1. Plant Kunitz Inhibitors
Although widely distributed in nature, plant Kunitz inhibitors (I3) are especially abundant in the Fabaceae (Leguminosae) family, which comprises the Mimosoideae, Caesalpinioideae, and Faboideae/Papilionoideae subfamilies
[1][10]. The Soybean Kunitz trypsin inhibitor (SKTI) was the first member of this family to be identified
[2][12], and was reported by M. Kunitz; inhibitors subsequently discovered that share similar characteristics are therefore named Kunitz inhibitors. Generally, Kunitz inhibitors are defense proteins, which exhibit different activities as highlighted
in this review, including antibacterial and antifungal activities, and act on inflammation, coagulation, thrombosis, and cancer.
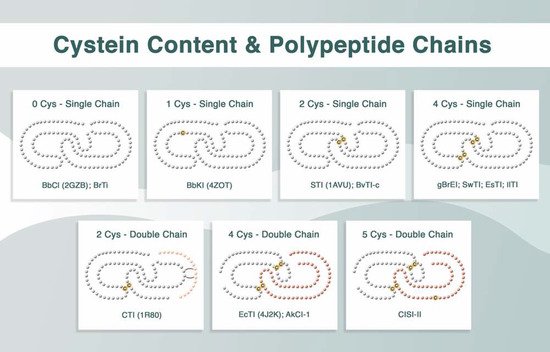
Plant Kunitz inhibitors generally exhibit highly conserved primary structures. These inhibitors usually have a reactive site located in the region that binds to the enzyme, and the formation of the enzyme–inhibitor complex occurs in 1:1 stoichiometry. Their reactive sites are frequently composed of Arg and Lys; however, they may occasionally contain Glu, Ala, or Val
[3][6]. These inhibitors can be single- or double-polypeptide-chain proteins, with variation in the number of cysteine residues and disulfide bridge patterns. The
Bauhinia bauhinioides cruzipain inhibitor (BbCI)
[4][13] and
Bauhinia rufa trypsin inhibitor (BrTI)
[5][14] are single-chain inhibitors lacking cysteine residues. The
B. bauhinioides kallikrein inhibitor (BbKI) is also a single-chain inhibitor; however, it contains one cysteine residue
[6][7][11,15]. The
B. rufa elastase inhibitor (gBrEI)
[8][16],
Swartzia pickellii trypsin inhibitor (SWTI)
[9][17],
Entada scandens trypsin inhibitor (ESTI)
[10][18], and
Inga laurina trypsin inhibitor (IlTI)
[11][19] are single-chain inhibitors with two cysteine residues. Although the
Copaifera langsdorffii inhibitor (ClI) also contains two cysteines, it is a double-chain inhibitor, with the chains linked through noncovalent bridges
[12][20]. The classic Kunitz-type inhibitors, soybean trypsin inhibitor (STI)
[13][21], and
Bauhinia variegata var. candida trypsin inhibitor (BvTI-C)
[14][22] are single-polypeptide-chain proteins that possess four cysteines. The
Enterolobium contortisiliquum trypsin inhibitor (EcTI)
[15][16][23,24] and
Acacia karroo trypsin inhibitor (AkTI)
[17][25] also contain four cysteines; however, they are double-polypeptide-chain proteins linked by two S–S bridges.
Canavalia lineata subtilisin inhibitor type II (CLSI-II) is a similar double-chain inhibitor linked by two S–S bridges, but contains five Cys residues, four of which are linked with bridges and one is a free cysteine
[18][26]. These profiles are summarized in
Figure 1.
Figure 1. Structural aspects of plant Kunitz inhibitors in terms of the number of cysteine residues (0, 1, 2, 4, or 5 Cys), S–S bridges (none, intrachain and/or interchain bridges), and polypeptide chain representation (single or double chains).
The same research group obtained rBbKIm based on the primary sequence of BbKI, but incorporated the RGD/RGE (Arg–Gly–Asp/Arg–Gly–Glu) motifs from BrTI. To this end, the mutations V21E, S24A, H25R, H27D, A28G, E127D, and Q130E were introduced into BbKI. The importance of these residues in the defense against predatory insects has been demonstrated [19][28]. This inhibitor also interfered with the viability of prostate cancer DU145 and PC3 cells, leading to cell death via the release of cytochrome c and caspase-3 activation [20][29].
Moreover, the
Ligusticum chuanxiong subtilisin/alpha-amylase inhibitor (LASI), expressed in
E. coli Rosetta and obtained from the rhizome of
L. chuanxiong (frequently used in Chinese medicine to treat headaches, cardiovascular diseases, rheumatic joint pain, menstrual disorders, and painful swelling), was also obtained as a recombinant protein. LASI inhibits mammalian alpha-amylase and bacterial subtilisin (which may be involved in the protection of plants against microorganisms) and also affects pest lepidopterans, which are all relevant for agricultural applications. Although LASI shares sequence similarities with other Kunitz protease inhibitors, such as
Cynara cardunculus (68%), it has a unique reactive site; consequently, LASI inhibits subtilisin but does not affect trypsin or chymotrypsin. Furthermore, LASI inhibits alpha-amylase from
Plutella xylostella,
Helicoverpa armigera, and
Spodoptera litura, thereby reducing the survival of these pests
[21][30].
The insecticidal properties of the
Cassia obtusifolia trypsin inhibitor (CoTI1) have also been described
[22][31]. CoTI1 was used to investigate stress tolerance and increase the resistance of crops against pests. It is a potent trypsin inhibitor (2.490 UI/mg activity) and also inhibits trypsin-like proteases from
H. armigera,
S. litura, and
S. exigua (1.770 UI/mg, 1.510 UI/mg, and 1.210 UI/mg, respectively). The amino acids L84, R86, and T88 in CoTI1 were identified, using in silico analysis, as being involved in trypsin inhibition, which was further confirmed by the loss of inhibitory activity (54%, 90%, and 53%, respectively) on mutating these residues to alanine.
Inhibition of human neutrophil elastase (HNE) is known to be beneficial in modulating inflammatory lung diseases. The
Caesalpinia echinata elastase inhibitor (CeEI), which inhibits HNE, has been used to study pulmonary inflammation
[23][32]. Native CeEI is a potent inhibitor of HNE (K
i 1.9 nM), cathepsin G (CatG, K
i 3.6 nM), and protease 3 (K
i 3.7 μM). Two recombinant isoinhibitors of CeEI, rCeEI-4, and rCeEI-5 were cloned and expressed in
E. coli. rCeEI-5 inhibited HNE 6.2 times more efficiently than CeEI, while rCeEI-4 exhibited 4.2-fold higher inhibition of chymotrypsin and 5.3-fold higher inhibition of HNE than CeCI. Synthetic, smaller functional peptides that inhibit HNE rCeEI-36 (K
i = 0.3 nM) and rCeEI-46 (K
i = 8.8 nM) were also obtained from the CeEI-4 sequence.
Apios americana, a legume tuber plant widely distributed across North America, is commonly used to make bread in Japan
[24][33]. This plant produces two Kunitz-type protease inhibitors,
Apios americana protease inhibitor 1 (AKPI-1) and
Apios americana protease inhibitor 2 (AKPI-2), which were cloned and expressed as recombinant proteins. rAKPI-2, characterized as chymotrypsin (K
i 6 µM) and trypsin inhibitor (K
i 0.4 µM), loses its property of trypsin inhibition when heated at 70 °C; however, the chymotrypsin inhibitory activity is maintained at 80% of its initial value at the same temperature.
Although most recombinant inhibitors are produced in bacterial hosts, it is also possible to do so with yeast, as achieved by Bunyatang et al.
[25][34]. The PKPI protease inhibitor, a bifunctional inhibitor that acts on protease and amylase, was cloned and transferred into
Pichia pastoris to produce a full-length protease inhibitor based on the
Hevea brasiliensis inhibitor (HbASI), which showed high activity against alpha-amylase (from
Aspergillus oryzae, IC
50 200 µg), subtilisin A (IC
50 380 µg), and trypsin (IC
50 325 µg), but no activity against chymotrypsin and human alpha-amylase. In addition, HbASI showed good stability over a wide temperature and pH range, and inhibited mycelial growth of
Phytophthora palmivora (0.2 µM), including 50% inhibition of zoospore .
2. Defense Proteins
Plants can detect events locally and transmit defense signals to build up systemic acquired resistance (SAR). A Kunitz protease inhibitor in the tea plant
Camellia sinensis (CsKPI1) was shown to be potentially involved in SAR based on its gene expression, which was strongly induced by insects through localized feeding, and in neighboring undamaged leaves. Jasmonic acid and CsKPI1 were detected simultaneously in damaged leaves; in addition, the jasmonic acid content also increased in neighboring leaves, suggesting its role as a vital hormonal signal that induces the expression of CsKPI1 in SAR
[26][67].
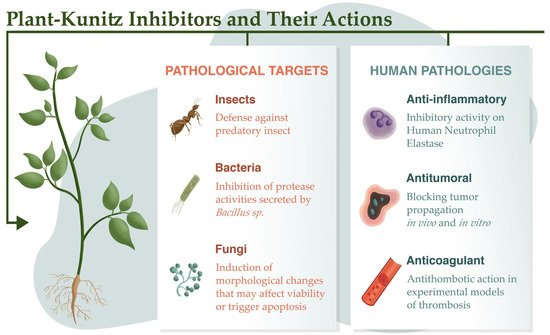
Based on their ability to function as defense proteins that protect against predator attacks, protease inhibitors have been used as insecticidal agents that act by inhibiting digestive proteases in insects, thereby controlling the availability of amino acids for the growth and development of larvae (
Figure 2). The Kunitz-type
Cassia leiandra trypsin inhibitor (ClTI) inhibits midgut digestive proteases (50% activity reduction at 4.65 µM) and larvae development (after 10 days at a final concentration of 15.4 µM), and delays adult emergence, in
Aedes aegypti, causing 44% mortality. These findings suggest that ClTI has the potential to reduce the
A. aegypti population without increasing the risk of developing insect resistance
[27][43]. Similarly, alocasin, purified from the rhizome of
Alocasia macrorrhiza, increased the mortality rate of
A. aegypti larvae and adult females (IC
50 = 0.17 mg/mL) by its action against the midgut enzymes of this insect. However, higher inhibitor concentrations lead to a reduction in the inhibitory effect, which may be attributed to the production of insect proteases insensitive to the inhibitor upon prolonged exposure
[28][38]. In another
study, proteolytic activity was restored on the 15th day after hatching of
Anticarsia gemmatalis larvae exposed to SKTI, after a decrease in the total proteolytic activity of trypsin and similar enzymes until the 12th day
[29][68]. On the other hand, the
Adenanthera pavonina Kunitz-type inhibitor (ApKTI) acts through a noncompetitive mechanism, thereby offering an advantage over such adaptation mechanisms as its affinity for trypsin is not affected by amino acid mutations in the enzyme active site, unlike other competitive protease inhibitors. Thus, ApKTI showed insecticidal activity even after 15 days of chronic exposure when included in the artificial diet of
Plodia interpunctella larvae
[30][36], and achieved a 60% reduction in the survival of neonatal
Anticarsia gemmatalis larvae
[31][37]. According to Sasaki et al.
[32][69],
A. aegypti larvae also do not use regulatory mechanisms to overcome the effects of ApKTI, and the inhibitor demonstrated degeneration of the microvilli of epithelial cells of the posterior midgut region, hypertrophy of the gastric cecal cells, and an augmented ectoperitrophic space in larvae. Interestingly, although trypsin and chymotrypsin-like serine proteases are involved in the initial protein digestion of Lepidoptera, ILTI (from
Inga laurina) inhibits trypsin in the midgut of
S. frugiperda more effectively compared to other protease inhibitors, such as SKTI and
Inga vera trypsin inhibitor (IVTI). Neither SKTI nor ILTI was active against chymotrypsin. Notably, ILTI was not degraded by enzymes in the midgut of insects, and was stable after elimination in feces, showing resistance to microbial metabolism
[33][59]. IVTI exerted midgut inhibition in
S. frugiperda,
C. cephalonica,
H. virescens, and
H. zea of 83%, 80%, 70%, and 64%, respectively
[34][60]. More recently, other inhibitors have been characterized, such as RsKI from
Rhynchosia sublobata, which is active against lepidopteran gut proteases, including those of
Achaea janata (IC
50 = 1.95 µg) and
Helicoverpa armigera (IC
50 = 59 µg)
[35][66], and the inhibitor CpPI 63, isolated from
Cajanus platycarpus seeds, a wild relative of pigeonpea
[36][70].
Figure 2. Uses of protease inhibitors as insecticides, and antibacterial and antifungal agents. The inhibitors are also active in mammal pathologies associated with inflammation, thrombosis, and tumorigenesis.
While protease inhibitors are important in defending against predators, they can also selectively assist in mutualistic relationships. For example, the ant
Pseudomyrmex ferrugineus uses
Acacia sp. hollow thorns as a nesting space, and while the
Acacia sp. provides food bodies and extrafloral nectar for ant larvae, the ant offers protection against herbivores that compete for vegetation. Thus, protease inhibitors extracted from
Acacia sp. food bodies are activated and convert the nutritive food reward into something difficult to digest for potential or optional exploiters, but not for mutualistic consumers, whose biochemistry takes advantage of this reward
[37][71].
Cowpeas are an important food source for millions of people worldwide. Many
studies have
been used
Callosobruchus maculatus [38][72] and
Zabrotes subfasciatus [39][73] as models to evaluate the effect of protease inhibitors because these beetles cause serious damage to the grain, making it unfit for human consumption. The
Pithecellobium dumosum Kunitz inhibitor (PdKI), purified from the seeds of the
P. dumosum tree, was evaluated against cysteine and serine proteases extracted from the larvae of coleopterans
C. maculatus and
Z. subfasciatus and the lepidopterans
Alabama argillacea and
Telchin licus. It completely inhibited bovine trypsin at 100 nM, with 50% inhibition against bovine chymotrypsin. PdKI showed specificity for serine proteases, especially against those from phytophagous insects, such as Coleoptera and Lepidoptera
[40][74].
Many factors are involved in the adaptability of insects to protease inhibitors, such as the alternative use of other classes of proteases, overexpression of proteases to minimize the inhibitory effect, or the degradation of the inhibitors by nontarget proteases, which are insensitive to the inhibitors. Thus, despite the potential applicability of protease inhibitors as effective compounds for the protection of crops against herbivory, most recent
onstudie
has beens have pointed to the importance of multitrophic approaches, taking into account not only target insect proteases, but also proteases from other organisms in the food chain, including the plant itself
[41][75].
Termites are one of the main wood-destroying pests that can invade and attack the wooden building structures in urban environments. While synthetic pesticides are traditionally used to prevent termite attacks, these compounds tend to harm humans and the environment. As an alternative, the trypsin inhibitor isolated from
Cassia grandis seeds (CgTI) can be employed, which exhibits termiticidal activity against
Nasutitermes corniger. However, the activity has been found to vary between termite castes, with workers (IC
50 = 0.685 mg/mL) being more resistant than soldiers (IC
50 = 0.765 mg/mL)
[42], which can be attributed to variation in enzymatic activities and digestive tracts between the castes. Similar to these results, EcTI was able to induce the death of worker termites, with an IC
50 of 0.242 mg/mL, whereas it did not exert significant inhibition in soldier termites. Notably, the molecule from the intestinal extract that binds to EcTI is a chitinase from
T. reesei, a symbiotic fungus present in the digestive tract of termites that is important in the cellulose degradation process. As this cellulose is exploited for the production of bioethanol, EcTI could be applied as a biotechnological tool in addition to being a natural active termiticide
[43][50].
In the same line of investigation, two distinct recombinant protease inhibitors of
B. bauhinioides origin, rBbKI (serine protease inhibitor) and rBbCI (cysteine protease inhibitor) were evaluated for their termiticidal activity. Despite sharing 82% structural similarity, the two inhibitors showed differing specificity for termite intestinal enzymes, and could be interesting tools for the characterization of
N. corniger enzymes. rBbKI showed termiticidal activity in workers, with an LC
50 of 0.9 mg/mL after four days, and did not affect the survival of soldiers, whereas rBbCI did not show any termiticidal activity on
N. corniger [44][64]. In another
study,
Araucaria angustifolia pine nut extract was shown to exhibit termiticidal activity against
N. corniger workers and soldiers at all tested concentrations
[45][76].
Finally, the bifunctional lectin and Kunitz inhibitor AEL, isolated from
Abelmoschus esculentus, was shown to exhibit activity against the fruit fly
Ceratitis capitata and the nematodes
Meloidogyne incognita and
Meloidogyne javanica, which infest plant roots. In the pupal stage of
M. incognita, 2 mg/mL AEL showed higher toxicity than organophosphates commonly used in agriculture, exemplifying the potential of protease inhibitors as promising insecticidal molecules
[46][35].
3. Antibacterial and Antifungal Activities
Fungi and bacteria release proteases into the media during infections, which facilitates their defense and survival. Inhibitors of these proteases can therefore hinder pathogen metabolism to improve human health
[47][77]. Plant inhibitors from the Kunitz family may be important in controlling fungal and bacterial growth, as demonstrated by the inhibition of proteases secreted by
Bacillus sp. (
Figure 2) and
Aspergillus flavus induced by extracts from seeds of
Cassia tora (L.)
syn Senna tora (L.)
Roxb [48][78]. In addition, the isolated protein fraction from
Erythrina poeppigiana interferes with the germination of
A. flavus spores
[49][79]. ClTI from
C. leiandra seeds inhibited the growth of
Candida albicans,
Candida tropicalis,
Candida parapsilosis, and
Candida krusei, with values of 5.13 μM, being most potent for
C. albicans, with an IC
50 of 2.10 μM
[50][80].
Some inhibitors show more restricted specificity, such as the
Inga edulis trypsin inhibitor (IeTI). Although it has no action against
C. albicans, it is a potent inhibitor of
C. tropicalis, as well as
Candida buinensis (87% inhibition with 400 μg/mL IeTI). The antifungal activity was found to be mediated by the alteration of the plasma membrane, which diverts energy metabolism from physiological functions to repair it, thereby inducing morphological changes that can affect cell viability or even trigger apoptosis through complexation with vital enzymes in this pathway (
Figure 2)
[51][58]. The same mechanism was described
in studies with
Enterolobium timbouva trypsin inhibitor (EtTI), suggesting that this inhibitor affects the integrity of the plasma membrane of yeasts, inducing the generation of intracellular ROS due to mitochondrial activation to maintain membrane potential, and consequently affecting the organelle structure. In addition, ROS generation is the main cause of DNA damage in yeast, and the mode of action of EtTI appears to involve the activation of apoptosis via a different pathway than traditional antifungal drugs
[52][53][56,57]. This mechanism is also similar to that used by
Pseudostellaria heterophylla trypsin inhibitor (PhTI), a 20.5 kDa thermostable inhibitor that has been reported to show potent activity against the pathogenic fungi
Candida gloeosporioides and
Fusarium oxysporum. The inhibitor also exhibits high stability, suggesting its potential for use as an antifungal protein in the food industry and agriculture
[54][63].