
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nthabiseng Nhlapo | -- | 1101 | 2022-05-12 14:41:22 | | | |
| 2 | Peter Tang | Meta information modification | 1101 | 2022-05-13 03:11:33 | | |
Video Upload Options
The features of the nanofibers (NFs) that are used to coat biomedical Ti-based implants are predominantly dependent on the type of polymer employed. Applicable polymers are categorized as either natural or synthetic based on their source and composition. Natural polymers, namely cellulose, collagen, gelatin, chitosan, chitin, dextrose and silk fibroin, have been electrospun into NF scaffolds.
1. Introduction
|
Polymer |
Properties |
Applications |
Degradation Rate |
Ref. |
|---|---|---|---|---|
|
Poly(ε-caprolactone) (PCL) |
Hydrophobic aliphatic polyester; slow degradation rate; bioactive; flexible mechanical properties; effectively entraps bactericidal material; semi-crystalline; semi-permeable |
Long-term implants; bone graft material; tissue engineering scaffolds; drug-delivery systems |
2–4 years |
|
|
Poly(lactic acid) (PLA) |
Hydrophobic aliphatic polyester; slow degradation rate; bioactive; tunable mechanical properties; crystalline; porous; stereoisomers: poly(L-lactide) (PLLA), poly(D-lactide) (PDLA), and poly(DL-lactide) (PDLLA) |
Biomedical coating; load-bearing applications; orthopedic fixation devices; tissue engineering; three-dimensional (3D) printed scaffolds; drug-delivery systems |
>24 months |
|
|
Poly(lactic-co-glycolic acid) (PLGA) |
Hydrophobic/hydrophilic balance; intermediate/adjustable degradation rate; PLA/PGA copolymer; crystalline; semi-permeable; low osteoinductivity |
Copolymer for development of bone substitute constructs; bone regeneration; orthopedic implants; tissue engineering |
6–12 months |
|
|
Poly(glycolic acid) (PGA) |
Hydrophilic aliphatic polyester; fast degradation rate; tunable material properties; crystalline; low solubility; semi-permeable |
Implants, tissue engineering; drug delivery; biological adhesives; open soft tissue wounds |
2–4 weeks |
|
|
Poly(ethylene oxide) (PEO) |
Hydrophilic; synthetic hydrogel |
Composite functional materials; hydrogel coatings; blood contact |
- |
|
|
Poly(vinyl alcohol) (PVA) |
Hydrophilic; fast degradation; gel-forming properties; good film-forming; good chemical resistance; semi-crystalline |
Implants; tissue engineering |
- |
2. Hybridization of Polymers
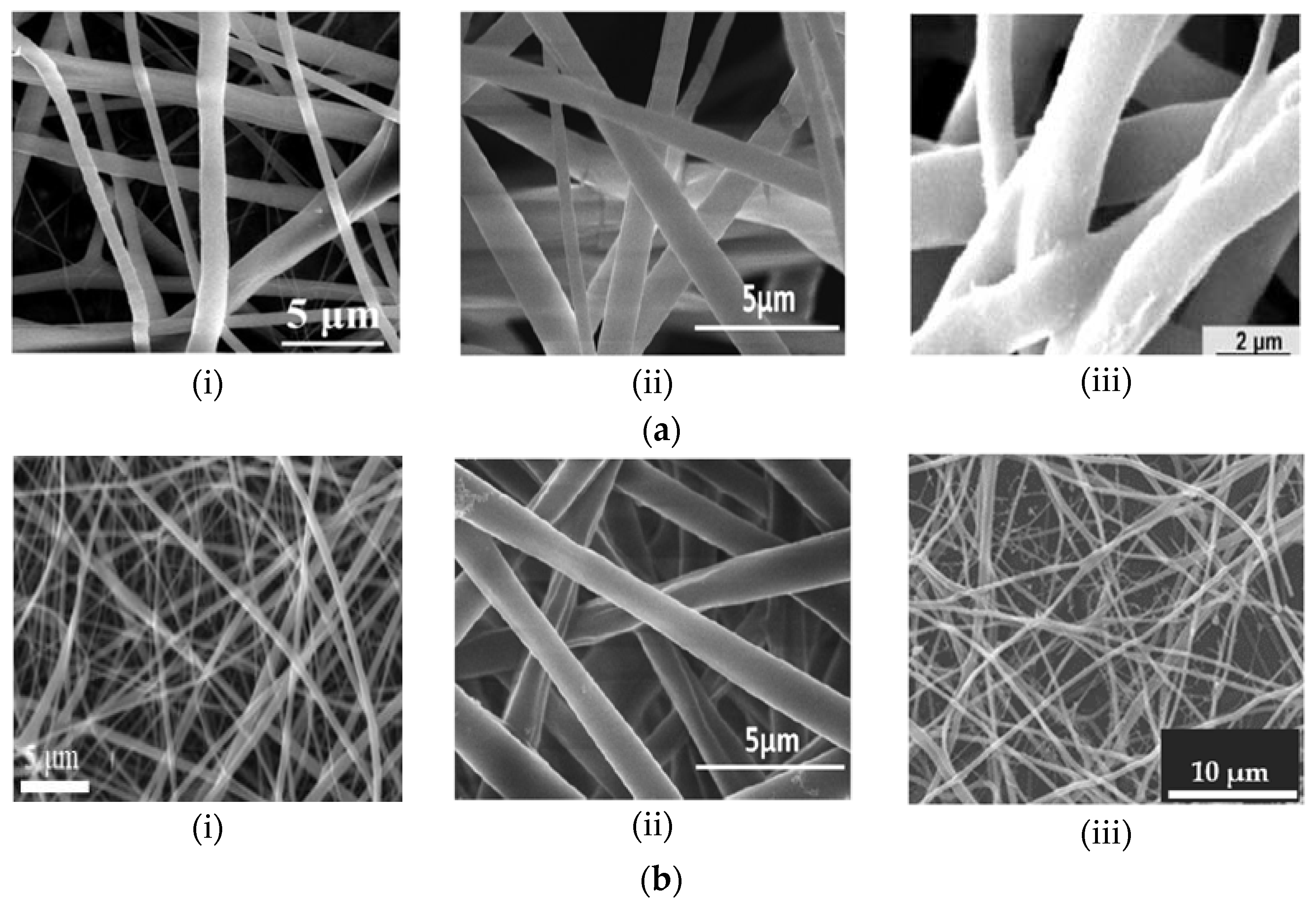
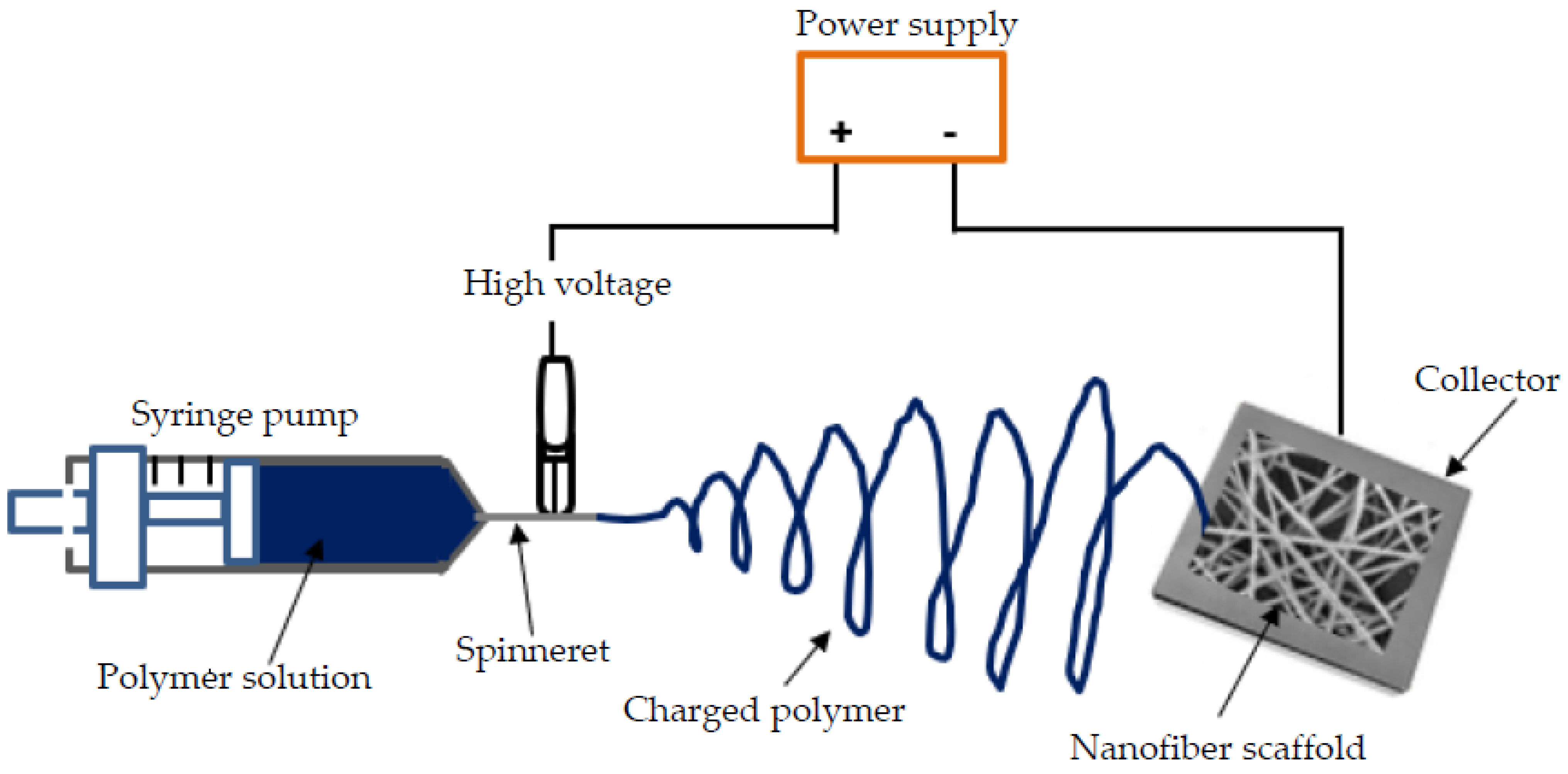
3. Electrospinning Technique
References
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Zhu, W.; Sakai, W.; Adachi, T.; Ohgitani, E.; Tsutsumi, N.; Mazda, O.; et al. Antibacterial and osteoconductive effects of chitosan/polyethylene oxide (PEO)/bioactive glass nanofibers for orthopedic applications. Appl. Sci. 2020, 10, 2360.
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659.
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145.
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative re-view of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105.
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959.
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36.
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of electrospun polymer-based nanofibers for biomedical applications: A perspective. Nanomaterials 2019, 9, 77.
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emergent Mater. 2019, 2, 169–179.
- Chong, S.-F.; Smith, A.A.A.; Zelikin, A.N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 2013, 9, 942–950.
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091.
- Montoro, S.R.; Medeiros, S.d.F.; Alves, G.M. Nanostructured hydrogel. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; Elsevier Inc.: Oxford, UK, 2014; pp. 325–355.
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Bragad, M.E.M.; et al. Po-rous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control Release 2019, 316, 331–348.
- Perumal, G.; Sivakumar, P.M.; Nandkumar, A.M.; Doble, M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110527.
- Chen, P. A Preliminary Discourse on Adhesion of Nanofibers Derived from Electrospun Polymers. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2013.
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176.
- Jeong, S.I.; Kim, B.-S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly(L-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946.
- Nathanael, A.J.; Oh, T.H. Biopolymer coatings for biomedical applications. Polymers 2020, 12, 3061.
- Ravichandran, R. Biomimetic Surface Modification of Dental Implant for Enhanced Osseointegration. Master’s Thesis, National University of Singapore, Singapore, 2009.
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. A 2008, 84, 291–299.
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid Polym. Sci. 2013, 291, 1541–1546.
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80.
- Tao, J. Effects of Molecular Weight and Solution Concentration on Electrospinning of PVA. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2003.
- Al Aboody, M.S. Electrospun fabrication and direct coating of bio-degradable fibrous composite on orthopedic titanium implant: Synthesis and characterizations. Mater. Res. Express 2021, 8, 015307.
- Jahanmard, F.; Croes, M.; Castilho, M.; Majed, A.; Steenbergen, M.J.; Lietaert, K.; Vogely, H.C.; van der Wal, B.C.H.; Stapel, D.A.C.; Malda, J.; et al. Bactericidal coating to prevent early and delayed implant-related infections. J. Control. Release 2020, 326, 38–52.
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577.
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C. Collagen/PCL nanofibers electrospun in green solvent by DOE assisted process. An in-sight into collagen contribution. Materials 2020, 13, 4698.
- Pham, Q.N.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211.
- Kiran, A.S.K.; Kumar, T.S.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocompo-site coatings. Nanomaterials 2018, 8, 860.
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745.
- Zhao, Y.; Qiu, Y.; Wang, H.; Chen, Y.; Jin, S.; Chen, S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int. J. Polym. Sci. 2016, 2016, 1–17.
- Ravichandran, R.; Ng, C.C.H.; Liao, S.; Pliszka, D.; Raghunath, M.; Ramakrishna, S.; Chan, C.K. Biomimetic surface modification of titanium surfaces for early cell capture by advanced electrospinning. Biomed. Mater. 2012, 7, 015001.
- Teo, W.-E. Electrospun Coated Metal Implants. Available online: http://electrospintech.com/coatedmetal.html (accessed on 14 January 2022).
- Lee, B.-Y.; Behler, K.; Kurtoglu, M.E.; Wynosky-Dolfi, M.A.; Rest, R.F.; Gogotsi, Y. Titanium dioxide-coated nanofibers for advanced filters. J. Nanoparticle Res. 2010, 12, 2511–2519.
- Abdal-hay, A.; Hwang, M.-G.; Lim, J.K. In vitro bioactivity of titanium implants coated with bicomponent hybrid biodegradable polymers. J. Sol-Gel Sci. Technol. 2012, 64, 756–764.
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Morris, T.; Wolf, R.; Vaughan, M. Effect of collagen-polycaprolactone nanofibers matrix coating on the In vitro cytocompatibility and in vivo bone responses of titanium. J. Med. Biol. Eng. 2018, 38, 197–210.
- Khandaker, M.; Riahinezhad, S.; Williams, W.R.; Wolf, R. Microgroove and collagen-poly(ε-caprolactone) nanofiber mesh coating improves the mechanical stability and osseointegration of titanium implants. Nanomaterials 2017, 7, 145.
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of nanofiber orientation on morphological and mechanical properties of electrospun chitosan mats. J. Healthc. Eng. 2018, 2018, 3651480.




