Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yeh-Han Wang | -- | 1125 | 2022-05-12 12:39:43 | | | |
| 2 | Camila Xu | Meta information modification | 1125 | 2022-05-13 03:46:56 | | | | |
| 3 | Camila Xu | + 23 word(s) | 1148 | 2022-05-20 08:44:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, Y.; Sung, M.; Li, C. Harmonization and Standardization of Panel-Based Tumor Mutational Burden. Encyclopedia. Available online: https://encyclopedia.pub/entry/22868 (accessed on 07 February 2026).
Wang Y, Sung M, Li C. Harmonization and Standardization of Panel-Based Tumor Mutational Burden. Encyclopedia. Available at: https://encyclopedia.pub/entry/22868. Accessed February 07, 2026.
Wang, Yeh-Han, Meng-Ta Sung, Chien-Feng Li. "Harmonization and Standardization of Panel-Based Tumor Mutational Burden" Encyclopedia, https://encyclopedia.pub/entry/22868 (accessed February 07, 2026).
Wang, Y., Sung, M., & Li, C. (2022, May 12). Harmonization and Standardization of Panel-Based Tumor Mutational Burden. In Encyclopedia. https://encyclopedia.pub/entry/22868
Wang, Yeh-Han, et al. "Harmonization and Standardization of Panel-Based Tumor Mutational Burden." Encyclopedia. Web. 12 May, 2022.
Copy Citation
Tumor mutational burden (TMB) refers to the total load of somatic mutations in tumor cells. It has been approved as a predictive biomarker for immune checkpoint inhibitors (ICIs), next-generation sequencing (NGS) TMB panels are being increasingly used clinically.
tumor mutational burden (TMB)
next-generation sequencing (NGS)
harmonization
1. Introduction
Along with the investigation of immune checkpoint inhibitors (ICIs), tumor mutational burden (TMB) has been developed to be a predictive biomarker in recent years. By definition, TMB refers to the total load of somatic mutations in tumor cells. As somatic mutations may cause specific tumor neoantigens, patients with a high TMB are likely to be responsive to immunotherapy [1][2][3][4]. A high TMB was first noted to be associated with the treatment response of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors in melanoma [5][6]. In the following years, TMB was employed in many clinical trials of anti-programmed cell death protein 1 (PD-1)/programmed cell death protein-ligand 1 (PD-L1) agents to treat various cancer types. Patients with a higher TMB tended to exhibit a better treatment response, but the testing methods and cutoffs of TMB varied across trials [3][7][8][9][10][11][12][13][14].
Several sequencing methods and multi-gene panels have been established to test TMB in academic, medical and diagnostic laboratories. Originally, the gold standard to calculate TMB was whole-exome sequencing (WES), where the total number of somatic mutations was calculated and reported. However, this is less feasible in most clinical settings because of its labor intensiveness and high cost, the lengthy turnaround time and the lack of computational or bioinformatics specialists. Panel-based TMB assays were thus developed by many laboratories and diagnostic companies. Generally, a TMB panel includes several hundreds of genes, and the somatic mutation load in tumors is estimated using specified bioinformatics algorithms. However, as the design varies across panels from gene selection to bioinformatics algorithms, no universal cutoff defines a high TMB status. The variation between TMB estimates can confuse clinicians and may hinder clinical decision making. Further harmonization and standardization are mandatory for the clinical implementation of panel-based TMB assays.
The standard is still unclear and sometimes confusing, although several TMB panels have been approved or cleared by the U.S. Food and Drug Administration (FDA). In the KEYNOTE-158 study, TMB was defined as a predictive biomarker, and a cutoff of 10 mutations per megabase (muts/Mb) to define a high TMB was proposed and further approved by the FDA as a tumor agnostic indication for the prescription of pembrolizumab [9][15]. A commercial laboratory-based panel, FoundationOne (F1) CDx (Foundation Medicine Inc.), was simultaneously approved as the companion diagnostic test for this indication. In addition, the Memorial Sloan Kettering Cancer Center (MSKCC) also developed an in-house cancer genomic profiling assay, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), which was cleared through the FDA 510(k) review in 2017 [16][17]. In the following years, several other next-generation sequencing (NGS) panels also obtained approval from the FDA. However, it is difficult to harmonize the inter-panel variation between NGS panels, regardless of whether they have regulatory authorization or clinical validation data from trials.
2. Harmonization and Standardization of Panel-Based TMB
2.1. Preanalytic Factors (Sample and DNA Issues)
Several preanalytic factors may influence the TMB estimation, primarily associated with DNA quality. Poor DNA quality, whether resulting from specimen handling, processing or archiving, is known to cause more false-positive calls of somatic mutations, which often feature a low allele frequency [18]. These false-positive mutations inappropriately increase the TMB estimates. Quy et al. noted that patients with high TMB estimates from specimens with a low DNA library concentration show no to less treatment benefits from anti-PD-L1 antibodies compared to the high concentration group [19]. While a low library concentration reflects poor DNA quality, the high TMB status determined by testing such specimens was more likely to be misclassified. In this research, significant increases in false-positive variant calls were also observed in formalin-fixed paraffin-embedded (FFPE) tissue specimens compared to frozen-fixed fresh tissue, a phenomenon that suggests that formalin potentially damages DNA tissue, especially with improper fixation [20]. Formalin-fixation-induced deamination is one of the preanalytic factors that leads to the overestimation of TMB.
Tumor fraction and input DNA quantity both affect the sensitivity of TMB testing, though their impacts vary in degrees between cancer types and panels. Some cancer types are known to have a higher TMB, such as NSCLC and melanoma, while others mostly express a low TMB. Generally, a low tumor fraction may cause missed calls of tumor somatic mutation and, thus, underestimation of TMB. However, panel-specific bioinformatics algorithms may partially compensate for these effects, and an acceptance criterion of tumor fraction has been verified in some TMB panels. The effects of a low tumor fraction and DNA input can be minimized through routine measures of laboratory quality assurance/quality control (QA/QC).
2.2. Sequencing Factors
Gene coverage and panel size are key factors affecting the performance of a TMB panel. Genes selected in TMB panels, together with their bioinformatics algorithm, primarily determine the accuracy and variability in TMB estimates [21]. As the genes selected in a panel are mostly cancer-related, their distribution and coverage in the genome are not randomly or evenly distributed. In addition, the prevalence of cancer gene mutations is also different across cancer types. Therefore, gene selection may cause internal bias for panel-based TMB estimates, which requires further calibration by the bioinformatics algorithm. Generally, the combination of gene selection and bioinformatics has been optimized in most laboratories and assays, and it has demonstrated comparability with WES TMB. However, the phase II FoCR harmonization study observed a tendency toward the overestimation of TMB when known pathogenic cancer genes were not excluded from the estimation [22]. Panel size was also critical to determine the variability in a TMB assay. Several studies indicate that smaller panels (<1 Mb) exhibit significant variability when correlating with WES TMB calculation [23][24][25][26].
2.3. Bioinformatics Factors
The initial clinical investigation of TMB was to calculate the sum of somatic mutations detected by WES. Several calculation strategies based on the inclusion types of mutations have been developed in different studies. Although attempts to include all somatic mutation types (e.g., synonymous, non-synonymous single-nucleotide variations (SNVs) and small insertions and deletions (indel)) for TMB calculations have been reported in several studies [14][27][28][29][30], the calculation that includes missense (non-synonymous) mutations only has been the mainstay approach for WES TMB [31]. Chang et al. investigated the impacts of the two calculation strategies above on the TMB value using CheckMate 026 data and found a perfect correlation between “all mutation types” and “missense mutation only” (Spearman’s rho = 0.99), but the former calculation exhibited 3.1-fold higher TMB values compared to the latter (median TMB: 540 muts for all mutation types while 170 muts for missense mutation only) [31]. As TMB panels only detect hundreds of genes (approximately accounting for 0.5–2 megabase of the genome across panels), various computational approaches have been optimized across laboratories or commercialized panels for a better correlation between panel-based TMB and WES TMB [26].
The core steps of the panel-based TMB calculation are shown in Figure 1.
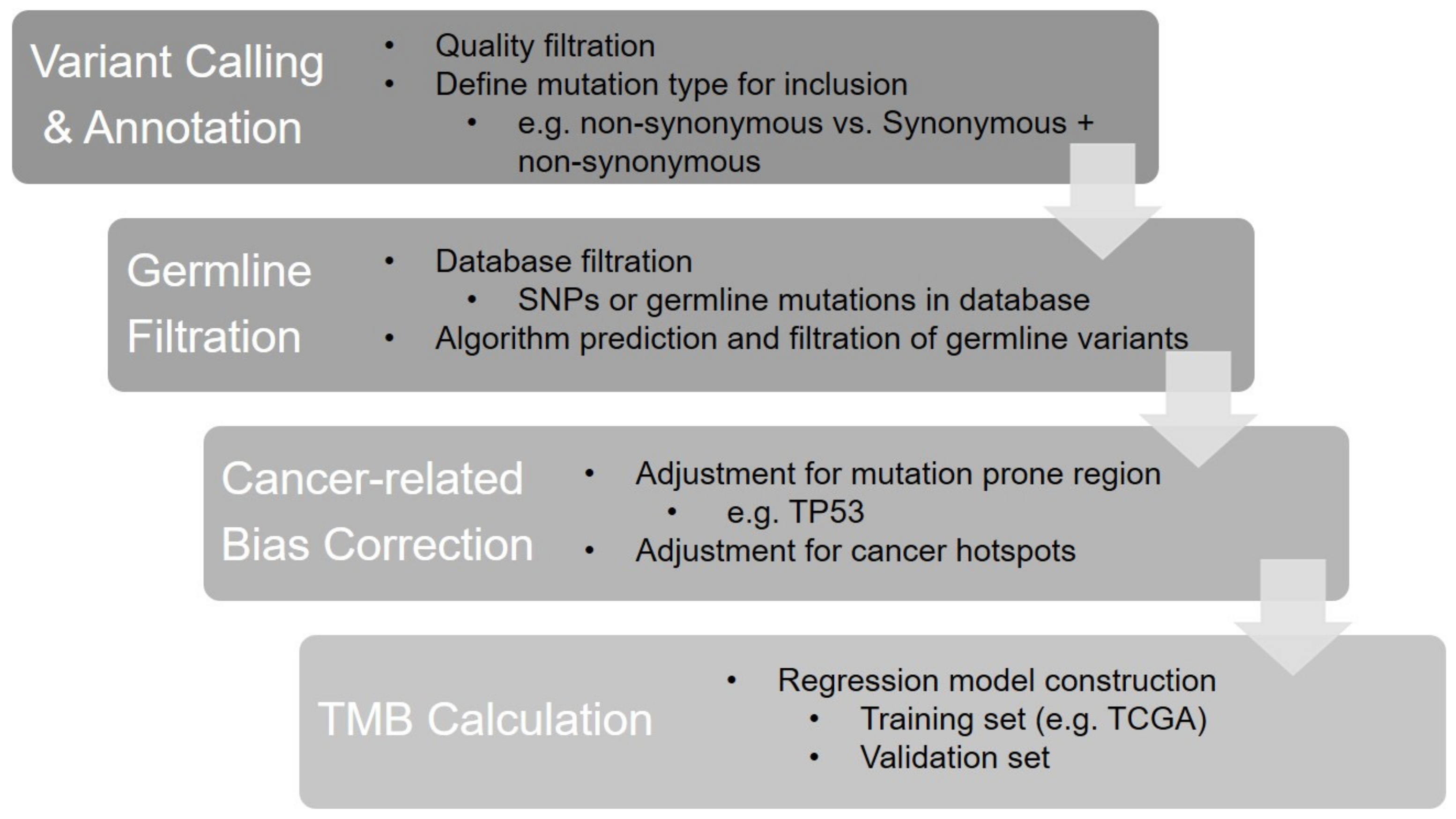 Figure 1. The bioinformatics algorithm of panel-based TMB calculation.
Figure 1. The bioinformatics algorithm of panel-based TMB calculation.References
- Tran, E.; Ahmadzadeh, M.; Lu, Y.C.; Gros, A.; Turcotte, S.; Robbins, P.F.; Gartner, J.J.; Zheng, Z.; Li, Y.F.; Ray, S.; et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015, 350, 1387–1390.
- Lee, C.H.; Yelensky, R.; Jooss, K.; Chan, T.A. Update on Tumor Neoantigens and Their Utility: Why It Is Good to Be Different. Trends Immunol. 2018, 39, 536–548.
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD–1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128.
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608.
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA–4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199.
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA–4 blockade in metastatic melanoma. Science 2015, 350, 207–211.
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier–Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104.
- Carbone, D.P.; Reck, M.; Paz–Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First–Line Nivolumab in Stage IV or Recurrent Non–Small–Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426.
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira–Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez–Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open–label, phase 2 KEYNOTE–158 study. Lancet Oncol. 2020, 21, 1353–1365.
- Powles, T.; Duran, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum–treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open–label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757.
- Rosenberg, J.E.; Hoffman–Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum–based chemotherapy: A single–arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920.
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman–Censits, J.; Perez–Gracia, J.L.; et al. Atezolizumab as first–line treatment in cisplatin–ineligible patients with locally advanced and metastatic urothelial carcinoma: A single–arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76.
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small–Cell Lung Cancer. Cancer Cell 2018, 33, 853–861.e4.
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; Al Bakir, M.; et al. Insertion–and–deletion–derived tumour–specific neoantigens and the immunogenic phenotype: A pan–cancer analysis. Lancet Oncol. 2017, 18, 1009–1021.
- Marcus, L.; Fashoyin–Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden–High Solid Tumors. Clin. Can. Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4685–4689.
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK–IMPACT): A Hybridization Capture–Based Next–Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. JMD 2015, 17, 251–264.
- Jibiki, T.; Nishimura, H.; Sengoku, S.; Kodama, K. Regulations, Open Data and Healthcare Innovation: A Case of MSK–IMPACT and Its Implications for Better Cancer Care. Cancers 2021, 13, 3448.
- Bettoni, F.; Koyama, F.C.; de Avelar Carpinetti, P.; Galante, P.A.F.; Camargo, A.A.; Asprino, P.F. A straightforward assay to evaluate DNA integrity and optimize next–generation sequencing for clinical diagnosis in oncology. Exp. Mol. Pathol. 2017, 103, 294–299.
- Quy, P.N.; Kanai, M.; Fukuyama, K.; Kou, T.; Kondo, T.; Yamamoto, Y.; Matsubara, J.; Hiroshima, A.; Mochizuki, H.; Sakuma, T.; et al. Association Between Preanalytical Factors and Tumor Mutational Burden Estimated by Next–Generation Sequencing–Based Multiplex Gene Panel Assay. Oncologist 2019, 24, e1401–e1408.
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002, 161, 1961–1971.
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713.
- Vega, D.M.; Yee, L.M.; McShane, L.M.; Williams, P.M.; Chen, L.; Vilimas, T.; Fabrizio, D.; Funari, V.; Newberg, J.; Bruce, L.K.; et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: Phase II of the Friends of Cancer Research TMB Harmonization Project. Ann. Oncol Off. J. Eur. Soc. Med. Oncol. ESMO 2021, 32, 1626–1636.
- Buchhalter, I.; Rempel, E.; Endris, V.; Allgauer, M.; Neumann, O.; Volckmar, A.L.; Kirchner, M.; Leichsenring, J.; Lier, A.; von Winterfeld, M.; et al. Size matters: Dissecting key parameters for panel–based tumor mutational burden analysis. Int. J. Cancer. 2019, 144, 848–858.
- Budczies, J.; Allgauer, M.; Litchfield, K.; Rempel, E.; Christopoulos, P.; Kazdal, D.; Endris, V.; Thomas, M.; Frohling, S.; Peters, S.; et al. Optimizing panel–based tumor mutational burden (TMB) measurement. Ann. Oncol Off. J. Eur. Soc. Med. Oncol. ESMO 2019, 30, 1496–1506.
- Allgauer, M.; Budczies, J.; Christopoulos, P.; Endris, V.; Lier, A.; Rempel, E.; Volckmar, A.L.; Kirchner, M.; von Winterfeld, M.; Leichsenring, J.; et al. Implementing tumor mutational burden (TMB) analysis in routine diagnostics–a primer for molecular pathologists and clinicians. Transl. Lung Cancer Res. 2018, 7, 703–715.
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825.
- Cancer Genome Atlas, N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337.
- Germano, G.; Lamba, S.; Rospo, G.; Barault, L.; Magri, A.; Maione, F.; Russo, M.; Crisafulli, G.; Bartolini, A.; Lerda, G.; et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017, 552, 116–120.
- Rospo, G.; Lorenzato, A.; Amirouchene–Angelozzi, N.; Magri, A.; Cancelliere, C.; Corti, G.; Negrino, C.; Amodio, V.; Montone, M.; Bartolini, A.; et al. Evolving neoantigen profiles in colorectal cancers with DNA repair defects. Genome Med. 2019, 11, 42.
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore–Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e7.
- Chang, H.; Sasson, A.; Srinivasan, S.; Golhar, R.; Greenawalt, D.M.; Geese, W.J.; Green, G.; Zerba, K.; Kirov, S.; Szustakowski, J. Bioinformatic Methods and Bridging of Assay Results for Reliable Tumor Mutational Burden Assessment in Non–Small–Cell Lung Cancer. Mol. Diagn. Ther. 2019, 23, 507–520.
More
Information
Subjects:
Medical Laboratory Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
717
Revisions:
3 times
(View History)
Update Date:
20 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




