
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Luiza Vilela Oliva | -- | 2671 | 2022-05-12 17:17:56 | | | |
| 2 | Amina Yu | -10 word(s) | 2661 | 2022-05-13 04:43:49 | | |
Video Upload Options
Plant Kunitz inhibitors generally exhibit highly conserved primary structures. These inhibitors usually have a reactive site located in the region that binds to the enzyme, and the formation of the enzyme–inhibitor complex occurs in 1:1 stoichiometry. Their reactive sites are frequently composed of Arg and Lys; they may occasionally contain Glu, Ala, or Val.
1. Plant Kunitz Inhibitors
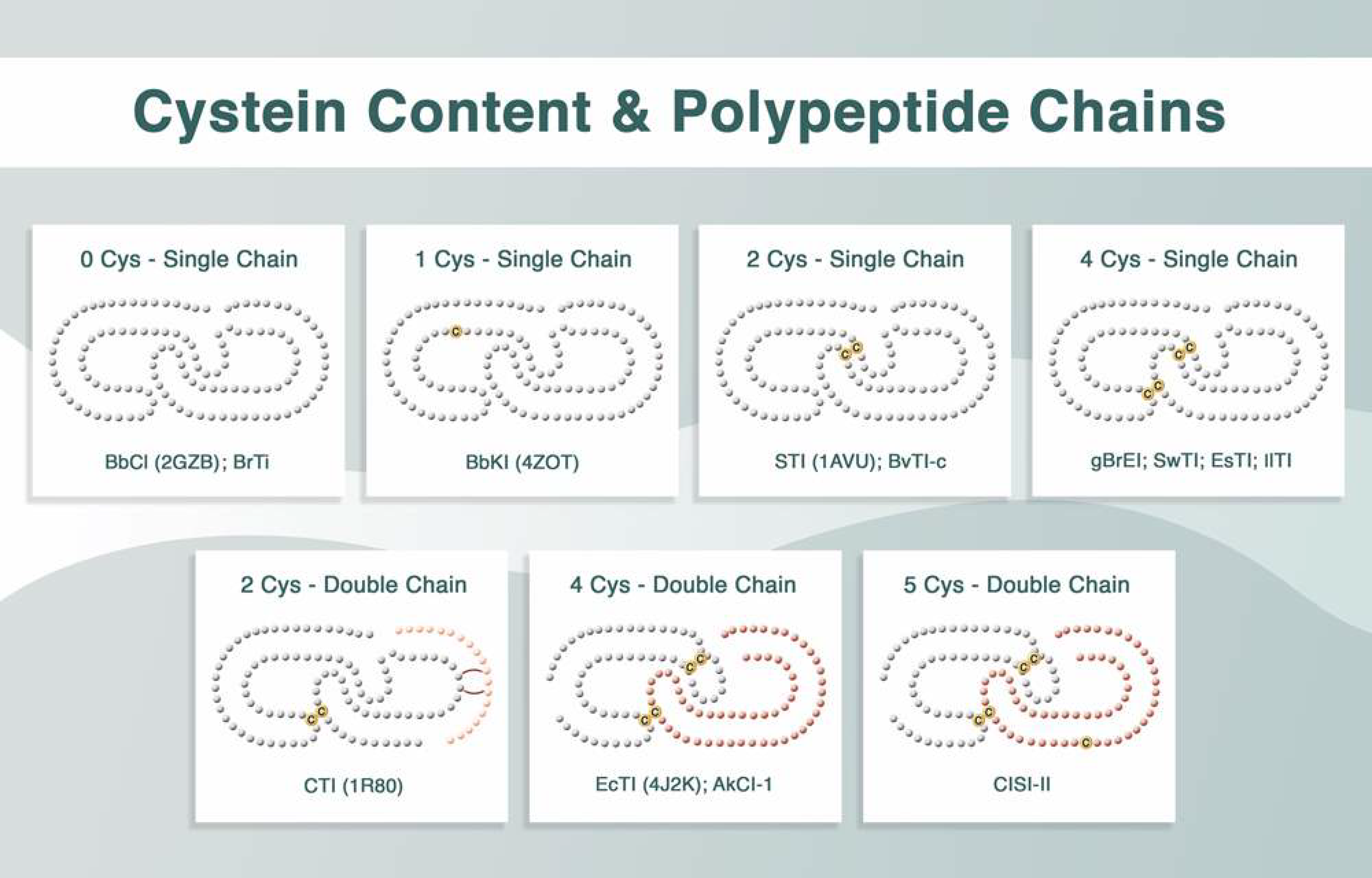
The same research group obtained rBbKIm based on the primary sequence of BbKI, but incorporated the RGD/RGE (Arg–Gly–Asp/Arg–Gly–Glu) motifs from BrTI. To this end, the mutations V21E, S24A, H25R, H27D, A28G, E127D, and Q130E were introduced into BbKI. The importance of these residues in the defense against predatory insects has been demonstrated [19]. This inhibitor also interfered with the viability of prostate cancer DU145 and PC3 cells, leading to cell death via the release of cytochrome c and caspase-3 activation [20].
2. Defense Proteins
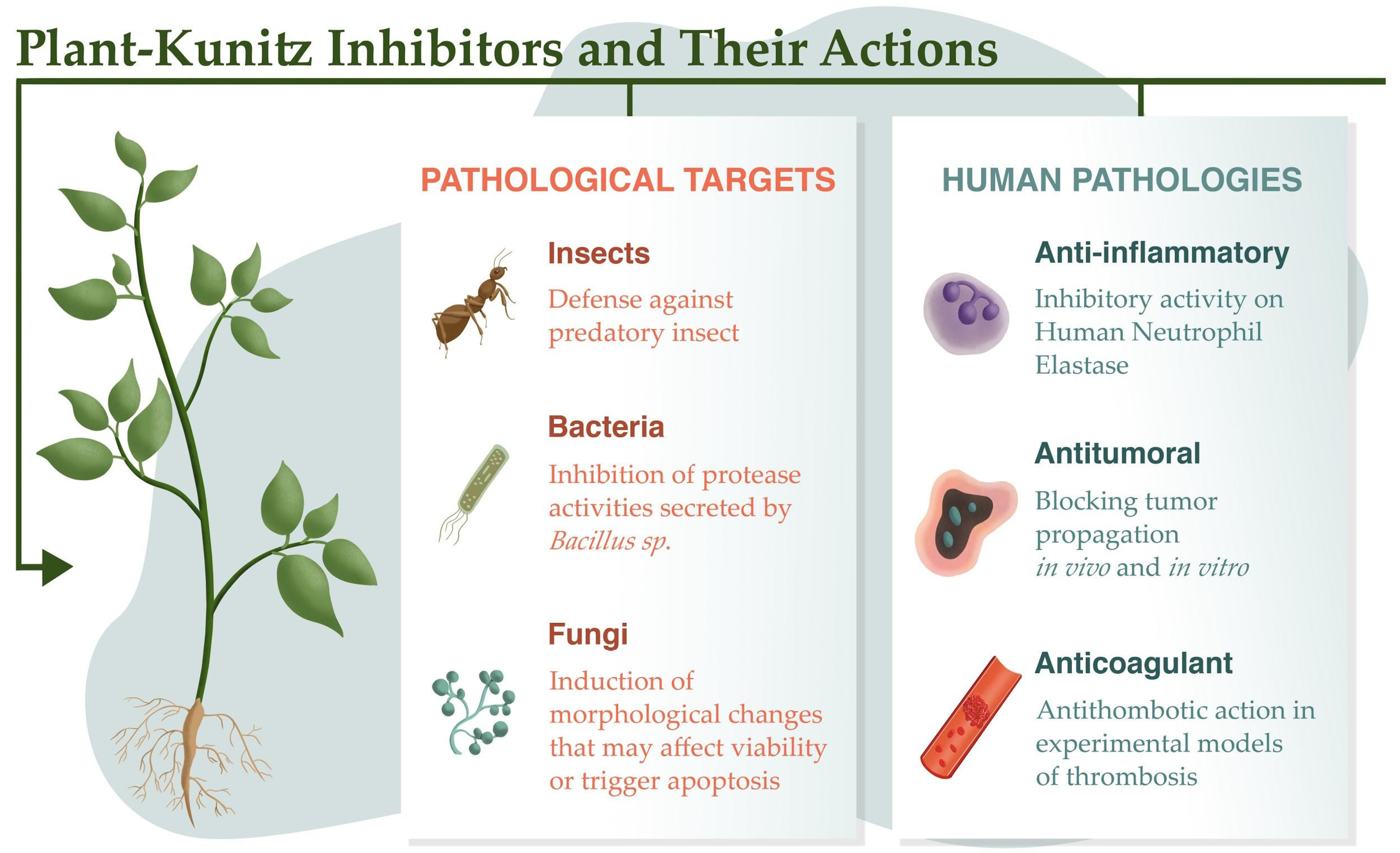
3. Antibacterial and Antifungal Activities
References
- Oliva, M.L.V.; Silva, M.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 2010, 92, 1667–1673.
- Kunitz, M. Crystallization of a Trypsin Inhibitor from Soybean. Science 1945, 101, 668–669.
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943.
- Hansen, D.; Macedo-Ribeiro, S.; Veríssimo, P.; Im, S.Y.; Sampaio, M.U.; Oliva, M.L.V. Crystal structure of a novel cysteinless plant Kunitz-type protease inhibitor. Biochem. Biophys. Res. Commun. 2007, 360, 735–740.
- Nakahata, A.M.; Bueno, N.R.; Rocha, H.A.; Franco, C.R.; Chammas, R.; Nakaie, C.R.; Jasiulionis, M.; Nader, H.B.; Santana, L.A.; Sampaio, M.U.; et al. Structural and inhibitory properties of a plant proteinase inhibitor containing the RGD motif. Int. J. Biol. Macromol. 2006, 40, 22–29.
- Zhou, D.; Hansen, D.; Shabalin, I.G.; Gustchina, A.; Vieira, D.F.; De Brito, M.V.; Araujo, A.P.U.; Oliva, M.L.; Wlodawer, A. Structure of BbKI, a disulfide-free plasma kallikrein inhibitor. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71 Pt 8, 1055–1062.
- Li, M.; Srp, J.; Gustchina, A.; Dauter, Z.; Mares, M.; Wlodawer, A. Crystal structures of the complex of a kallikrein inhibitor from Bauhinia bauhinioides with trypsin and modeling of kallikrein complexes. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 56–69.
- Sumikawa, J.T.; Nakahata, A.M.; Fritz, H.; Mentele, R.; Sampaio, M.U.; Oliva, M.L. A Kunitz-Type Glycosylated Elastase Inhibitor with One Disulfide Bridge. Planta Med. 2006, 72, 393–397.
- Cavalcanti, M.D.S.M.; Oliva, M.L.; Fritzc, H.; Jochumc, M.; Mentelec, R.; Sampaiob, M.; Coelho, L.C.; Batista, I.F.; Sampaio, C.A. Characterization of a Kunitz Trypsin Inhibitor with One Disulfide Bridge Purified from Swartzia pickellii. Biochem. Biophys. Res. Commun. 2002, 291, 635–639.
- Lingaraju, M.H.; Gowda, L.R. A Kunitz trypsin inhibitor of Entada scandens seeds: Another member with single disulfide bridge. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 850–855.
- Macedo, M.L.; Garcia, V.A.; Freire, M.D.; Richardson, M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry 2007, 68, 1104–1111.
- Krauchenco, S.; Nagem, R.A.; da Silva, J.A.; Marangoni, S.; Polikarpov, I. Three-dimensional structure of an unusual Kunitz (STI) type trypsin inhibitor from Copaifera langsdorffii. Biochimie 2004, 86, 167–172.
- Song, H.K.; Suh, S.W. Kunitz-type soybean trypsin inhibitor revisited: Refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998, 275, 347–363.
- Di Ciero, L.; Oliva, M.L.; Torquato, R.; Köhler, P.; Weder, J.K.; Novello, C.J.; Sampaio, C.A.; Oliveira, B.; Marangoni, S. The complete amino acid sequence of a trypsin inhibitor from Bauhinia variegata var. candida seeds. J. Protein Chem. 1998, 17, 827–834.
- Batista, I.F.; Nonato, M.C.; Bonfadini, M.R.; Beltramini, L.M.; Oliva, M.L.; Sampaio, M.U.; Sampaio, C.A.; Garratt, R.C. Preliminary crystallographic studies of EcTI, a serine proteinase inhibitor from Enterolobium contortisiliquum seeds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 602–604.
- Zhou, D.; Lobo, Y.; Batista, I.F.; Marques-Porto, R.; Gustchina, A.; Oliva, M.L.; Wlodawer, A. Crystal Structures of a Plant Trypsin Inhibitor from Enterolobium contortisiliquum (EcTI) and of Its Complex with Bovine Trypsin. PLoS ONE 2013, 8, e62252.
- Patthy, A.; Molnár, T.; Porrogi, P.; Naude, R.; Gráf, L. Isolation and characterization of a protease inhibitor from Acacia karroo with a common combining loop and overlapping binding sites for chymotrypsin and trypsin. Arch. Biochem. Biophys. 2015, 565, 9–16.
- Terada, S.; Katayama, H.; Noda, K.; Fujimura, S.; Kimoto, E. Amino Acid Sequences of Kunitz Family Subtilisin Inhibitors from Seeds of Canavalia lineata. J. Biochem. 1994, 115, 397–404.
- Sumikawa, J.T.; Brito, M.V.; Araújo, A.P.U.; Macedo, M.L.; Oliva, M.L.V.; Miranda, A. Action of Bauhinia-derivated compounds on Callosobruchus maculatus development. Adv. Exp. Med. Biol. 2009, 611, 563–564.
- Ferreira, J.G.; Diniz, P.M.M.; de Paula, C.A.A.; Lobo, Y.A.; Gamero, E.J.P.; Paschoalin, T.; Nogueira-Pedro, A.; Maza, P.K.; Toledo, M.S.; Suzuki, E.; et al. The Impaired Viability of Prostate Cancer Cell Lines by the Recombinant Plant Kallikrein Inhibitor. J. Biol. Chem. 2013, 288, 13641–13654.
- Yu, J.-H.; Li, Y.-Y.; Xiang, M.; Zhu, J.-Q.; Huang, X.-H.; Wang, W.-J.; Tan, R.; Zhou, J.-Y.; Liao, H. Molecular cloning and characterization of α-amylase/subtilisin inhibitor from rhizome of Ligusticum chuanxiong. Biotechnol. Lett. 2016, 39, 141–148.
- Liu, Z.; Zhu, Q.; Li, J.; Zhang, G.; Jiamahate, A.; Zhou, J.; Liao, H. Isolation, structure modeling and function characterization of a trypsin inhibitor from Cassia obtusifolia. Biotechnol. Lett. 2015, 37, 863–869.
- Cruz-Silva, I.; Gozzo, A.J.; Nunes, V.A.; Tanaka, A.S.; Araujo, M.D.S. Bioengineering of an elastase inhibitor from Caesalpinia echinata (Brazil wood) seeds. Phytochemistry 2021, 182, 112595.
- Liu, J.; Yonekura, M.; Kouzuma, Y. Purification, cDNA cloning and characterization of Kunitz-type protease inhibitors from Apios americana tubers. Biosci. Biotechnol. Biochem. 2020, 84, 563–574.
- Bunyatang, O.; Chirapongsatonkul, N.; Bangrak, P.; Henry, R.; Churngchow, N. Molecular cloning and characterization of a novel bi-functional α-amylase/subtilisin inhibitor from Hevea brasiliensis. Plant Physiol. Biochem. 2016, 101, 76–87.
- Zhu, J.; He, Y.; Yan, X.; Liu, L.; Guo, R.; Xia, X.; Cheng, D.; Mi, X.; Samarina, L.; Liu, S.; et al. Duplication and transcriptional divergence of three Kunitz protease inhibitor genes that modulate insect and pathogen defenses in tea plant (Camellia sinensis). Hortic. Res. 2019, 6, 126.
- Dias, L.P.; Oliveira, J.T.; Rocha-Bezerra, L.C.; Sousa, D.O.; da Costa, H.P.S.; Araujo, N.M.; Carvalho, A.F.; Tabosa, P.M.; Monteiro-Moreira, A.C.; Lobo, M.D.; et al. A trypsin inhibitor purified from Cassia leiandra seeds has insecticidal activity against Aedes aegypti. Process Biochem. 2017, 57, 228–238.
- Vajravijayan, S.; Pletnev, S.; Pletnev, V.Z.; Nandhagopal, N.; Gunasekaran, K. Crystal structure of a novel Kunitz type inhibitor, alocasin with anti-Aedes aegypti activity targeting midgut proteases. Pest Manag. Sci. 2018, 74, 2761–2772.
- Mendonça, E.G.; Barros, R.A.; Cordeiro, G.; Silva, C.R.; Campos, W.G.; Oliveira, J.A.; Oliveira, M.G.A. Larval development and proteolytic activity of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) exposed to different soybean protease inhibitors. Arch. Insect Biochem. Physiol. 2020, 103, e21637.
- De Oliveira, C.; Flores, T.M.D.O.; Cardoso, M.H.; Oshiro, K.G.N.; Russi, R.; de França, A.; Dos Santos, E.A.; Franco, O.L.; De Oliveira, A.S.; Migliolo, L. Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties. Molecules 2019, 24, 4344.
- Meriño-Cabrera, Y.; Mendes, T.A.D.O.; Castro, J.; Barbosa, S.L.; Macedo, M.; Oliveira, M.G.A. Noncompetitive tight-binding inhibition of Anticarsia gemmatalis trypsins by Adenanthera pavonina protease inhibitor affects larvae survival. Arch. Insect Biochem. Physiol. 2020, 104, e21687.
- Sasaki, D.Y.; Jacobowski, A.C.; de Souza, A.P.; Cardoso, M.H.; Franco, O.L.; Macedo, M.L.R. Effects of proteinase inhibitor from Adenanthera pavonina seeds on short- and long term larval development of Aedes aegypti. Biochimie 2015, 112, 172–186.
- Machado, S.W.; De Oliveira, C.; Zério, N.G.; Parra, J.; Macedo, M. Inga laurina trypsin inhibitor (ILTI) obstructs Spodoptera frugiperda trypsins expressed during adaptive mechanisms against plant protease inhibitors. Arch. Insect Biochem. Physiol. 2017, 95, e21393.
- Bezerra, C.S.; de Oliveira, C.F.R.; Machado, O.L.T.; de Mello, G.S.V.; Pitta, M.G.R.; Rêgo, M.J.B.M.; Napoleão, T.H.; Paiva, P.M.G.; Ribeiro, S.D.F.F.; Gomes, V.M.; et al. Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: A multifunctional Kunitz inhibitor. Process Biochem. 2016, 51, 792–803.
- Mohanraj, S.S.; Gujjarlapudi, M.; Lokya, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and characterization of Bowman-Birk and Kunitz isoinhibitors from the seeds of Rhynchosia sublobata (Schumach.) Meikle, a wild relative of pigeonpea. Phytochemistry 2019, 159, 159–171.
- Swathi, M.; Mishra, P.K.; Lokya, V.; Swaroop, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and Partial Characterization of Trypsin-Specific Proteinase Inhibitors from Pigeonpea Wild Relative Cajanus platycarpus L. (Fabaceae) Active against Gut Proteases of Lepidopteran Pest Helicoverpa armigera. Front. Physiol. 2016, 7, 388.
- Orona-Tamayo, D.; Wielsch, N.; Blanco-Labra, A.; Svatos, A.; Farías-Rodríguez, R.; Heil, M. Exclusive rewards in mutualisms: Ant proteases and plant protease inhibitors create a lock-key system to protect Acacia food bodies from exploitation. Mol. Ecol. 2013, 22, 4087–4100.
- Sanon, A.; Zakaria, I.; Clémentine, L.D.B.; Niango, B.M.; Honora, N.R.C. Potential of Botanicals to Control Callosobruchus maculatus (Col.: Chrysomelidae, Bruchinae), a Major Pest of Stored Cowpeas in Burkina Faso: A Review. Int. J. Insect. Sci. 2018, 10, 1–8.
- Mallqui, K.S.V.; Oliveira, E.E.; Guedes, R.N.C. Competition between the bean weevils Acanthoscelides obtectus and Zabrotes subfasciatus in common beans. J. Stored Prod. Res. 2013, 55, 32–35.
- Rufino, F.P.; Pedroso, V.M.; Araujo, J.N.; de França, A.F.; Rabelo, L.M.; Migliolo, L.; Kiyota, S.; Santos, E.A.; Franco, O.L.; Oliveira, A.S. Inhibitory effects of a Kunitz-type inhibitor from Pithecellobium dumosum (Benth) seeds against insect-pests’ digestive proteinases. Plant Physiol. Biochem. 2013, 63, 70–76.
- Rumpold, B.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–24.
- Brandão-Costa, R.; Araújo, V.F.; Porto, A. CgTI, a novel thermostable Kunitz trypsin-inhibitor purified from Cassia grandis seeds: Purification, characterization and termiticidal activity. Int. J. Biol. Macromol. 2018, 118 Pt B, 2296–2306.
- Ferreira, R.; Napoleão, T.H.; Silva-Lucca, R.A.; Silva, M.; Paiva, P.M.G.; Oliva, M.L.V. The effects of Enterolobium contortisiliquum serine protease inhibitor on the survival of the termite Nasutitermes corniger, and its use as affinity adsorbent to purify termite proteases. Pest Manag. Sci. 2019, 75, 632–638.
- Ferreira, R.S.; Brito, M.V.; Napoleão, T.H.; Silva, M.; Paiva, P.; Oliva, M. Effects of two protease inhibitors from Bauhinia bauhinoides with different specificity towards gut enzymes of Nasutitermes corniger and its survival. Chemosphere 2019, 222, 364–370.
- Sallai, R.C.; Salu, B.R.; Silva-Lucca, R.A.; Alves, F.L.; Napoleão, T.H.; Paiva, P.M.G.; Ferreira, R.D.S.; Sampaio, M.U.; Oliva, M.L.V. Biotechnological Potential of Araucaria angustifolia Pine Nuts Extract and the Cysteine Protease Inhibitor AaCI-2S. Plants 2020, 9, 1676.
- De Lacerda, J.T.J.G.; Lacerda, R.R.E.; Assunção, N.A.; Tashima, A.K.; Juliano, M.A.; dos Santos, G.A. New insights into lectin from Abelmoschus esculentus seeds as a Kunitz-type inhibitor and its toxic effects on Ceratitis capitata and root-knot nematodes Meloidogyne spp. Process Biochem. 2017, 63, 96–104.
- Müller, V.; Bonacci, G.; Batthyány, C.; Amé, M.V.; Carrari, F.; Gieco, J.; Asis, R. Peanut Seed Cultivars with Contrasting Resistance to Aspergillus parasiticus Colonization Display Differential Temporal Response of Protease Inhibitors. Phytopathology 2017, 107, 474–482.
- Tripathi, V.R.; Kumar, S.; Garg, S.K. A study on trypsin, Aspergillus flavus and Bacillus sp. protease inhibitory activity in Cassia tora (L.) syn Senna tora (L.) Roxb. seed extract. BMC Complement. Altern. Med. 2011, 11, 56.
- Barros, K.M.A.; Sardi, J.D.C.O.; Maria-Neto, S.; Macedo, A.J.; Ramalho, S.R.; de Oliveira, D.G.L.; Macedo, M.L.R. A new Kunitz trypsin inhibitor from Erythrina poeppigiana exhibits antimicrobial and antibiofilm properties against bacteria. Biomed. Pharmacother. 2021, 144, 112198.
- Araújo, N.M.; Dias, L.P.; Costa, H.P.; Sousa, D.O.; Vasconcelos, I.M.; de Morais, G.A.; Oliveira, J.T. ClTI, a Kunitz trypsin inhibitor purified from Cassia leiandra Benth. seeds, exerts a candidicidal effect on Candida albicans by inducing oxidative stress and necrosis. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 183032.
- Dib, H.X.; De Oliveira, D.G.L.; De Oliveira, C.F.R.; Taveira, G.B.; Mello, E.D.O.; Verbisck, N.V.; Chang, M.; Corrêa, D., Jr.; Gomes, V.M.; Macedo, M.L.R. Biochemical characterization of a Kunitz inhibitor from Inga edulis seeds with antifungal activity against Candida spp. Arch. Microbiol. 2018, 201, 223–233.
- De Oliveira, C.F.; Oliveira, C.T.; Taveira, G.B.; Mello, E.D.O.; Gomes, V.M.; Macedo, M.L.R. Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. Int. J. Biol. Macromol. 2018, 119, 645–653.
- Mehmood, S.; Imran, M.; Ali, A.; Munawar, A.; Khaliq, B.; Anwar, F.; Saaed, Q.; Buck, F.; Hussain, S.; Saeed, A.; et al. Model prediction of a Kunitz-type trypsin inhibitor protein from seeds of Acacia nilotica L. with strong antimicrobial and insecticidal activity. Turk. J. Biol. 2020, 44, 188–200.
- Cai, X.; Xie, X.; Fu, N.; Wang, S. Physico-Chemical and Antifungal Properties of a Trypsin Inhibitor from the Roots of Pseudostellaria heterophylla. Molecules 2018, 23, 2388.




