Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aseni Ediriweera | -- | 2273 | 2022-05-17 09:30:27 | | | |
| 2 | Rita Xu | Meta information modification | 2273 | 2022-05-17 09:48:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ediriweera, A.; Karunarathna, S.; Yapa, N.; Schaefer, D.; , .; Suwannarach, N.; Xu, J. Ectomycorrhizal Mushrooms. Encyclopedia. Available online: https://encyclopedia.pub/entry/22997 (accessed on 09 February 2026).
Ediriweera A, Karunarathna S, Yapa N, Schaefer D, , Suwannarach N, et al. Ectomycorrhizal Mushrooms. Encyclopedia. Available at: https://encyclopedia.pub/entry/22997. Accessed February 09, 2026.
Ediriweera, Aseni, Samantha Karunarathna, Neelamanie Yapa, Doug Schaefer, , Nakarin Suwannarach, Jianchu Xu. "Ectomycorrhizal Mushrooms" Encyclopedia, https://encyclopedia.pub/entry/22997 (accessed February 09, 2026).
Ediriweera, A., Karunarathna, S., Yapa, N., Schaefer, D., , ., Suwannarach, N., & Xu, J. (2022, May 17). Ectomycorrhizal Mushrooms. In Encyclopedia. https://encyclopedia.pub/entry/22997
Ediriweera, Aseni, et al. "Ectomycorrhizal Mushrooms." Encyclopedia. Web. 17 May, 2022.
Copy Citation
Environmental changes and heavy metal pollution are some of the consequences of anthropogenic activities. Many ecosystems, including edaphic ecosystems, suffer from the effects of pollution. The accurate assessment of soil heavy metal contamination leads to better approaches for remediating soils. The exploration of different ways, including biological methods, to conduct environmental monitoring is still ongoing.
contamination
ectomycorrhiza
heavy metal
1. Introduction
Heavy metals (HM) and metalloids are metallic elements having a specific density of more than 5 g cm−3, such as mercury (Hg), chromium (Cr), cadmium (Cd), arsenic (As), and lead (Pb) [1]. Heavy metals are adversely affecting living organisms even at low concentrations through bio-accumulation in the food chain [2][3]. Heavy metals are characterized by a long half-life, and they are highly persistent in the environment with the potential for accumulation [4]. The artificial radionuclides or isotopes of different heavy metals cause significant hazards in terms of soil pollution. There are two main sources globally, which are responsible for the presence of different radionuclides in the environment [5]. Nuclear weapon tests and nuclear reactor catastrophes such as Chernobyl in 1986 are the main sources that emitted considerable amounts of α, β, and γ-radionuclides into the atmosphere. For instance, the long-life gamma radioisotope cesium 137Cs is considered an important indicator of radioactive pollution due to its long half-life (30.07 years) [5][6]. Caridi et al. [7] explain that radionuclides are strongly absorbed into the sediments and retained for a long time, resulting in genetic mutations, the development of diseases, and soil infertility.
Although cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), and zinc (Zn) are also grouped under the HM category, at optimum concentrations, they are beneficial for plant growth and development [8]. Nevertheless, the same metals become toxic when they exceed certain threshold concentrations [8][9]. Even though HM occurs naturally in the crust of the Earth, anthropogenic activities mostly contribute to the HM prevalence in air, soil, and water [10][11]. Since these metals are non-biodegradable, they persist in the environment [12]. Therefore, heavy metal contamination and pollution are major environmental hazards, which, when amplified by inadequate human intervention, could become a serious concern.
Conventional farming practices, increasing industrialization, and the substantial use of fossil fuels have led to high concentrations of heavy metals in the environment [13]. Furthermore, the large quantities of synthetic fertilizers and other agro-chemicals used in farming systems lead to substantially high concentrations of heavy metals in soil and water [1]. In acidic soils, HM becomes highly bioavailable due to competition with H+ for binding sites and increased solubility [14][15]. Considering these threats, scientists have focused on finding solutions to minimize the adverse effects caused by heavy metals. In the HM remediation processes in contaminated environments, demarcation of the contamination, identification, and quantification of the HM, specifically in their ionic form, are inevitably important [16][17][18].
From an ecological perspective, different organisms, including microorganisms, are naturally occurring bioindicators. Bioindicators are used to assess the environmental quality and detect positive or negative changes and their subsequent effects on biotic and abiotic components of the environment [19]. Bioindicator organisms possess the potential to absorb particular pollutants from the surrounding environment and indicate the presence of a particular pollutant within the organism [20]. Although different types of organisms are used as bioindicators in ecological monitoring, ectomycorrhizal mushrooms are well known among them due to their capability of absorbing heavy metals from the surrounding environment [18].
Mycorrhizae are generally considered mutualistic symbioses between plant roots and some fungi [21]. These symbioses are characterized by the bi-directional movement of nutrients where carbon flows to the fungus from the plant and inorganic nutrients move to the plant from the fungus, thereby providing a critical bond between the plant root and soil [22]. In the humid tropics, the following two major types of mycorrhizal associations of trees have been reported: ectomycorrhiza (EM) and arbuscular mycorrhizal fungi (AM) [23]. Both EM and AM are recognized as heavy metal accumulators [24][25][26].
Nevertheless, the ectomycorrhizal fungi have been identified as a group of organisms that intensely affect ecosystems by facilitating nutrient and water uptake, structuring soil, maintaining food webs, protecting the root systems of trees from pathogenic organisms, extreme environmental conditions, and phytoremediation of contaminated soils [27][28][29]. Besides that, the vast diversity of EM fungi helps nature to withstand changes in environmental factors due to heavy pollution and global climate change. Moreover, many EM fungi play a major role in bearing commercial value for edible fruiting bodies and in producing metabolites useful in industries [28][30]. Therefore, conservation, broader appreciation, and widening applications of EM fungi are necessary due to their extensive intervention in the successful functioning of ecosystems.
2. Ectomycorrhizal Fungi
Ectomycorrhizal mushrooms include approximately 10,000 species, mainly belonging to Basidiomycetes, Ascomycetes, and Zygomycetes that form associations with host plants [31][32] and were found to have evolved 130 million years ago [31]. Caesalpiniaceae, Pinaceae, Fagaceae, and Dipterocarpaceae are the major families known to host EM mushrooms in tropical, subtropical, temperate, and boreal forests [33]. Among the different classes of EM fungi, Agaricomycetes are found to be the dominant class in forming mycorrhizal symbiosis [34].
Ectomycorrhizal mushrooms possess a dual lifestyle in soil, being symbionts and facultative saprotrophs [35]. Due to the ecological symbiosis maintained by EM mushrooms with the root systems of trees, the number of interactions maintained with the inhabited environment is higher compared to purely saprotrophic mushrooms [36].
Ectomycorrhizal fungi are capable of enclosing their hyphae, convolving the root tips, and creating a hyphal mantle. Within the mantle, hyphae grow between cortical and epidermal cells where the lumen of roots is not penetrated [37]. This formation results in a structure called the Hartig net (Figure 1). A Hartig net makes an interface for nutrient and water exchange between fungi and roots [35]. Meanwhile, the hyphae extend out of the fungal mantle to the soil and explore the faraway regions of the rhizosphere, and increase the nutrients available for the host [35]. Hence, EM mushrooms act as an integral part of plant nutrition. The plants intern, transferring carbohydrates to the fungus [35]. Apart from sharing nutrients, the host plant also receives benefits, including increased tolerance for salt and drought stresses [38], tolerance to heavy metals [38][39], and resistance to plant pathogens [40][41]. Furthermore, EM mushrooms protect their hosts [42][43], mitigating drought stress and producing vitamins and hormones for plant development [31][44].
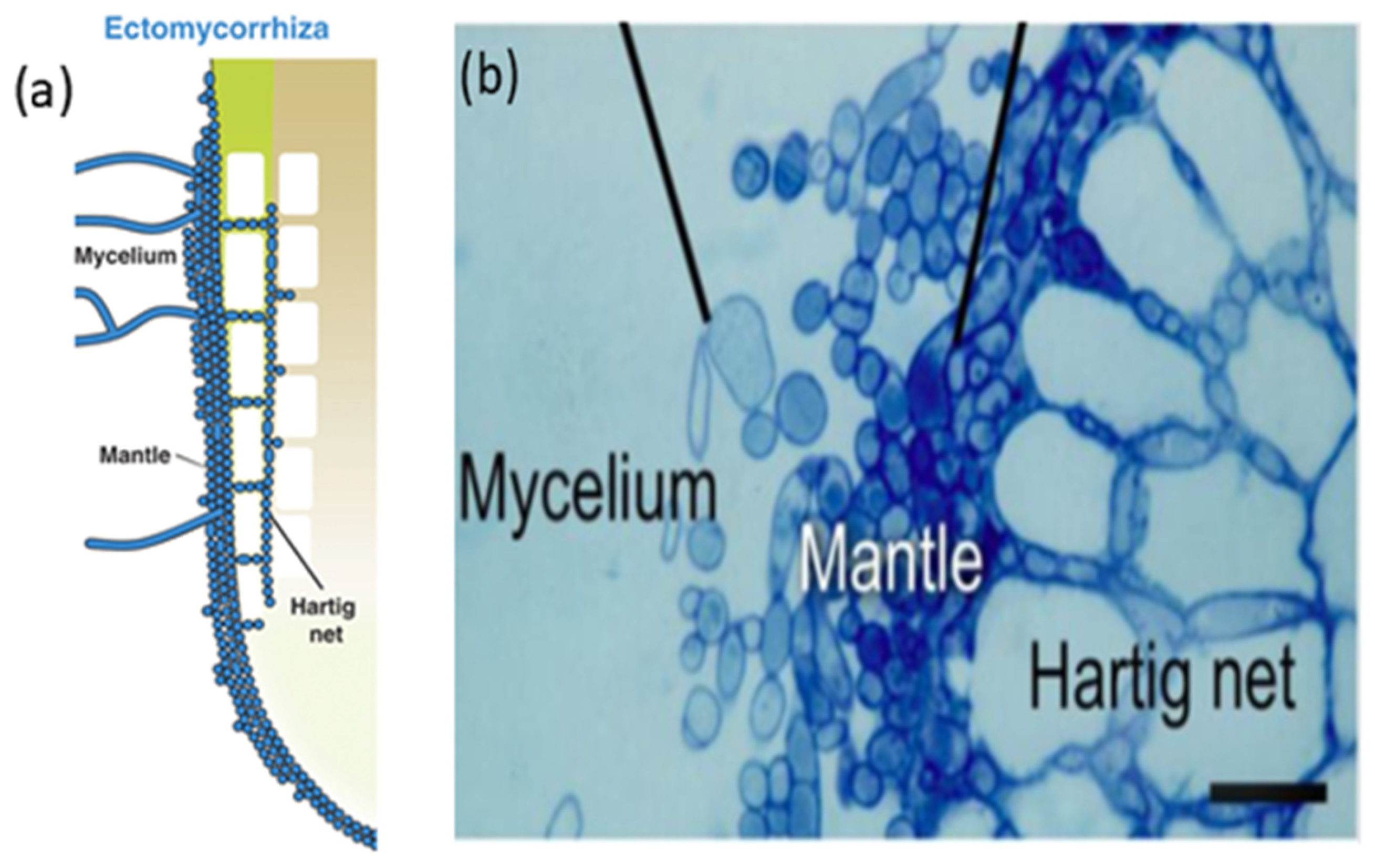
Figure 1. (a) Illustration of root colonization structures in ectomycorrhiza (b) Hartig net formation of EM fungi. Outbreak of hyphae of mycelium in intracellular space with no penetration in lumen [37] Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis.
Ectomycorrhizal fungi possess crucial symbiotic relationships with plants that grow on heavy metal contaminated sites such as AM and influence plants to alleviate heavy metal toxicity [45][46]. The enhanced Cd tolerance of Paxillus involutus by Populus canescens [38] and Cu and Cd tolerance of Eucalyptus tereticornis by Pisolithus albus [47] were some of the evidences that EM helped for host heavy metal tolerance. Furthermore, the alleviated Cd toxicity of Pinus pinaster seedlings was found with the EM fungi Suillus bovinus and Suillus granulatus [48]. Inocybe curvipes enhanced the Pb and Zn tolerance of the Masson pine trees [49]. According to Krznaric et al. [50], pine trees that have an association with Suillus luteus possess the ability of heavy metal tolerance.
In contrast to AM, the symbiotic compatibility and stress tolerance of EM are species-specific to some extent, and therefore, knowing EM fungal community dynamics can lead to understanding the processes of forest ecosystems and help to facilitate the tools of bioindicators in different environmental stress conditions, including heavy metal toxicity due to contamination of soil [51]. Furthermore, the changes in EM fungal dynamics can therefore be correlated with altered tree responses to stress conditions, including heavy metal toxicity [52]. According to Milenge et al. [53], environmental stress factors could affect photosynthesis and, hence, reduce the sugar availability to EM, which might lead to changes in EM dynamics. Similarly, due to stress factors, the changes in the EM community might alter plant nutrient uptake and photosynthesis and affect plant performance [52]. As a consequence, both EM fungi and the host plant together can act as bioindicators.
3. The Role of Ectomycorrhizas in Heavy Metal Stress Tolerance of Host Plants
3.1. Heavy Metal Deposition in EM Fungi
Heavy metals are absorbed by EM fungi from soil solutions. Soil contamination by heavy metals occurs mainly due to natural phenomena and anthropogenic activities. Heavy metal deposition of Basidiomycetes varies depending on temperature, humidity, nature of metal, soil pH, substrate, mushroom species, and also ecosystem processes [54][55][56]. Due to the sensitivity of EM fungi to heavy metal contaminants, EM mushrooms can be used as an active and passive biomonitoring tool for metal deposition [57][58]. The estimations of biological effects caused by heavy metals are measured by monitoring the population dynamics of EM mushrooms, community variations, and morphological changes [59][60]. Reduced hyphal extension, morphological changes in mycelia, biomass reduction, and increased hyphal branching are major indications of declines of some EM species due to metal stress [61][62].
The mobility of the heavy metal changes due to the nature of metallic compounds since the availability of cations depends mainly on the anions. Ni, Co, Pb, and Cr have less mobility compared to Cd and Hg [9][63][64]. The ectomycorrhizal fungi Suillus granulatus, Lactarius deliciosus, Tuber melanosporium, and Tuber brumale showed higher Cu biosorption with surplus supplies of potassium (K) and three [65].
The EM mushrooms are capable of withholding heavy metals absorbed by soil solutions and, hence, protecting the host plants. This mechanism of the EM mushrooms relies mainly on the capacity of the fungus to continue proliferation through the substrate, producing new biomass in the presence of high metal concentrations [65][66][67][68]. The deposition levels of heavy metals in EM fungi have been extensively studied in different regions and contamination levels [64][69]. Lactarius deliciosus, Russula delica, and Russula albida from Canakkale, Turkey were found to have deposited the higher concentrations of Cd, Cu, and Pb [24][64][70][71], while Zn was deposited in Russula delica. A higher level of Cr was recorded in Lactarius deliciosus [64][70][71][72]. Russula albida was found to absorb higher Ni, Cr, Mn, and Zn concentrations, and it was in the range of 1–5 mg kg−1 [72].
Akin et al. [64] carried out two different experiments to compare the absorption of Cu and Zn by Baorangia bicolor in Çanakkale, Turkey. Baorangia bicolor was compared with Retiboletus fuscus, Russula delica, and Russula crustosa for Cu absorption and found that R. fuscus and R. delica absorbed more Cu compared to B. bicolor. A comparison of B. bicolor with R. crustosa and Lactarius representaneus for Zn absorption resulted in a higher absorption rate of R. crustosa and L. representaneus. Further, Imleria badia showed higher sensitivity to Pb by absorbing a concentration of 0.448 ± 0.03 mg kg−1 of biomass [64][70][71][73].
Crane et al. [15] revealed the response of EM fungi towards Hg concentration and showed that Amanita muscaria, Coccobotrys xylophilus, Laccaria laccata, Piloderma bicolour, Pisolithus arhizus, and Suillus decipiens are influenced by Hg at the immature stage of their growth, reducing the growth of the fruiting bodies. The changes in morphology depend on the concentrations of Hg and the exposure time. Among hyper-accumulating mycorrhizal mushrooms, such as Gomphidius glutinosus, Craterellus tubaeformis, and Laccaria amethystina, which are all associated with pines, G. glutinosus has been seen to absorb the most via the mycelium and concentrate radioactive cesium (Cs) more than 10,000-fold over ambient background levels [74].
Studies carried out in Slovakia, Turkey, and Northern Poland revealed that different EM fungi such as Boletus edulis and Paxillus involutus hold considerably higher concentrations of Hg, Pb, Cd, and Cu [15][75][76][77]. Tuzen et al. [78] and Dermirbas et al. [58] revealed that Tricholoma terreum is one of the EM fungi studied for retention of different metal ions. Furthermore, Yilmaz et al. [79] explained that T. terreum tolerates and holds a higher number of metal ions compared to other EM fungi, and his results explained that the highest contents of HM were represented by Fe (744), Zn (179), and Cu (51) mg kg−1. Durken et al. [80] demonstrated that Paxillus rubicundulus can hold Pb (0.69), Cd (0.78), Hg (0.21), Fe (37.0), Cu (51.0), Mn (10.8), Zn (16.8) mg kg−1 in a study conducted in Turkey.
It was reported from Northern Greece and Turkey that Boletus sp. and Hydnum repandum, Russula delica, Tricholoma terreum, Butyriboletus appendiculatus, Leccinum scabrum, Psilocybe coronilla, Tricholoma scalpturatum, and Suillus granulatus are a few edible EM fungi that withhold Pb, Cd, Hg, Cu, Mn, Zn, and Fe in higher quantities compared to other edible EM fungi [80][81][82][83][84].
3.2. Heavy Metal Deposition in Edible EM Mushrooms
The deposition of heavy metals in mushrooms is an important concern for edible mushrooms. Wild edible fungi are belonging to several trophic groups as saprotrophic or termite associated (growing in mutualistic relation with termites) or ectomycorrhizal [84][85]. Some EM genera, i.e., edible Amanita., Lactarius, Lactifluus, and Russula, are common in some forests. These edible EM fungi are harvested and consumed by people [85]. Boletus edulis (Bull.), Tricholoma matsutake (S. Ito and S. Imai), Lyophyllum shimeji, T. bakamatsutake Hongo, T. portentosum (Fr.), Rhizopogon roseolus (Corda) Th. Fr., Suillus grevillei (Klotzsch) Singer, Boletus edulis Bull., Amanita caesareoides Lj. N. Vassiljeva, Entoloma sepium (Noulet and Dass.) Richon and Roze, Cantharellus cibarius Fr., and Tuber indicum Cooke & Massee, Cantharellus cibarius, and Lactarius hatsudake are some of the examples of edible mycorrhizal mushrooms [84]. Heavy metal pollution causes detrimental effects on humans, other organisms, and the environment. Hence, it is important to study and investigate the metal content and accumulation in local wild mushrooms since they are a significant nutritional source in many countries [70].
Heavy metal contents of six edible EM mushroom species viz. Cyanoboletus pulverulentus, Cantharellus cibarius, Lactarius quietus, Russula xerampelina, and Suillus grevillea were estimated by Arvay et al. [86]. The highest mean concentrations of some metal elements were recorded in S. grevillei as 107, 104, 81.6, and 434 mg/kg (dried mass basis) for Zn, Cu, Mn, and Fe, respectively. Furthermore, the highest content of Co was found in L. quietus at 0.90 mg/kg (dm). Mleczek et al. [81] stated that toxic metals (Al, Cr, Hg, Ni, As, and Pb) amounts are higher in wild mushrooms compared to cultivated mushrooms.
References
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic Effects of Heavy Metals and Pesticides in Living Systems. Front. Chem. 2017, 5, 70.
- Gupta, A.; Jyotis, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as Potential Tool for Remediation of Heavy Metals: A Review. J. Microb. Biochem. Technol. 2016, 8, 364–372.
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M.S. Assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal(loid) contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 318–326.
- Grant, C.A.; Sheppard, S.C. Fertilizer Impacts on Cadmium Availability in Agricultural Soils and Crops. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 210–228.
- Szarlowicz, K.; Reczynski, W.; Misiak, R.; Kubica, B. Radionuclides and heavy metal concentrations as complementary tools for studying the impact of industrialization on the environment. J. Radioanal. Nucl. Chem. 2013, 298, 1323–1333.
- Yap, C.K.; Al-Mutairi, K.A. Ecological-Health Risk Assessments of Heavy Metals (Cu, Pb, and Zn) in Aquatic Sediments from the ASEAN-5 Emerging Developing Countries: A Review and Synthesis. Biology 2022, 11, 7.
- Caridi, F.; Testagrossa, B.; Acri, G. Elemental composition and natural radioactivity of refractory materials. Environ. Earth Sci. 2021, 80, 170.
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161.
- Alloway, B.J. Cadmium. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie and Son Ltd.: Glasgow, Scotland, 1990; pp. 100–124.
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49.
- Chandrajith, R.; Seneviratna, S.; Wickramaarachchi, K.; Attanayake, T.; Aturaliya, T.N.C.; Dissanayake, C.B. Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: Study of a chronic kidney disease area in Sri Lanka. Environ. Earth Sci. 2009, 60, 193–201.
- Jabeen, F.; Chaudhry, A.S. Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from Indus River in Pakistan. Environ. Monit. Assess. 2010, 166, 641–651.
- Aslam, M.; Verma, D.K.; Dhakerya, R.; Rais, S.; Alam, M.; Ansari, F.A. Bio-indicator: A comparative study on uptake and accumulation of heavy metals in some plant leaves. Res. J. Environ. Earth Sci. 2012, 4, 1060–1070.
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75.
- Crane, S.; Dighton, J.; Barkay, T. Growth responses to and accumulation of mercury by ectomycorrhizal fungi. Fungal Biol. 2010, 114, 873–880.
- Falandysz, J.; Frankowska, A.; Jarzyńska, G.; Dryżałowska, A. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J. Environ. Sci. Health Part B 2011, 46, 231–246.
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2012, 97, 477–501.
- Nowakowski, P.; Renata, M.; Jolanta, S.; Puścion-Jakubik, A.; Mielcarek, K.; Borawska, M.; Socha, K. Evaluation of toxic element content and health risk assessment of edible wild mushrooms. J. Food Compos. Anal. 2020, 96, 103698.
- Kuldeep, S.; Prodyut, B. Lichen as a Bio-indicator tool for assessment of climate and air pollution vulnerability: Review. Int. Res. J. Environ. Sci. 2015, 4, 107–117.
- Attaullah, M.; Nawaz, M.A.; Ilahi, I.; Ali, H.; Jan, T.; Khwaja, S.; Hazrat, A.; Ullah, I.; Ullah, Z.; Ullah, S.; et al. Honey as a bioindicator of environmental organochlorine insecticides contamination. Braz. J. Microbiol. 2021, 83, e250373.
- O’Connor, P.J.; Sally, E.S.; Smith, A.F. Arbuscular mycorrhizal associations in the southern Simpson Desert. Aust. J. Bot. 2001, 49, 493–499.
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304.
- Massicotte, H.B.; Melville, L.H.; Peterson, R.L. Scanning Electron Microscopy of Ectomycorrhizae Potential and Limitations. Scanning Microsc. 1987, 3, 58.
- Kalac, P.; Svoboda, L. Review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281.
- Vetter, J. Arsenic content of some edible mushroom species. Eur. Food Res. Technol. 2004, 219, 71–74.
- Svoboda, L.; Havlickova, B.; Kalač, P. Contents of cadmium, mercury and lead in edible mushrooms growing in a historical silver mining area. Food Chem. 2006, 96, 580–585.
- Amaranthus, M. The Importance and Conservation of Ectomycorrhizal Fungal Diversity in Forest Ecosystems; United States Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1998.
- Boroujeni, D.S.; Hemmatinezhad, B. Review of Application and Importance of Ectomycorrhiza Fungi and their Role in the Stability of Ecosystems. Biosci. Biotechnol. Res. Asia 2015, 12, 153–158.
- Policelli, N.; Horton, T.R.; Hudon, A.T.; Patterson, T.R.; Bhatnagar, J.M. Back to Roots: The Role of Ectomycorrhizal Fungi in Boreal and Temperate Forest Restoration. Front. For. Glob. Chang. 2020, 3, 97.
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal Fungi: Participation in Nutrient Turnover and Community Assembly Pattern in Forest Ecosystems. Forests 2020, 11, 453.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: London, UK, 1997; p. 605.
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic press: San Diego, CA, USA, 2010; p. 800.
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456.
- Martin, F.; Nehls, U. Harnessing ectomycorrhizal genomics for ecological insights. Curr. Opin. Plant Biol. 2009, 12, 508–515.
- Azul, A.M.; Sousa, J.P.; Agerer, R.; Martín, M.P.; Freitas, H. Land use practices and ectomycorrhizal fungal communities from oak woodlands dominated by Quercus suber L. considering drought scenarios. Mycorrhiza 2009, 20, 73–88.
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48.
- Ma, Y.; He, J.; Ma, C.; Luo, J. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus canescens. Plant Cell Environ. 2014, 37, 627–642.
- Huang, J.; Nara, K.; Lian, C.; Zong, K. Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana Lamb.) in Pb–Zn mine sites of central south China. Mycorrhiza 2012, 22, 589–602.
- Harrison, M.J. Signaling in the Arbuscular Mycorrhizal Symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42.
- Bradshaw, B. Salinity Tolerance of Selected Ectomycorrhizal Fungi (Pisolithus tinctorius Pers.) and Ectomycorrhizal Eucalypts. B.Sc. Thesis, Edith Cowan University, Perth, Australia, June 2000.
- Perrin, R.; Garbaye, J. Influence of ectomycorrhizae on infectivity of Pythium-infested soils and substrates. Plant Soil 1983, 71, 345–351.
- Jones, M.; Hutchinson, T.C. The effect of mycorrhizal infection on the response of Betula papyrifera to nickel and copper. New Phytol. 1986, 102, 429–442.
- Borchers, J.G.; Perry, D.A. The influence of soil texture and aggregation on carbon and nitrogen dynamics in southwest Oregon forests and clear cuts. Can. J. For. Res. 1992, 22, 298–305.
- Godbold, D.L.; Jentschke, G.; Winter, S.; Marschner, P. Ectomycorrhizas and amelioration of metal stress in forest trees. Chemosphere 1998, 36, 757–762.
- Jentschke, G.; Godbold, D.L. Metal toxicity and ectomycorrhizas. Physiol. Plant. 2000, 109, 107–116.
- Reddy, M.S.; Kour, M.; Aggarwal, S.; Ahuja, S. Metal induction of a Pisolithus albus metallothionein and its potential involvement in heavy metal tolerance during mycorrhizal symbiosis. Environ. Microbiol. 2016, 18, 2446–2454.
- Sousa, N.R.; Ramos, M.A.; Marques, A.P.; Castro, P.M. The effect of ectomycorrhizal fungi forming symbiosis with Pinus pinaster seedlings exposed to cadmium. Sci. Total Environ. 2012, 414, 63–67.
- Krznaric, E.; Verbruggen, N.; Wevers, J.H.L.; Carleer, R. Cd tolerant Suillus luteus: A fungal insurance for pines exposed to cadmium. Environ. Pollut. 2009, 157, 1581–1588.
- Kułdo, E.; Jarzyńska, G.; Gucia, M.; Falandysz, J. Mineral constituents of edible parasol mushroom Macrolepiota procera (Scop. ex Fr.) Sing and soils beneath its fruiting bodies collected from a rural forest area. Chem. Pap. 2014, 68, 484–492.
- Milenge, K.H.; Nshimba, S.M.H.; Masumbuko, N.C.; Nabahungu, N.L.; Degreef, J.; De Kesel, A. Host plants and edaphic factors influence the distribution and diversity of ectomycorrhizal fungal fruiting bodies within rainforests Tshopo, Democratic Republic of the Congo. Afr. J. Ecol. 2019, 57, 247–259.
- Leake, J.R. Is diversity of ECM fungi important for ecosystem function? New Phytol. 2001, 152, 1–8.
- Kernaghan, K. Mycorrhizal diversity: Cause and effect? Pedobiologia 2005, 49, 511–520.
- Seeger, R. Toxische schwermetalle in Pilzen. Dtsch. Apoth. Ztg. 1982, 122, 1835–1844.
- Svoboda, L.; Zimmermannová, K.; Kalač, P. Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci. Total Environ. 2000, 246, 61–67.
- Chen, Y.P.; Liu, Q.; Liu, Y.J.; Jia, F.A.; He, X.H. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci. Rep. 2014, 4, 4287.
- García, M.A.; Alonso, J.; Fernández, M.I.; Melgar, M.J. Lead Content in Edible Wild Mushrooms in Northwest Spain as Indicator of Environmental Contamination. Arch. Environ. Contam. Toxicol. 1998, 34, 330–335.
- Demirbaş, A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chem. 2001, 75, 453–457.
- Melgar, M.J.; Alonso, J.; Pérez-López, M.; Garcia, M.A. Influence of some factors in toxicity and accumulation of cadmium from edible wild macrofungi in NW Spain. J. Environ. Sci. Health Part B 1998, 33, 439–455.
- Falandysz, J.; Brzostowski, A.; Nosewicz, M.; Danisiewicz, D.; Frankowska, A.; Apanasewicz, D.; Bielawski, L. Mercury in edible mushrooms from the area of the Trojmiejski Landscape Park. Bromatol. Chem. Toksykol. 2000, 33, 177–182.
- Ruhling, A.; Baath, E.; Nordgren, A.; Soderstrom, B. Fungi in metal contaminated soil near the Gusum Brass Mill, Sweden. J. Hum. Environ. Stud. 1984, 13, 34–36.
- Fomina, M.; Alexander, I.; Colpaert, J.V.; Gadd, G. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 851–866.
- Kalač, P.; Svoboda, L.; Havlíčková, B. Contents of cadmium and mercury in edible mushrooms. J. Appl. Biomed. 2004, 2, 15–20.
- Akin, C.; Munevver, C.; Mahmut, C. The heavy metal content of wild edible mushroom samples collected in Canakkale Province, Turkey. Biol. Trace Elem. Res. 2010, 134, 212–219.
- Poitou, M.; Oliveier, J.M. Effect of copper on mycelium of three edible ectomycorrhizal fungi. Agric. Ecosyst. Environ. 1990, 28, 403–408.
- Colpaert, J.V.; Van, A.J.A. Zinc toxicity in ectomycorrhizal Pinus sylvestris. Plant Soil 1992, 143, 201–211.
- Denny, H.J.; Wilkins, D.A. Zinc tolerance in Betula spp. IV. The mechanism of ectomycorrhizal amelioration of zinc toxicity. New Phytol. 1987, 106, 545–553.
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322.
- Zhang, Q.; Huang, J.; Wang, F.; Xu, J. Mercury Distribution and Deposition in Glacier Snow over Western China. Environ. Sci. Technol. 2008, 46, 5404–5413.
- Işiloğlu, M.M.M.; Merdivan, M.; Yilmaz, F. Heavy Metal Contents in Some Macrofungi Collected in the Northwestern Part of Turkey. Arch. Environ. Contam. Toxicol. 2001, 41, 1–7.
- Zhang, D.; Gao, T.; Pei, M.A.; Ying, L.; Pengcheng, S.U. Bioaccumulation of Heavy Metal in Wild Growing Mushrooms from Liangshan Yi Nationality Autonomous Prefecture, China. Wuhan Univ. J. Nat. Sci. 2008, 13, 267–272.
- Zhu, X.; Song, F.; Liu, F. Arbuscular Mycorrhizal Fungi and Tolerance of Temperature Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017; pp. 163–194.
- Kalac, P.; Wittingerova, M.; Staskova, I. The contents of seven biogenic trace elements in edible mushrooms. Potravin. Vedy 1989, 7, 131–136.
- Michelot, D.; Siobud, E.; Dore, J.C.; Viel, C. Update on Metal Content Profiles in mushrooms-toxicological implycations and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012.
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Ten Speed Press, Penguin Random House LLC: New York, NY, USA, 2005.
- Kalač, P.; Burda, J.; Stašková, I. Concentrations of lead, cadmium, mercury and copper in mushrooms in the vicinity of a lead smelter. Sci. Total Environ. 1991, 105, 109–119.
- Kalac, P.; Niznaska, M.; Staskova, I. Concentration of Mercury, Copper Cadmium and Lead in Fruiting Bodies of Edible Mushrooms in the Vicinity of Mercury and Copper Smelter. Sci. Total Environ. 1996, 177, 251–258.
- Tüzen, M.; Özdemir, M.; Demirbaş, A. Study of heavy metals in some cultivated and uncultivated mushrooms of Turkish origin. Food Chem. 1998, 63, 247–251.
- Yilmaz, F.; Isiloglu, M.; Merdivan, M. 2003—Heavy metal levels in some macrofungi. Turk. J. Bot. 2003, 27, 45–56.
- Durkan, N.; Ugulu, I.; Unver, M.C.; Dogan, Y.; Baslar, S. Concentrations of trace elements aluminum, boron, cobalt and tin in various wild edible mushroom species from Buyuk Menderes River Basin of Turkey by ICP-OES. Trace Elem. Electrolytes 2011, 28, 242–248.
- Mleczek, M.; Siwulski, M.; Mikołajczak, P.; Gąsecka, M. Differences in Cu content in selected mushroom species growing in the same unpolluted areas in Poland. J. Environ. Sci. Health 2015, 50, 659–666.
- Ouzouni, P.; Riganakos, K. Nutritional value and metal content profile of Greek wild edible fungi. Acta Aliment. 2007, 36, 99–110.
- Soylak, M.; Saracoglu, S.; Tuzen, M.; Mendil, D. Determination of trace metals in mushroom samples from Kayseri, Turkey. Food Chem. 2005, 92, 649–652.
- Yamada, A. Utility of mycorrhizal mushrooms as food resources in Japan. J. Fac. Agric. Shinshu Univ. 2002, 1, 1–7.
- Kamalebo, H.M.; De Kesel, A. Wild edible ectomycorrhizal fungi: An underutilized food resource from the rainforests of Tshopo province (Democratic Republic of the Congo). J. Ethnobiol. Ethnomed. 2020, 16, 8.
- Arvay, J.; Tomáš, J.; Hauptvogl, M.; Massányi, P.; Harangozo, L.; Tóth, T.; Stanovič, R.; Bryndzová, S.; Bumbalová, M. Human exposure to heavy metals and possible public health risks via consumption of wild edible mushrooms from Slovak paradise national park, Slovakia. J. Environ. Sci. Health. 2015, 50, 833–843.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
17 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





