Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiang-Ping Chu | -- | 2474 | 2022-05-18 15:41:29 | | | |
| 2 | Rita Xu | Meta information modification | 2474 | 2022-05-19 03:31:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chu, X.; , . KCNQ Channels. Encyclopedia. Available online: https://encyclopedia.pub/entry/23076 (accessed on 07 February 2026).
Chu X, . KCNQ Channels. Encyclopedia. Available at: https://encyclopedia.pub/entry/23076. Accessed February 07, 2026.
Chu, Xiang-Ping, . "KCNQ Channels" Encyclopedia, https://encyclopedia.pub/entry/23076 (accessed February 07, 2026).
Chu, X., & , . (2022, May 18). KCNQ Channels. In Encyclopedia. https://encyclopedia.pub/entry/23076
Chu, Xiang-Ping and . "KCNQ Channels." Encyclopedia. Web. 18 May, 2022.
Copy Citation
The broad distribution of voltage-gated potassium channels (VGKCs) in the human body makes them a critical component for the study of physiological and pathological function. Within the KCNQ family of VGKCs, these aqueous conduits serve an array of critical roles in homeostasis, especially in neural tissue.
KCNQ channels
neural plasticity
1. Introduction
Ion channels serve as an aqueous conduit for several nuanced cellular processes to maintain the homeostatic direction of the body. Moreover, there are over 400 genes that encode for at least one ion channel subunit [1][2]. The various mechanisms for alternative splicing make for an enormous variety of subunit combinations designed for appropriate physiological functions. Among these, the largest and most diverse group of ion channels are potassium (K+) channels [2][3]. These channels are composed of tetrameric integral membrane regions, which form an aqueous pore for K+ to permeate across the membrane. This ion serves a critical role in maintaining electrical gradients during the repolarization of action potentials and maintaining the negative resting membrane potential [3][4].
Voltage-gated potassium channels (VGKCs, also Kv) form a broad distribution of channels in the nervous system as well as other tissues. Structurally, Kv channels are also a tetramer integral membrane pore-forming alpha subunit but also contain six transmembrane segmental helices, classified as S1–S6. In addition, the S1–S4 transmembrane segmental helices compose the actual voltage sensation region, and the latter two (S5–S6) units are the actual gate of the channel, as depicted in Figure 1. The voltage sensation region (S1–S4) is supple in its ability to adapt to shifting membrane potentials by creating a conformational shift. This shift spreads through the pore-forming subunit via interactions with the S4 transmembrane segments. In addition, this segment is also protected during depolarization of the action potential (AP). This protection is due to the presence of the acidic residues on S1 and S2 transmembrane segments, which limits deterrence [3][4][5].
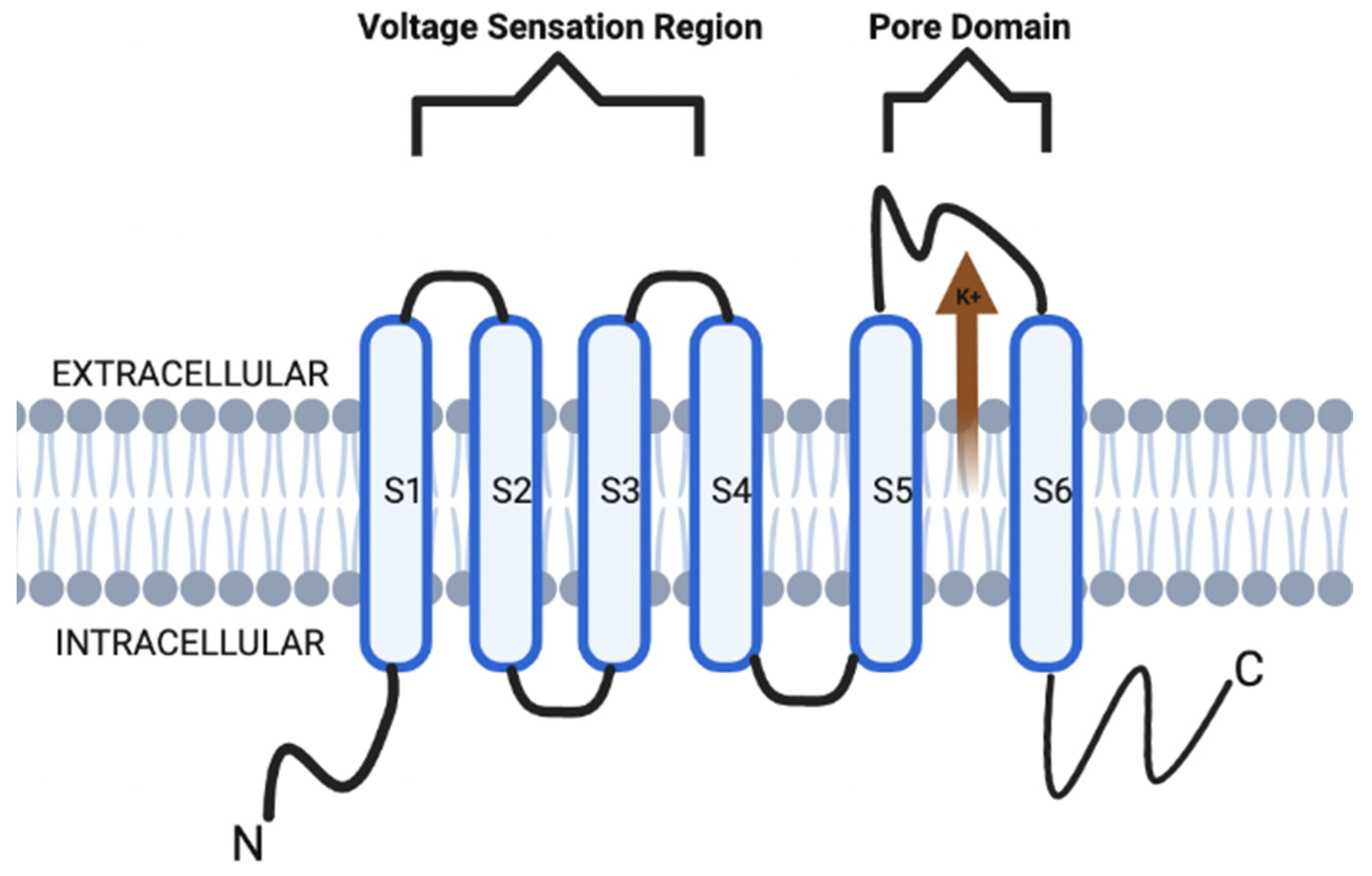
Figure 1. KCNQ channel structure is composed of six transmembrane segmental helices, classified as S1–S6. In addition, the S1–S4 transmembrane segmental helices compose the actual voltage sensation region, and the latter two (S5–S6) units are the actual gate of the channel.
Within the family of Kv channels, there are subfamilies that can be grouped according to the N- and C-terminal domains and encoded genes [5][6]. The importance behind the subfamily grouping lies in the Kv proteins, which can be functionally divergent with different membrane sensitivity potentials, gating interactions, and dynamic responses [4]. These subfamilies of Kv channels are all encoded by 40 genes, and current literature establishes exactly 12 subfamilies of Kv channels as a product of this gene encoding (e.g., Kv1–12) [6].
Historically, some of the earliest studies on voltage-gated ion channels (VGICs) were on the contemporary Kv7 subfamily [5][6]. Moreover, the understanding of the Kv7 subfamily was not immediate upon discovery. Rather, the literature initially focused on a concept known as the M channel. This channel was initially termed due to its activity as a low-threshold non-inactivating K+ channel [7]. They were named “M channels” as such because of pilot literature that showcased their inhibition via muscarinic acetylcholine receptors (mAChR) stimulation [5]. Today, the subunits of the subfamily Q Kv7 K+ (KCNQ) channel family are now known to be part of M channels and are a key target as the basis for pharmacological treatment modalities for a broad spectrum of neurological disorders. This is because Kv7 have been shown to be stimulated by membrane potentials that are more negative than the AP threshold due to their activity as a low-threshold non-inactivating K+ channel [5][6][7].
Structurally, the KCNQ channels are similar to their Kv channel relatives (Figure 1). However, the emphasis on these channels is in their ability to utilize their glycine residues to contribute to a major part of their K+ ion preference [8][9]. Specifically, the channels have glycine residues which utilize their carbonyl oxygen branches to form a shell that is specific for the size of K+ ions compared to Ca2+ and Na+ ions [9][10].
The KCNQ channels are responsible for the M currents during physiological processes, which is important in the regulation of various neuronal excitability [10]. The basis of which is formed by several different KCNQ isoforms forming heterotrimeric channels. The M-current is a non-inactivating sub-threshold current [9][10]. The increases in neuronal excitability have resulted from physiological modulation, pharmacological inhibition, and genetic mutations that affect the M-current [9][10][11]. The Kv7 channels can transiently induce the suppression of the M-current such that they limit the firing frequency of neurons [10][11]. Furthermore, it is the Kv7.2 and Kv7.3 channels which are specifically involved in the regulation of M-current, and some other channels can also play minor contributory roles [10][11][12][13].
With regards to the actual opening and closing of the KCNQ channel, there are several mechanisms. For example, KCNQ channels can open via binding of the phosphatidylinositol 4,5-bisphosphate (PIP2) ligand. The direct binding of gamma-aminobutyric acid (GABA) to the KCNQ channel can directly increase the likelihood that a KCNQ channel will open and allow K+ permeation. This mechanism seems to be GABA-specific as such a conformation has not been identified in KCNQ channels activated by other means. Secondly, inositol 1,4,5-trisphosphate (IP3)-mediated intracellular calcium signals promote PIP2 synthesis and, via calmodulin, will suppress the M-current [14][15]. In regard to neuronal KCNQ channels, their importance lies in the ability to modulate neurotransmitter release and somatic excitation in the nervous system. Robust production of PIP2 via hydrolysis agonizes four receptors in the sympathetic neurons of the superior cervical ganglion (e.g., M1, AT1, B2, and P2Y). Modulation of this system occurs via competitive or allosteric regulation of the membrane transport protein affinities for PIP2 molecules [1][5][6][7][8][9][10][11][16][17][18][19][20][21].
With this array of physiological properties found in KCNQ channels, there has been a growth in the literature on KCNQ channel property modifications for therapeutic treatment modalities, as well as the role of these channels in various pathological processes. Specifically, the alteration or loss of function (LOF) by these KCNQ (i.e., channelopathies) highlight their importance in physiological function in the body.
There are various phenotypic presentations of these channelopathies as most are due to genetic etiology amongst whichever genes are involved and the location of the channels, as depicted in Table 1 [17][18][19][20][21]. The most common genes involved in channelopathies are KCNQ1-5 (without consideration of spliced variants) [14][17][21]. KCNQ1 is most expressed in cardiac and cochlear tissue [14][22]. Specifically, cardiac KCNQ1 LOF mutations are associated with type 1 long-QT syndrome [22][23][24][25][26][27][28][29][30]. Cochlear KCNQ1 pathology involves the autosomal recessive long-QT syndrome (Jervell Lange-Nielsen syndrome), which is associated with potassium channelopathy leading to bilateral sensorineural hearing loss as well as the cardiac arrhythmia [26][27][28][29]. KCNQ2 is most expressed in the fetal cerebellum, hippocampus, and medulla [30][31]. Genetic mutation in KCNQ2 is often associated with benign familial neonatal seizures and early-onset epileptic encephalopathy [9][32][33][34][35][36][37][38][39][40][41][42][43][44][45]. KCNQ3 is also most expressed in the fetal cerebellum, hippocampus, and medulla [9][30]. In addition, KCNQ3 mutations are often associated with channelopathies in conjunction with KCNQ2 [33], but additional literature also supports KCNQ mutations in bipolar disorder [36] and various thyroid disorders [37]. Similar to KCNQ1 expression, KCNQ4 is most expressed in the cochlear hair cells but also in trigeminal ganglia [14]. This plays a key role in maintaining the K+ gradient for channel mechanosensation to carry K+ into hair cells to stimulate auditory sensation [14][38]. KCNQ4 mutations are often associated with auditory hearing loss and have therefore been a key target in developing pharmacotherapeutic options for hearing loss [39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54]. KCNQ5 is most expressed in neural tissue, including the retinal pigment epithelium [48]. However, the lack of recent literature on the profile of these encoded channel subfamilies suggests that there may be unknown channelopathies related to vision homeostasis [14][48]. The expression of these genes is more often in association with other KCNQ genes than what was separately outlined. In addition to KCNQ5, KCNQ1 and KCNQ4 are also often encoded to channels in the neuronal retina and may also have a degree of contribution to its physiological function [9][14][30]. Despite this, the importance of highlighting single gene encoding remains key to approaching neural pathophysiology [2][8][14]. Given this importance, the aim of this entry is to provide an up-to-date understanding of the contemporary work of KCNQ channels in order to provide greater emphasis on KCNQ’s involvement in various pathophysiological processes distributed throughout the human body.
Table 1. Expression distribution and associated pathologies with channel genes.
| Gene | Expression Distribution | Associated Pathologies |
|---|---|---|
| KCNQ1 | Cochlea | Type 1 long QT syndrome |
| Heart | ||
| KCNQ2 | Cerebellum | Benign familial neonatal seizures |
| Hippocampus Medulla |
Early onset epileptic encephalopathy | |
| KCNQ3 | Cerebellum | Benign familial neonatal seizures |
| Hippocampus Medulla |
Early onset epileptic encephalopathy Bipolar Disorder |
|
| KCNQ4 | Cochlea Trigeminal ganglia |
Deafness |
| KCNQ5 | Retinal pigment epithelium | * |
* No major associated pathologies. Of note, this table is not comprehensive to all expression and pathological distributions of these genes.
2. Modulation of Synaptic Plasticity by KCNQ Channels
There has been a greater development in the role of KCNQ channels among neuronal networks in the past decade. This has led to its consideration for potential pharmacotherapeutic applications [14]. The ability for neuronal modification, or neural plasticity, is a key area of focus in understanding the foundations of learning and memory functions. Anatomically, the origin of the literature on neural plasticity can be further refined by discussing the concept of synaptic plasticity. This concept focuses on hippocampal formation and two principal cell types: pyramidal neurons and granular cells. Specifically, the pyramidal neurons are composed of diverse branching of dendritic neurons, which are responsible for synaptic communication with other neurons [49][50]. The morphological formation of these neurons within the hippocampus leads to the further subfield classification of pyramidal cells in what is known as Cornu Ammonus (CA), divided into CA1, CA2, and CA3 [49][50]. These regions serve an important role in localizing KCNQ channel function in synaptic plasticity [49][51][52][53][54][55][56][57][58][59][60][61]. Within synaptic plasticity, two major models involved in the application of neural plasticity are long-term potentiation (LTP) and long-term depression (LTD) [59]. These models are activity-dependent, and the literature establishes their role in namesake enhancement or reduction in synaptic efficiency. Historically, LTP was initially found in animal models, which found a sustained enhancement in the hippocampus following high-frequency electrode stimulation. LTD was later recognized after laboratory models found the opposite effect following low-frequency simulations [61][62][63][64][65]. At the cellular level, the literature suggests there are numerous factors that play a role in creating the genres of synaptic efficiency and, ultimately, neural plasticity [63][64][65][66][67][68].
Historically, the literature establishes high concentrations of KCNQ2–5 channels in the perisomatic CA1 hippocampal regions [50]. Within dendritic CA1 regions, the current generated by KCNQ channels may not serve as robust of a role as seen in pyramidal CA1 regions [14][50]. Moreover, it has been seen that modulation of KCNQ currents via linopirdine and XE991 do not create effects on synaptic excitability. Rather, it has been shown that the axonal KCNQ channels create a backpropagation into the dendritic CA1 regions [14][65][66][67][68]. This may suggest that the quantity of KCNQ channels in the dendrites does not play as robust of a role in synaptic excitability as the axonal KCNQ channels do themselves [64][65][66][67][68]. This makes axonal KCNQ channels the greater focus of study.
It was initially found that pharmacologic KCNQ channel inhibition via linopirdine reduced spike frequency adaptation (SFA) in CA1 pyramidal neurons in vitro, but only after the initial spike [49][50]. Following this initial discovery, it was also found that KCNQ channel modulation also plays a role in after hyperpolarization, which ultimately supports the notion that KCNQ channels contribute to AP [66]. In addition, muscarinic channel inhibition (i.e., KCNQ) has been shown to stimulate an array of homeostatic neuroplastic changes in synaptic efficiency. This array, in combination with KCNQ’s contribution to AP, may suggest that this array occurs at different time points, which allows for understanding that a temporal process of these neuroplastic changes occurs rather than a synced process in the hippocampus [67]. If the behavior of KCNQ channels occurs in a temporal process, this can make way for a greater understanding of the role of KCNQ via LTP and, therefore, memory development.
LTP genre can be categorized as either dependent or independent of N-methyl-D-aspartate (NMDA) receptors. Within the NMDA receptor-dependent form of LTP, it is suggested that KCNQ inhibition via XE991 stimulates the opening of NMDA receptors mediated channels during LTP by stimulating the depolarization after AP firing when performed via theta-burst stimulation [65]. This behavior may suggest that XE991 inhibition could serve a pharmacotherapeutic role in improving memory. However, the literature regarding the modulation of KCNQ channels regarding memory and LTP is still not completely understood [62][63][64][65][66][67][68]. With regard to LTP in the presence of acute stress, it is well understood that stress impairs spatial memory retrieval. Flupirtine-induced activation of KCNQ channels in the CA1 region is found to have a neuroprotective effect on spatial memory retrieval in the case of acute stress. The mechanism behind these protective effects is suggested to be through the Akt/GSK-3β and Erk1/2 signaling pathways, and animal models have shown flupirtine treatments resulted in decreased expression of apoptosis factors (i.e., Bax) and upregulation of hippocampal p-Erk1/2 [66][67][68]. Likewise, literature establishes beneficial effects on memory via KCNQ pharmacological inhibition as well. In addition to the aforementioned effects on LTP by XE991, this inhibitor has also improved cognitive impairment secondary to acetylcholine (ACh) depletion animal model induced by the neuroexcitatory kainic acid [64][65][66][67][68]. The discovery of this behavior has led to a growing body of literature on potential therapeutic applications in Alzheimer’s Disease due to its nature as a cholinergic deficiency-related cognitive impairment [66][67]. In addition, the inhibition of KCNQ channels via linopirdine is also well-established in enhancing cognition via increased ACh release [63][65][66][67][68][69][70][71][72][73][74][75][76][77].
In contrast, while LTP typically occurs after a brief high-intensity stimulation of a postsynaptic neuron, LTD can be caused by prolonged low-intensity stimulation or simulation that occurs after the firing of an AP [69]. This leads to insufficient depolarization due to this lower level of stimulation. This does not generate a removal of the magnesium blockage of the NMDA receptor [78][79][80][81][82]. However, there is evidence that this stimulation is enough to open some NMDA receptors to allow for calcium ions into the cell. These cellular calcium levels are thought to activate a cellular cascade to remove α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [77][78][79][80][81]. This reduces the postsynaptic glutamate receptor density, which decreases synapse efficiency and, therefore, memory and learning development. Despite the developments in literature dedicated to LTD, there is little literature on the effects that KCNQ channels have on this mechanism compared to LTP.
Other than the pharmacological inhibition of KCNQ channels, the inhibition by genetic proxy also serves a role in neural plasticity with regard to cognition. Animal models have shown epileptic seizures in addition to cognitive spatial memory impairment in cases of mutant or LOF genes that encode for KCNQ2 [82][83][84][85][86][87][88][89]. These effects brought upon by genetic inhibition challenge the protective and cognitive improvements seen in pharmacologic forms of KCNQ inhibition. This is where the literature ought to focus in order to determine if secondary factors that are not understood in these animal models also contribute to the memory impairment (i.e., hippocampal morphology) [90][91][92][93][94][95]. The epileptic phenotype, in conjunction with the cognitive impairment of these genetic models, may suggest additional psychomotor exploration [95][96][97][98][99][100][101][102][103][104].
References
- Camerino, D.C.; Desaphy, J.F.; Tricarico, D.; Pierno, S.; Liantonio, A. Therapeutic approaches to ion channel diseases. Adv. Genet. 2008, 64, 81–145.
- Abbott, G.W. KCNQs: Ligand- and voltage-gated potassium channels. Front. Physiol. 2020, 11, 583.
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576.
- Häfner, S.; Sandoz, G. Photopharmacological approaches for dissecting potassium channel physiology. Curr. Opin. Pharmacol. 2022, 63, 102178.
- Mandal, K. Review of PIP2 in cellular signaling, functions and diseases. Int. J. Mol. Sci. 2020, 21, 8342.
- Harraz, O.F. PIP2: A critical regulator of vascular ion channels hiding in plain sight. Proc. Natl. Acad. Sci. USA 2020, 117, 20378–20389.
- Krajnik, A.; Brazzo, J.A.; Vaidyanathan, K.; Das, T.; Redondo-Muñoz, J.; Bae, Y. Phosphoinositide signaling and mechanotransduction in cardiovascular biology and disease. Front. Cell Dev. Biol. 2020, 8, 595849.
- McLean, M.A.; Stephen, A.G.; Sligar, S.G. PIP2 influences the conformational dynamics of membrane-bound KRAS4b. Biochemistry 2019, 58, 3537–3545.
- Jespersen, T.; Grunnet, M.; Olesen, S.P. The KCNQ1 potassium channel: From gene to physiological function. Physiology 2005, 20, 408–416.
- Liu, W.X.; Deng, E.Z.; Chen, W.; Lin, H. Identifying the subfamilies of voltage-gated potassium channels using feature selection technique. Int. J. Mol. Sci. 2014, 15, 12940–12951.
- Ranjan, R.; Logette, E.; Marani, M.; Herzog, M.; Tâche, V.; Scantamburlo, E.; Buchillier, V.; Markram, H. A Kinetic map of the homomeric voltage-gated potassium channel (Kv) family. Front. Cell. Neurosci. 2019, 13, 358.
- Brown, D.A.; Adams, P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980, 283, 673–676.
- Delmas, P.; Brown, D.A. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 2005, 6, 850–862.
- Wang, J.J.; Li, Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol. Sin. 2016, 37, 25–33.
- Eren-Koçak, E.; Dalkara, T. Ion channel dysfunction and neuroinflammation in migraine and depression. Front. Pharmacol. 2021, 12, 777607.
- Sacco, T.; Tempia, F. A-type potassium currents active at subthreshold potentials in mouse cerebellar Purkinje cells. J. Physiol. 2002, 543, 505–520.
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195.
- Jentsch, T.J. Neuronal KCNQ potassium channels: Physiology and role in disease. Nat. Rev. Neurosci. 2000, 1, 21–30.
- Lehmann-Horn, F.; Jurkat-Rott, K. Voltage-gated ion channels and hereditary disease. Physiol. Rev. 1999, 79, 1317–1372.
- Jurkat-Rott, K.; Lehmann-Horn, F. Human muscle voltage-gated ion channels and hereditary disease. Curr. Opin. Pharmacol. 2001, 1, 280–287.
- Felix, R. Channelopathies: Ion channel defects linked to heritable clinical disorders. J. Med. Genet. 2000, 37, 729–740.
- Robbins, J. KCNQ potassium channels: Physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 2001, 90, 1–19.
- Wang, Z.; Wang, L.; Liu, W.; Hu, D.; Gao, Y.; Ge, Q.; Liu, X.; Li, L.; Wang, Y.; Wang, S.; et al. Pathogenic mechanism and gene correction for LQTS-causing double mutations in KCNQ1 using a pluripotent stem cell model. Stem Cell Res. 2019, 38, 101483.
- Wei, H.; Wu, J.; Liu, Z. Studying KCNQ1 mutation and drug response in type 1 long QT syndrome using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Methods Mol. Biol. 2018, 1684, 7–28.
- Ma, D.; Wei, H.; Lu, J.; Huang, D.; Liu, Z.; Loh, L.J.; Islam, O.; Liew, R.; Shim, W.; Cook, S.A. Characterization of a novel KCNQ1 mutation for type 1 long QT syndrome and assessment of the therapeutic potential of a novel IKs activator using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2015, 6, 39.
- Wuriyanghai, Y.; Makiyama, T.; Sasaki, K.; Kamakura, T.; Yamamoto, Y.; Hayano, M.; Harita, T.; Nishiuchi, S.; Chen, J.; Kohjitani, H.; et al. Complex aberrant splicing in the induced pluripotent stem cell-derived cardiomyocytes from a patient with long QT syndrome carrying KCNQ1-A344Aspl mutation. Heart Rhythm 2018, 15, 1566–1574.
- García Gozalo, M.; Bermejo Arnedo, I.; De Vera McMullan, P. KCNQ1 gene mutation and epilepsy in patient with long QT syndrome. Med. Clin. 2021, 157, 456–457.
- Marstrand, P.; Almatlouh, K.; Kanters, J.K.; Graff, C.; Christensen, A.H.; Bundgaard, H.; Theilade, J. Effect of moderate potassium-elevating treatment in long QT syndrome: The TriQarr potassium study. Open Heart 2021, 8, e001670.
- Zhang, R.; Ding, C.; Wang, H. Treatment on arrhythmia electric storm in a Jervell and Lange-Nielsen syndrome patient by ablation of the triggering premature ventricular contraction: A case report. Ann. Palliat. Med. 2021, 10, 4938–4943.
- Giudicessi, J.R.; Ackerman, M.J. Prevalence and potential genetic determinants of sensorineural deafness in KCNQ1 homozygosity and compound heterozygosity. Circ. Cardiovasc. Genet. 2013, 6, 193–200.
- Qiu, Y.; Chen, S.; Wu, X.; Zhang, W.J.; Xie, W.; Jin, Y.; Xie, L.; Xu, K.; Bai, X.; Zhang, H.M.; et al. Jervell and Lange-Nielsen syndrome due to a novel compound heterozygous KCNQ1 mutation in a Chinese family. Neural Plast. 2020, 2020, 3569359.
- Vyas, B.; Puri, R.D.; Namboodiri, N.; Nair, M.; Sharma, D.; Movva, S.; Saxena, R.; Bohora, S.; Aggarwal, N.; Vora, A.; et al. KCNQ1 mutations associated with Jervell and Lange-Nielsen syndrome and autosomal recessive Romano-Ward syndrome in India-expanding the spectrum of long QT syndrome type 1. Am. J. Med. Genet. 2016, 170, 1510–1519.
- Yang, Q.; Tan, Q.Q.; Lan, C.J.; Lv, B.Z.; Zhou, G.M.; Zhong, W.Q.; Gu, Z.M.; Mao, Y.M.; Liao, X. The changes of KCNQ5 expression and potassium microenvironment in the retina of myopic guinea pigs. Front. Physiol. 2021, 12, 790580.
- Mönnig, G.; Schulze-Bahr, E.; Wedekind, H.; Eckardt, L.; Kirchhof, P.; Funke, H.; Kotthoff, S.; Vogt, J.; Assmann, G.; Breithardt, G.; et al. Clinical aspects and molecular genetics of the Jervell- and Lange-Nielsen Syndrome. Z. Kardiol. 2002, 91, 380–388.
- Kanaumi, T.; Takashima, S.; Iwasaki, H.; Itoh, M.; Mitsudome, A.; Hirose, S. Developmental changes in KCNQ2 and KCNQ3 expression in human brain: Possible contribution to the age-dependent etiology of benign familial neonatal convulsions. Brain Dev. 2008, 30, 362–369.
- Devaux, J.J.; Kleopa, K.A.; Cooper, E.C.; Scherer, S.S. KCNQ2 is a nodal K+ channel. J. Neurosci. 2004, 24, 1236–1244.
- Mary, L.; Nourisson, E.; Feger, C.; Laugel, V.; Chaigne, D.; Keren, B.; Afenjar, A.; Billette, T.; Trost, D.; Cieuta-Walti, C.; et al. Pathogenic variants in KCNQ2 cause intellectual deficiency without epilepsy: Broadening the phenotypic spectrum of a potassium channelopathy. Am. J. Med. Genet. A 2021, 185, 1803–1815.
- Vanoye, C.G.; Desai, R.R.; Ji, Z.; Adusumilli, S.; Jairam, N.; Ghabra, N.; Joshi, N.; Fitch, E.; Helbig, K.L.; McKnight, D.; et al. High-throughput evaluation of epilepsy-associated KCNQ2 variants reveals functional and pharmacological heterogeneity. JCI Insight 2022, 7, e156314.
- Hu, C.; Liu, D.; Luo, T.; Wang, Y.; Liu, Z. Phenotypic spectrum and long-term outcome of children with genetic early-infantile-onset developmental and epileptic encephalopathy. Epileptic Disord. 2022.
- Kim, K.W.; Kim, K.; Kim, H.J.; Kim, B.I.; Baek, M.; Suh, B.C. Posttranscriptional modulation of KCNQ2 gene expression by the miR-106b microRNA family. Proc. Natl. Acad. Sci. USA 2021, 118, e2110200118.
- Monni, L.; Kraus, L.; Dipper-Wawra, M.; Soares-Da-Silva, P.; Maier, N.; Schmitz, D.; Holtkamp, M.; Fidzinski, P. In vitro and in vivo anti-epileptic efficacy of eslicarbazepine acetate in a mouse model of KCNQ2-related self-limited epilepsy. Br. J. Pharmacol. 2022, 179, 84–102.
- Nissenkorn, A.; Kornilov, P.; Peretz, A.; Blumkin, L.; Heimer, G.; Ben-Zeev, B.; Attali, B. Personalized treatment with retigabine for pharmacoresistant epilepsy arising from a pathogenic variant in the KCNQ2 selectivity filter. Epileptic Disord. 2021, 23, 695–705.
- Lee, I.C.; Chang, T.M.; Liang, J.S.; Li, S.Y. KCNQ2 mutations in childhood nonlesional epilepsy: Variable phenotypes and a novel mutation in a case series. Mol. Genet. Genom. Med. 2019, 7, e00816.
- Milh, M.; Lacoste, C.; Cacciagli, P.; Abidi, A.; Sutera-Sardo, J.; Tzelepis, I.; Colin, E.; Badens, C.; Afenjar, A.; Coeslier, A.D.; et al. Variable clinical expression in patients with mosaicism for KCNQ2 mutations. Am. J. Med. Genet. A 2015, 167, 2314–2318.
- Lazo, P.A.; García, J.L.; Gómez-Puertas, P.; Marcos-Alcalde, Í.; Arjona, C.; Villarroel, A.; González-Sarmiento, R.; Fons, C. Novel dominant KCNQ2 exon 7 partial in-frame duplication in a complex epileptic and neurodevelopmental delay syndrome. Int. J. Mol. Sci. 2020, 21, 4447.
- Kaminsky, Z.; Jones, I.; Verma, R.; Saleh, L.; Trivedi, H.; Guintivano, J.; Akman, R.; Zandi, P.; Lee, R.S.; Potash, J.B. DNA methylation and expression of KCNQ3 in bipolar disorder. Bipolar Disord. 2015, 17, 150–159.
- Mittal, K.; Rafiq, M.A.; Rafiullah, R.; Harripaul, R.; Ali, H.; Ayaz, M.; Aslam, M.; Naeem, F.; Amin-Ud-Din, M.; Waqas, A.; et al. Mutations in the genes for thyroglobulin and thyroid peroxidase cause thyroid dyshormonogenesis and autosomal-recessive intellectual disability. J. Hum. Genet. 2016, 61, 867–872.
- Rim, J.H.; Choi, J.Y.; Jung, J.; Gee, H.Y. Activation of KCNQ4 as a therapeutic strategy to treat hearing loss. Int. J. Mol. Sci. 2021, 22, 2510.
- Lee, S.Y.; Choi, H.B.; Park, M.; Choi, I.S.; An, J.; Kim, A.; Kim, E.; Kim, N.; Han, J.H.; Kim, M.Y.; et al. Novel KCNQ4 variants in different functional domains confer genotype- and mechanism-based therapeutics in patients with nonsyndromic hearing loss. Exp. Mol. Med. 2021, 53, 1192–1204.
- Thorpe, R.K.; Walls, W.D.; Corrigan, R.; Schaefer, A.; Wang, K.; Huygen, P.; Casavant, T.L.; Smith, R.J.H. AudioGene: Refining the natural history of KCNQ4, GSDME, WFS1, and COCH-associated hearing loss. Hum. Genet. 2022, 141, 877–887.
- Yen, T.T.; Chen, I.C.; Hua, M.W.; Wei, C.Y.; Shih, K.H.; Li, J.L.; Lin, C.H.; Hsiao, T.H.; Chen, Y.M.; Jiang, R.S. A KCNQ4 c.546C>G Genetic variant associated with late onset non-syndromic hearing loss in a Taiwanese population. Genes 2021, 12, 1711.
- Kojima, T.; Wasano, K.; Takahashi, S.; Homma, K. Cell death-inducing cytotoxicity in truncated KCNQ4 variants associated with DFNA2 hearing loss. Dis. Model Mech. 2021, 14, dmm049015.
- Peixoto-Pinheiro, B.; Vona, B.; Löwenheim, H.; Rüttiger, L.; Knipper, M.; Adel, Y. Age-related hearing loss pertaining to potassium ion channels in the cochlea and auditory pathway. Dis. Model Mech. 2021, 473, 823–840.
- Borgini, M.; Mondal, P.; Liu, R.; Wipf, P. Chemical modulation of Kv7 potassium channels. RSC Med. Chem. 2021, 12, 483–537.
- Xiong, Q.; Sun, H.; Li, M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat. Chem. Biol. 2007, 3, 287–296.
- Tompson, D.J.; Buraglio, M.; Andrews, S.M.; Wheless, J.W. Adolescent clinical development of ezogabine/retigabine as adjunctive therapy for partial-onset seizures: Pharmacokinetics and tolerability. J. Pediatr. Pharmacol. Ther. 2016, 21, 404–412.
- Gunthorpe, M.J.; Large, C.H.; Sankar, R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012, 53, 412–424.
- Bayasgalan, T.; Stupniki, S.; Kovács, A.; Csemer, A.; Szentesi, P.; Pocsai, K.; Dionisio, L.; Spitzmaul, G.; Pál, B. Alteration of mesopontine cholinergic function by the lack of KCNQ4 subunit. Front. Cell. Neurosci. 2021, 15, 707789.
- Niu, X.; Yu, K.; He, B. Transcranial focused ultrasound induces sustained synaptic plasticity in rat hippocampus. Brain Stimul. 2022, 15, 352–359.
- Caragea, V.M.; Manahan-Vaughan, D. Bidirectional regulation of hippocampal synaptic plasticity and modulation of cumulative spatial memory by dopamine D2-like receptors. Front. Behav. Neurosci. 2022, 15, 803574.
- Sahu, G.; Turner, R.W. The molecular basis for the calcium-dependent slow afterhyperpolarization in CA1 hippocampal pyramidal neurons. Front. Physiol. 2021, 12, 759707.
- Bentzen, B.H.; Schmitt, N.; Calloe, K.; Dalby, B.W.; Grunnet, M.; Olesen, S.P. The acrylamide (S)-1 differentially affects Kv7 (KCNQ) potassium channels. Neuropharmacology 2006, 51, 1068–1077.
- Blom, S.M.; Schmitt, N.; Jensen, H.S. The acrylamide (S)-2 as a positive and negative modulator of Kv7 channels expressed in Xenopus laevis oocytes. PLoS ONE 2009, 4, e8251.
- Zhang, X.; An, H.; Li, J.; Zhang, Y.; Liu, Y.; Jia, Z.; Zhang, W.; Chu, L.; Zhang, H. Selective activation of vascular Kv 7.4/Kv 7.5 K+ channels by fasudil contributes to its vasorelaxant effect. Br. J. Pharmacol. 2016, 173, 3480–3491.
- Zhang, X.; Yang, D.; Hughes, B.A. KCNQ5/K(v)7.5 potassium channel expression and subcellular localization in primate retinal pigment epithelium and neural retina. Am. J. Physiol. Cell Physiol. 2011, 301, C1017–C1026.
- Fogwe, L.A.; Reddy, V.; Mesfin, F.B. Neuroanatomy, Hippocampus; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482171 (accessed on 15 January 2020).
- Hu, H.; Vervaeke, K.; Storm, J.F. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J. Neurosci. 2007, 27, 1853–1867.
- Cooper, E.C.; Harrington, E.; Jan, Y.N.; Jan, L.Y. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J. Neurosci. 2001, 21, 9529–9540.
- Tzingounis, A.V.; Heidenreich, M.; Kharkovets, T.; Spitzmaul, G.; Jensen, H.S.; Nicoll, R.A.; Jentsch, T.J. The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 10232–10237.
- Boscia, F.; Elkjaer, M.L.; Illes, Z.; Kukley, M. Altered expression of ion channels in white matter lesions of progressive multiple sclerosis: What do we know about their function? Front. Cell. Neurosci. 2021, 15, 685703.
- Schultz, C.; Engelhardt, M. Anatomy of the hippocampal formation. Front. Neurol. Neurosci. 2014, 34, 6–17.
- Ito, H.T.; Schuman, E.M. Functional division of hippocampal area CA1 via modulatory gating of entorhinal cortical inputs. Hippocampus 2012, 22, 372–387.
- Sun, X.C.; Li, L.; Zhang, M.; Li, W.B.; Li, Q.J.; Zhao, L. Division of CA1, CA3 and DG regions of the hippocampus of Wistar rat. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2012, 28, 189–192.
- Watson, C.; Binks, D. Elongation of the CA1 field of the septal hippocampus in ungulates. J. Comp. Neurol. 2019, 527, 818–832.
- Van Groen, T.; Wyss, J.M. Extrinsic projections from area CA1 of the rat hippocampus: Olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp. Neurol. 1990, 302, 515–528.
- De La Rosa-Prieto, C.; Ubeda-Banon, I.; Mohedano-Moriano, A.; Pro-Sistiaga, P.; Saiz-Sanchez, D.; Insausti, R.; Martinez-Marcos, A. Subicular and CA1 hippocampal projections to the accessory olfactory bulb. Hippocampus 2009, 19, 124–129.
- Bliss, T.V.; Cooke, S.F. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66 (Suppl. 1), 3–17.
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356.
- Kemp, A.; Manahan-Vaughan, D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl. Acad. Sci. USA 2004, 101, 8192–8197.
- Hirano, T. Long-term depression and other synaptic plasticity in the cerebellum. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 183–195.
- Wiegert, J.S.; Pulin, M.; Gee, C.E.; Oertner, T.G. The fate of hippocampal synapses depends on the sequence of plasticity-inducing events. Elife 2018, 7, e39151.
- Sakurai, M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J. Physiol. 1987, 394, 463–480.
- Wiegert, J.S.; Oertner, T.G. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. USA 2013, 110, E4510–E4519.
- Lezmy, J.; Gelman, H.; Katsenelson, M.; Styr, B.; Tikochinsky, E.; Lipinsky, M.; Peretz, A.; Slutsky, I.; Attali, B. M-current inhibition in hippocampal excitatory neurons triggers intrinsic and synaptic homeostatic responses at different temporal scales. J. Neurosci. 2020, 40, 3694–3706.
- Petrovic, M.M.; Nowacki, J.; Olivo, V.; Tsaneva-Atanasova, K.; Randall, A.D.; Mellor, J.R. Inhibition of post-synaptic Kv7/KCNQ/M channels facilitates long-term potentiation in the hippocampus. PLoS ONE 2012, 7, e30402.
- Huang, P.; Li, C.; Fu, T.; Zhao, D.; Yi, Z.; Lu, Q.; Guo, L.; Xu, X. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav. Brain Res. 2015, 288, 1–10.
- Stanton, P.K. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus 1996, 6, 35–42.
- McCutchen, E.; Scheiderer, C.L.; Dobrunz, L.E.; McMahon, L.L. Coexistence of muscarinic long-term depression with electrically induced long-term potentiation and depression at CA3-CA1 synapses. J. Neurophysiol. 2006, 96, 3114–3121.
- Milner, A.J.; Cummings, D.M.; Spencer, J.P.; Murphy, K.P. Bi-directional plasticity and age-dependent long-term depression at mouse CA3-CA1 hippocampal synapses. Neurosci. Lett. 2004, 367, 1–5.
- Fontán-Lozano, A.; Suárez-Pereira, I.; Delgado-García, J.M.; Carrión, A.M. The M-current inhibitor XE991 decreases the stimulation threshold for long-term synaptic plasticity in healthy mice and in models of cognitive disease. Hippocampus 2011, 21, 22–32.
- Milh, M.; Roubertoux, P.; Biba, N.; Chavany, J.; Spiga Ghata, A.; Fulachier, C.; Collins, S.C.; Wagner, C.; Roux, J.C.; Yalcin, B.; et al. A knock-in mouse model for KCNQ2-related epileptic encephalopathy displays spontaneous generalized seizures and cognitive impairment. Epilepsia 2020, 61, 868–878.
- Baculis, B.C.; Zhang, J.; Chung, H.J. The role of Kv7 channels in neural plasticity and behavior. Front. Physiol. 2020, 11, 568667.
- Thomann, P.A.; Seidl, U.; Brinkmann, J.; Hirjak, D.; Traeger, T.; Wolf, R.C.; Essig, M.; Schroder, J. Hippocampal morphology and autobiographic memory in mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 507–515.
- Hirjak, D.; Wolf, R.C.; Remmele, B.; Seidl, U.; Thomann, A.K.; Kubera, K.M.; Schröder, J.; Maier-Hein, K.H.; Thomann, P.A. Hippocampal formation alterations differently contribute to autobiographic memory deficits in mild cognitive impairment and Alzheimer’s disease. Hippocampus 2017, 27, 702–715.
- Li, X.T. Alzheimer’s disease therapy based on acetylcholinesterase inhibitor/blocker effects on voltage-gated potassium channels. Metab. Brain Dis. 2022, 37, 581–587.
- Spoleti, E.; Krashia, P.; La Barbera, L.; Nobili, A.; Lupascu, C.A.; Giacalone, E.; Keller, F.; Migliore, M.; Renzi, M.; D’Amelio, M. Early derailment of firing properties in CA1 pyramidal cells of the ventral hippocampus in an Alzheimer’s disease mouse model. Exp. Neurol. 2021, 350, 113969.
- Moriguchi, S.; Inagaki, R.; Fukunaga, K. Memantine improves cognitive deficits via KATP channel inhibition in olfactory bulbectomized mice. Mol. Cell. Neurosci. 2021, 117, 103680.
- Djebari, S.; Iborra-Lázaro, G.; Temprano-Carazo, S.; Sánchez-Rodríguez, I.; Nava-Mesa, M.O.; Múnera, A.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. G-Protein-gated inwardly rectifying potassium (Kir3/GIRK) channels govern synaptic plasticity that supports hippocampal-dependent cognitive functions in male mice. J. Neurosci. 2021, 41, 7086–7102.
- Ashrafuzzaman, M. Mitochondrial ion channels in aging and related diseases. Curr. Aging Sci. 2022.
- Islas, Á.A.; Scior, T.; Torres-Ramirez, O.; Salinas-Stefanon, E.M.; Lopez-Lopez, G.; Flores-Hernandez, J. Computational molecular characterization of the interaction of acetylcholine and the NMDA receptor to explain the direct glycine-competitive potentiation of NMDA-mediated neuronal currents. ACS Chem. Neurosci. 2022, 13, 229–244.
- Cocozza, G.; Garofalo, S.; Capitani, R.; D’Alessandro, G.; Limatola, C.; Taylor, K.I. Microglial potassium channels: From homeostasis to neurodegeneration. Biomolecules 2021, 11, 1774.
- Sharma, K.; Pradhan, S.; Duffy, L.K.; Yeasmin, S.; Bhattarai, N.; Schulte, M.K. Role of receptors in relation to plaques and tangles in Alzheimer’s disease pathology. Int. J. Mol. Sci. 2021, 22, 12987.
- Hirni, D.I.; Kivisaari, S.L.; Monsch, A.U.; Taylor, K.I. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer’s disease. Neuropsychologia 2013, 51, 930–937.
- Bomilcar, I.; Bertrand, E.; Morris, R.G.; Mograbi, D.C. The seven selves of dementia. Front. Psychiatry 2021, 12, 646050.
More
Information
Subjects:
Physiology; Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




