Pharmacogenetics (PGx) is an emerging field of pharmacology focusing on how gene variations affect the patient’s response to treatment. Pharmacogenetics is a promising tool to optimize the selection and dosing of medications, including urate-lowering therapies (ULTs) among patients with gout. The global prevalence of gout is rising, and it disproportionately affects specific racial groups and individuals with select socioeconomic status. Genetic and experimental findings have provided evidence that genetic polymorphisms associated with serum urate pathology are also of pharmacogenetic interest. Patients with gout present with several comorbidities, warranting the use of several acute and long-term medications that increase their pill burden and the risk of adverse drug events. Implementing PGx testing can identify individuals who are more or less likely to benefit from a given treatment, improve medication adherence, and reduce pill burden.
1. Background
Pharmacogenetics (PGx) is an emerging field of pharmacology focusing on how gene variations affect the patient’s response to treatment. Pharmacogenetics leverages patient genetics to ascertain the response to pharmacotherapy, including gout treatments. Including pharmacogenetics in clinical practice could enable providers to make optimal and informed decisions about drug selection, dose modifications, and treatment options. The ultimate goals of PGx are to individualize medicine and improve patient treatment outcomes by minimizing the risk of adverse drug events
[1]. Indeed, pharmacogenetics could usher in a new era in targeted therapy to reduce the risks associated with the trial-error prescribing strategies. Moreover, pharmacogenetics could improve adherence to treatment by identifying optimal responders or those at risk for drug toxicity
[1].
Hyperuricemia (HU), a risk factor for gout, may accumulate in articular and non-articular tissue structures, forming monosodium urate (MSU) crystals
[2]. Urate underexcretion is considered the predominant pathogenesis of hyperuricemia. The increased dietary intake of purine-rich sources, endogenous cell turnover, and decreased extrarenal elimination of serum uric acid (SUA) can also contribute to the pathogenesis of high urate levels. Multiple risk factors associated with HU include diet, comorbid diseases, certain drugs, and genetics
[2][3].
Patients with gout may present with acute inflammatory arthritis, subcutaneous accumulation of MSU crystals (i.e., tophi), joint damage, and chronic gouty arthritis
[2]. Other non-articular clinical features, such as renal or kidney stones, may also result from chronic HU
[4]. Further, HU has been significantly associated with the incidence of hypertension (HTN) in adults aged ≥ 40 years
[5]. Additionally, it is associated with a 20% increased prevalence of HTN
[6], and a higher risk of insulin resistance
[7]. Based on those observations, reducing urate levels has become an important therapeutic target beyond gout management, and it potentially prevents and maximizes the treatment of other comorbid conditions
[8].
2. Genetics of Hyperuricemia and Gout
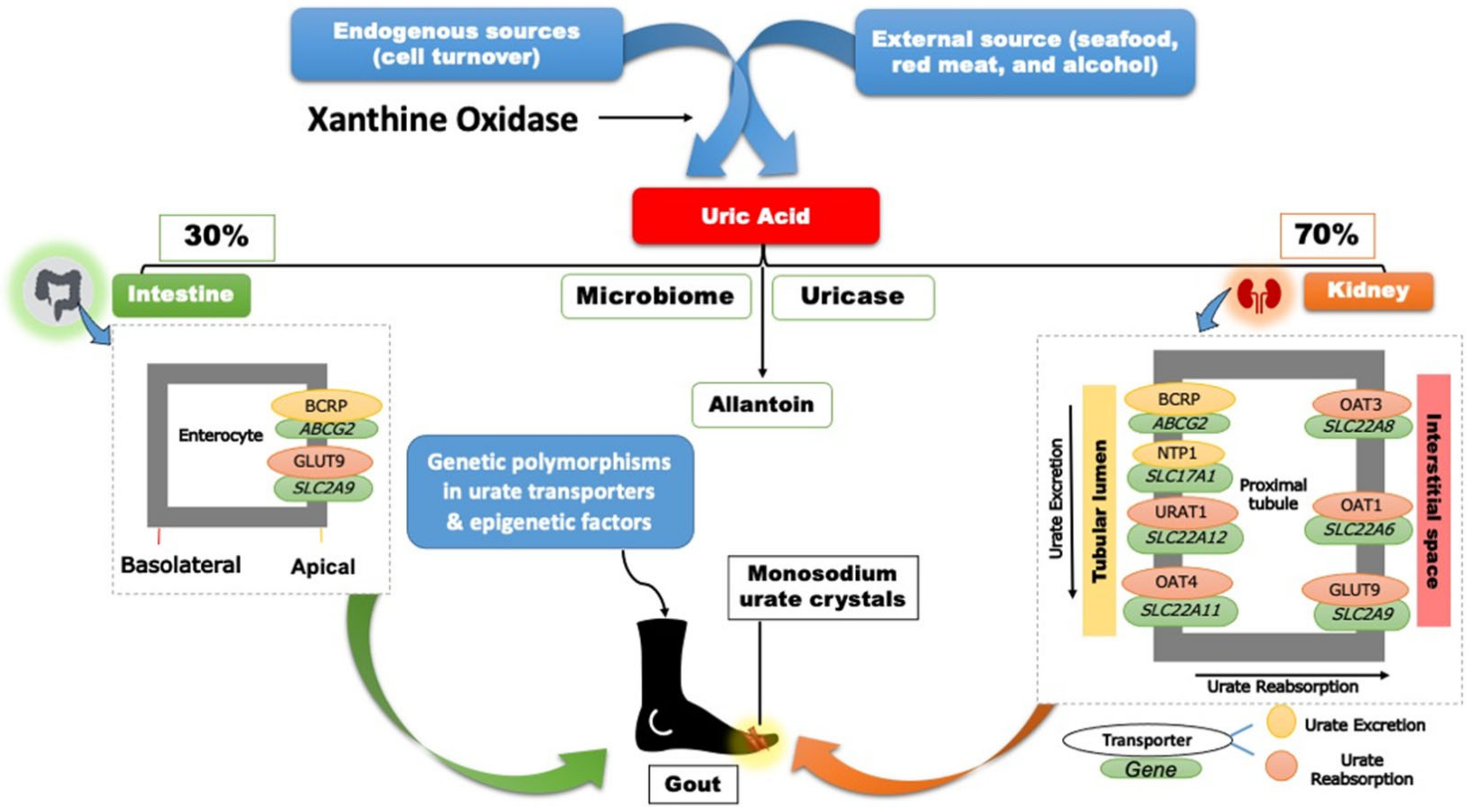
Two-thirds of SUA is eliminated through the renal proximal tubule (RPT), while the remaining one-third is eliminated through the small intestine and metabolized by the gut microflora (
Figure 1)
[9].
Figure 1. Regulation and handling of uric acid.
Approximately, ninety percent of UA, processed by the kidney, is reabsorbed through the proximal tubular cells
[10]. Though several aspects of UA elimination and reabsorption remain unknown, extensive population genetic studies, particularly genome-wide association studies (GWAS), have identified significant genetic polymorphisms in the UA disposition pathway
[10][11][12][13]. Variations in genes regulating UA excretion (
ABCG2,
SLC17A1), UA reabsorption (
SLC22A12,
SLC2A9, and
SLC22A11), and a lipid metabolizing gene (
GCKR), as well as a scaffolding protein (
PDZK1) have all been linked to SUA levels (
Figure 1)
[10].
The major urate transporter proteins encoded by the above genes are involved in various functions in the UA disposition pathway. For instance, the solute carrier family 22 member 12 (
SLC22A12) encodes the kidney-specific urate transporter URAT1, which is found on the apical surface of the renal proximal tubule epithelial cells
[14]. Secondly, the apical ATP-binding cassette transporter G2 (ABCG2) (i.e., breast cancer resistance protein) is involved in urate excretion into the distal renal tubule
[15][16]. Thirdly, the key player in transporting UA into the interstitial space and circulation is the GLUT9 (
SLC2A9)
[17][18]. Other transporters identified in GWAS, including OAT1, OAT3, and OAT4, are thought to play minor roles in the urate transportome (
Figure 1)
[15]. Considering the inhibition of urate reabsorption as a therapeutic target in managing gout, the interplay between the single nucleotide polymorphisms (SNPs) within these transporters and the uricosuric pharmacotherapies highlights the potential of pharmacogenetics to guide and personalize drug therapy in patients with gout and HU.
3. Gout Management Pharmacotherapy
The pharmacotherapy management of gout includes rapid and effective control of the inflammation in acute gout flares, continued ULT to prevent future flares, and ultimately improve gout treatment outcomes
[2]. Contemporary gout treatment guidelines recommend allopurinol as the preferred first-line treatment for managing chronic gout. Pharmacotherapies, such as non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids, are also appropriate first-line agents to manage gout flares
[2]. Pharmacotherapies, including interleukin-1 inhibitors (i.e., canakinumab and rilonacept), are also used to control gout flares when alternatives are contra-indicated or ineffective. Social and environmental factors, as well as diet and genetics, could affect a patient’s adherence and response to ULTs. As the field of pharmacogenomics continues to evolve, multiple studies have evaluated the effect of gene variants, including
G6PD,
HLA-B*58:01, and
CYP2C9, on predicting the response to ULTs and possible adverse drug reactions (
Table 1)
[2][19].
Table 1. Pharmacogenetics Summary of Gout Treatment and CPIC Guideline Level of Evidence.
| Drug |
Mapped Genes |
Effect |
Clinical Outcomes |
CPIC Guideline Level of Evidence a |
References |
| Xanthine oxidase inhibitors (XO) |
| Allopurinol or Oxypurinol |
HLA-B |
Safety |
HLA-B*58:01 allele significantly increases the risk of allopurinol-induced serious cutaneous reaction |
A |
[2][20] |
| AOX |
Response |
rs3731722 A>G is associated with a better response to the standard dose of allopurinol (300 mg/day) vs. non-carriers |
NA |
[21] |
| ABCG2 |
Response/PK |
rs2231142 C>A (Q141K) is associated with poor response to allopurinol |
NA |
[22] |
| SLC22A12 |
Response/PK |
rs505802 C>T may influence the response to allopurinol and the PK of oxypurinol as they are substrates for the URAT1 |
NA |
[23][24][25] |
| Febuxostat |
UGT1A1 |
Response/PK |
rs34650714 C>T is associated with lower doses of febuxostat |
NA |
[21] |
| Uricosuric Agents |
| Probenecid |
SLC22A12 |
Response |
Homozygous or heterozygous for the mutant allele (G774A) have impaired response to loading tests of probenecid |
NA |
[26][27] |
| ABCB1 |
PK |
rs1045642 C>T could influence the PK effect of probenecid as an inhibitor when co-administered with Beta-lactam |
NA |
[28] |
| G6PD |
Safety |
Possible hematologic adverse reactions in G6PD deficient patients |
B |
[29] |
| Benzbromarone |
CYP2C9 |
Safety |
Carriers of the no-function allele (CYP2C9*3) have reduced metabolic activity leading to prolonged exposure to benzbromarone relative to normal metabolizers |
NA |
[30][31] |
| Recombinant Uricase |
| Pegloticase |
G6PD |
Safety |
Risk of hemolysis or methemoglobinemia in G6PD deficient patients |
B |
[32] |
| Non-steroidal anti-inflammatory drugs (NSAIDs) |
| Ibuprofen, celecoxib, and other NSAIDs |
CYP2C9 |
Safety/PK |
Increased risk of NSAID-related GI bleeding in no-function allele (*3) carriers relative to normal function, as well as reduced metabolism and prolonged exposure to ibuprofen and celecoxib in CYP2C9 poor metabolizers |
A (ibuprofen and celecoxib); C (indomethacin, diclofenac, naproxen) |
[33][34] |
| Anti-inflammatory |
| Colchicine |
CYP2D6 |
Response |
Diminished response to colchicine in CYP2D6*4 variant carriers |
NA |
[35] |
| ABCB1 |
Inconsistent evidence wherein one study indicates good response in the T allele carriers of the SNP rs10455642 C>T, while another study suggests no response with the T allele |
NA |
[36][37] |
| SEPHS1 |
Safety |
The risk allele G of rs74795203 A>G significantly increases the risk of gastrointestinal adverse events by 2.5-fold with using colchicine |
NA |
[38] |
| KIF13A, RNU6-793Pb |
Safety |
The risk allele A of rs6916345 G>A (intergenic) was significantly associated with a ~2-fold increased risk of gastrointestinal adverse events with colchicine compared with the G allele |
NA |
[38] |
| Corticosteroids |
| Injectable triamcinolone acetonide |
HCG22 |
Safety |
The G and T alleles of rs3873352 C>G and rs2523864 C>T, respectively, increase the risk of steroid-induced ocular hypertension |
NA |
[39] |
| IL-1 inhibitor |
| Anakinra |
IL1RN |
Response |
SNP cluster in strong linkage disequilibrium associated with poor response to anakinra |
NA |
[40] |