
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanna Giordano | -- | 2852 | 2022-05-13 09:27:37 | | | |
| 2 | Beatrix Zheng | + 11 word(s) | 2863 | 2022-05-13 10:01:29 | | |
Video Upload Options
Mesothelin (MSLN) is a glycoprotein located in the mesothelial lining of the body’s cavities and in many neoplasms. It is anchored to the cell membrane by a glycosylphosphatidylinositol linkage. The mesothelin gene was first cloned by Chang and Pastan, and it encodes a precursor protein that is processed to yield a 40 kDa mesothelin protein and a 31 kDa soluble fragment. The human soluble fragment, named the megakaryocyte-potentiating factor (MPF), has been reported to have megakaryocyte-potentiating activity in mouse bone marrow. In normal tissue, the physiological and biological functions of MSLN are still uncertain. Molecular biology studies demonstrated that a lack of MSLN in an MSLN knockout mouse model did not affect development, growth, or reproduction. Conversely, MSLN is considered to be involved in several mechanisms of cancer pathogenesis. In ovarian carcinomas, it has been demonstrated that the binding of MSLN with its partner MUC16 (CA125) may play a role in cell adhesion, facilitating intra-peritoneal ovarian cancer metastasis.
1. Mesothelin as a New Cancer Biomarker for the Diagnosis and Prognosis of Ovarian Carcinomas
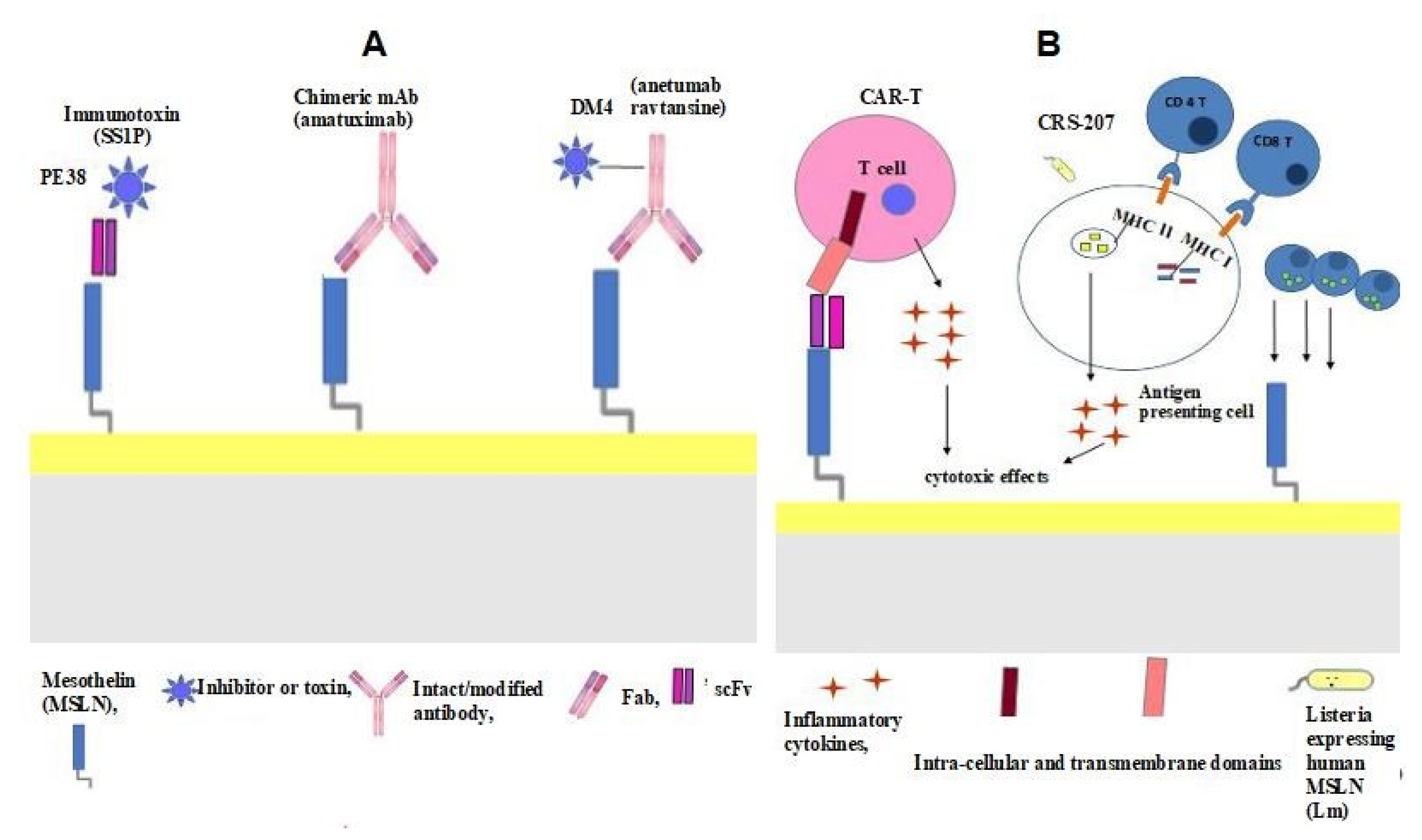
2. Mesothelin as a Therapeutic Target
| Clinical Trials gov Identifier | Agent | Phase | Status | Disease Setting | Recruiting Centers |
|---|---|---|---|---|---|
| NCT00066651 | SS1P | I | Completed | Advanced Cervical, ovarian Fallopian tube, pacreatic, peritoneal, lung, head and neck cancer | Unites States |
| NCT01521325 | MORAb-009 (Chimeric Anti-Mesothelin mAb) amatuximab |
I | Completed | Ovarian carcinoma, Mesothelioma, Pancreatic Cancer, Non Small Cell Lung | United States |
| NCT01413451 | MORAb-009 (Chimeric Anti-Mesothelin mAb) amatuximab |
Early Phase I | Terminated without efficacy in patienets with Ovarian Carcinoma | Ovarian carcinoma Mesothelioma, Pancreatic Cancer, Non Small Cell Lung cancer expressing mesothelin | United States |
| NCT01439152 | BAY-94 9343 (Anti-MesothelinAntibody Drug Conjugate) Anetumab ravtansine |
I | Completed | Invasive epithelial ovarian, primary serous peritoneal fallopian tube cancer | United States |
| NCT02751918 | BAY-94 9343 (Anti-MesothelinAntibody Drug Conjugate) Anetumab ravtansine + pegyleted liposomal doxorubicin |
Ib | Completed | Invasive or metastatic, predominantly epithelial platinum-resistant ovarian, fallopian tube, or primary serous peritoneal cancer | United States Belgium Moldova Spain |
| NCT03814447 | CAR-T-meso | Early Phase I | Recruitment | Refractory-Relapsed Ovarian Cancer | China |
| NCT03608618 | CAR-T-meso+ intraperitoneal MCY-M11 | Early Phase I | Recruitment | Advanced Ovarian cancer and mesthelioma | United States |
| NCT00585845 | CRS-207 | I | Terminated | Ovarian Carcinoma, Mesothelioma, Non small-cell Lung carcinoma, Pancratic carcinoma, who have failed or who are not candidates for standard treatments | United States |
| NCT02575807 | CRS-207 + alfaPD-1 + IDO1 inhibitor (Epacadostat) | I /II | Terminated low enrollment and lack of cinical activity | Platinum-resistant ovarian, fallopian or seous peritoneal cancer | United States |
2.1. Anti-Mesothelin Immunotoxin SS1P
SS1P comprises an anti-MSLN immunotoxin obtained from immunised mice and fused to a truncated form of Pseudomonas exotoxin A (PE38) (Figure 2A). SS1P binding to MSLN forms a complex that is internalised by endocytosis and PE, translocated in the cytosol, and kills the cells that catalyse protein synthesis, thereby initiating programmed cell death [14] (Figure 2A). In vitro studies have demonstrated the cytotoxic effect of SS1P on the neoplastic cells of patients affected by ovarian carcinomas [14]. In a phase I clinical trial (ClinicalTrials.gov identifier: NCT00066651), patients with ovarian carcinomas presented with stable disease.
2.2. MORAb-009 (Chimeric Anti-Mesothelin mAb)
2.3. Anti-Mesothelin Antibody–Drug Conjugate (BAY-94 9343)
2.4. Chimeric Antigen Receptor T cell (CAR T) Therapy
2.5. Vaccines
References
- Kakimoto, S.; Miyamoto, M.; Einama, T.; Matsuura, H.; Iwahashi, H.; Ishibashi, H.; Sakamoto, T.; Hada, T.; Takano, M. Co-Expression of Mesothelin and CA125 Is Associated with the Poor Prognosis of Endometrial Serous Carcinoma and Mixed Carcinomas Including Serous Carcinoma. Pathol. Oncol. Res. 2020, 26, 2299–2306.
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20.
- Casey, M.J.; Synder, C.; Bewtra, C.; Narod, S.A.; Watson, P.; Lynch, H.T. Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol. Oncol. 2005, 97, 457–467.
- Rebbeck, T.R.; Lynch, H.T.; Neuhausen, S.L.; Narod, S.A.; Van’t Veer, L.; Garber, J.E.; Evans, G.; Isaacs, C.; Daly, M.B.; Matloff, E.; et al. Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N. Engl. J. Med. 2002, 346, 1616–1622.
- Jiang, Y.; Zhao, J.; Zhang, L.; Tian, S.; Yang, T.; Wang, L.; Zhao, M.; Yang, Q.; Wang, Y.; Yang, X. Evaluation of the Efficacy and Safety of PARP Inhibitors in Advanced-Stage Epithelial Ovarian Cancer. Front. Oncol. 2020, 10, 954.
- Crum, C.P.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9.
- Patrono, M.G.; Corzo, C.; Iniesta, M.; Ramirez, P.T. Management of Preinvasive Lesions. Clin. Obstet. Gynecol. 2017, 60, 771–779.
- Lee, Y.; Medeiros, F.; Kindelberger, D.; Callahan, M.J.; Muto, M.G.; Crum, C.P. Advances in the recognition of tubal intraepithelial carcinoma: Applications to cancer screening and the pathogenesis of ovarian cancer. Adv. Anat. Pathol. 2006, 13, 1–7.
- Lamberts, L.E.; Menke-van der Houven van Oordt, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin. Cancer Res. 2016, 22, 1642–1652.
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261.
- Ruan, G.; Ye, L.; Liu, G.; An, J.; Sehouli, J.; Sun, P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: An updated systematic review and meta-analysis. Onco Targets Ther. 2018, 11, 521–528.
- Gourley, C.; Balmaña, J.; Ledermann, J.A.; Serra, V.; Dent, R.; Loibl, S.; Pujade-Lauraine, E.; Boulton, S.J. Moving from poly (ADP-Ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J. Clin. Oncol. 2019, 37, 2257–2269.
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565.
- Hassan, R.; Lerner, M.R.; Benbrook, D.; Lightfoot, S.A.; Brackett, D.J.; Wang, Q.C.; Pastan, I. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin. Cancer Res. 2002, 8, 3520–3526.
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.M.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319.
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.M.; Steinberg, S.M.; Fowler, D.H.; et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci. Transl. Med. 2013, 23, 208ra147.
- Hassan, R.; Ebel, W.; Routhier, E.L.; Patel, R.; Kline, J.B.; Zhang, J. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007, 7, 20.
- Hassan, R.; Schweizer, C.; Lu, K.F.; Schuler, B.; Remaley, A.T.; Weil, S.C. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: Implications for cancer therapy. Lung Cancer 2010, 68, 455–459.
- Hassan, R.; Cohen, S.J.; Phillips, M.; Pastan, I.; Sharon, E.; Kelly, R.J.; Schweizer, C.; Weil, S.; Laheru, D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin expressing cancers. Clin. Cancer Res. 2010, 16, 6132–6138.
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014, 20, 5927–5936.
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548.
- Chen, H.; Lin, Z.; Arnst, K.E.; Miller, D.D.; Li, W. Tubulin Inhibitor-Based Antibody-Drug Conjugates for Cancer Therapy. Molecules 2017, 22, 1281.
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20.
- Quanz, M.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Stelte-Ludwig, B.; Golfier, S.; Elbi, C.; Mumberg, D.; Ziegelbauer, K.; Schatz, C.A. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expression human ovarian cancer models. Oncotarget 2018, 9, 34103–34121.
- Hung, C.F.; Xu, X.; Li, L.; Ma, Y.; Jin, Q.; Viley, A.; Allen, C.; Natarajan, P.; Shivakumar, R.; Peshwa, M.V.; et al. Development of Anti-Human Mesothelin-Targeted Chimeric Antigen Receptor Messenger RNA-Transfected Peripheral Blood Lymphocytes for Ovarian Cancer Therapy. Hum. Gene Ther. 2018, 29, 614–625.
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014, 2, 112–120.
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009, 106, 3360–3365.
- Sandaltzopoulos, R.; Scholler, N.; Powell, D.J., Jr. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol. Ther. 2012, 20, 633–643.
- Banville, A.C.; Wouters, M.C.A.; Oberg, A.L.; Goergen, K.M.; Maurer, M.J.; Milne, K.; Ashkani, J.; Field, E.; Ghesquiere, C.; Jones, S.J.M.; et al. Co-expression patterns of chimeric antigen receptor (CAR)-T cell target antigens in primary and recurrent ovarian cancer. Gynecol. Oncol. 2021, 160, 520–529.
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, 22.
- Huang, R.Y.; Eppolito, C.; Lele, S.; Shrikant, P.; Matsuzaki, J.; Odunsi, K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015, 6, 27359–27377.
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880.
- Schoutrop, E.; El-Serafi, I.; Poiret, T.; Zhao, Y.; Gultekin, O.; He, R.; Moyano-Galceran, L.; Carlson, J.W.; Lehti, K.; Hassan, M.; et al. Mesothelin-Specific CAR T Cells Target Ovarian Cancer. Cancer Res. 2021, 81, 3022–3035.
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127.
- Tanyi, J.L.; Stashwick, C.; Plesa, G.; Morgan, M.A.; Porter, D.; Maus, M.V.; June, C.H. Possible Compartmental Cytokine Release Syndrome in a Patient With Recurrent Ovarian Cancer After Treatment With Mesothelin-targeted CAR-T Cells. J. Immunother. 2017, 40, 104–107.
- Hu, Y.; Sun, J.; Wu, Z.; Yu, J.; Cui, Q.; Pu, C.; Liang, B.; Luo, Y.; Shi, J.; Jin, A.; et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J. Hematol. Oncol. 2016, 9, 70.
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448.
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544.
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra25.
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46.
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies. Front. Immunol. 2021, 12, 642316.
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin. Cancer Res. 2012, 18, 858–868.
- Golub, S.H.; O’Connell, T.X.; Morton, D.L. Correlation of in vivo and in vitro assays of immunocompetence in cancer patients. Cancer Res. 1974, 34, 1833–1837.
- Flickinger, J.C., Jr.; Rodeck, U.; Snook, A.E. Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines 2018, 6, 48.




