J. Environ. Res. Public Health 2020, 17, 3669.
- Gonzalez-Montesinos, J.L.; Fernandez-Santos, J.R.; Vaz-Pardal, C.; Ponce-Gonzalez, J.G.; Marin-Galindo, A.; Arnedillo, A. Effects of a rehabilitation programme using a nasal inspiratory restriction device in copd. Int. J. Environ. Res. Public Health 2021, 18, 4207.
