
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Casado | -- | 3552 | 2022-05-11 10:08:37 | | | |
| 2 | Conner Chen | -4 word(s) | 3548 | 2022-05-12 03:20:09 | | | | |
| 3 | Conner Chen | -1 word(s) | 3547 | 2022-05-12 03:51:13 | | |
Video Upload Options
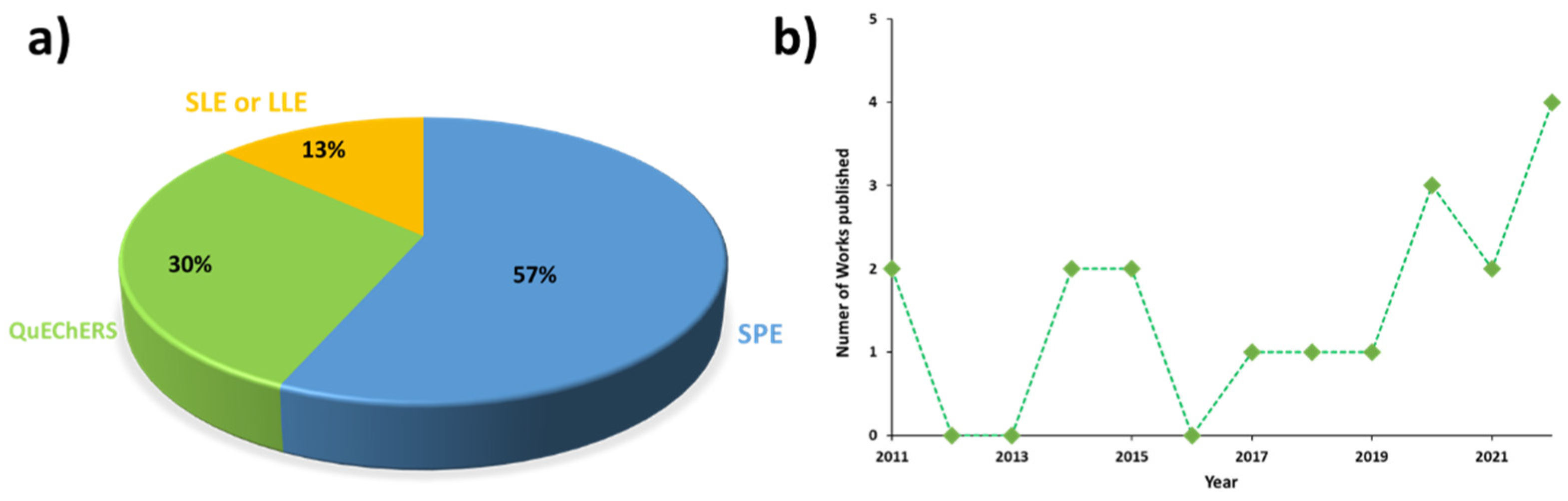
The identification of concerning high levels of pyrrolizidine alkaloids (PAs) in a wide variety of food products has raised the occurrence of these natural toxins as one of the main current issues of the food safety field. Consequently, a regulation with maximum concentration levels of these alkaloids has been published to monitor their occurrence in several foodstuffs. According to legislation, the analytical methodologies developed for their determination must include multiresidue extractions with high selectivity and sensitivity, as a set of 21 + 14 PAs should be simultaneously monitored. However, the multiresidue extraction of these alkaloids is a difficult task due to the high complexity of food and feed samples. Accordingly, although solid-phase extraction is still the technique most widely used for sample preparation, the QuEChERS method can be a suitable alternative for the simultaneous determination of multiple analytes, providing green extraction and clean-up of samples in a quick and cost-effective way.
1. Introduction
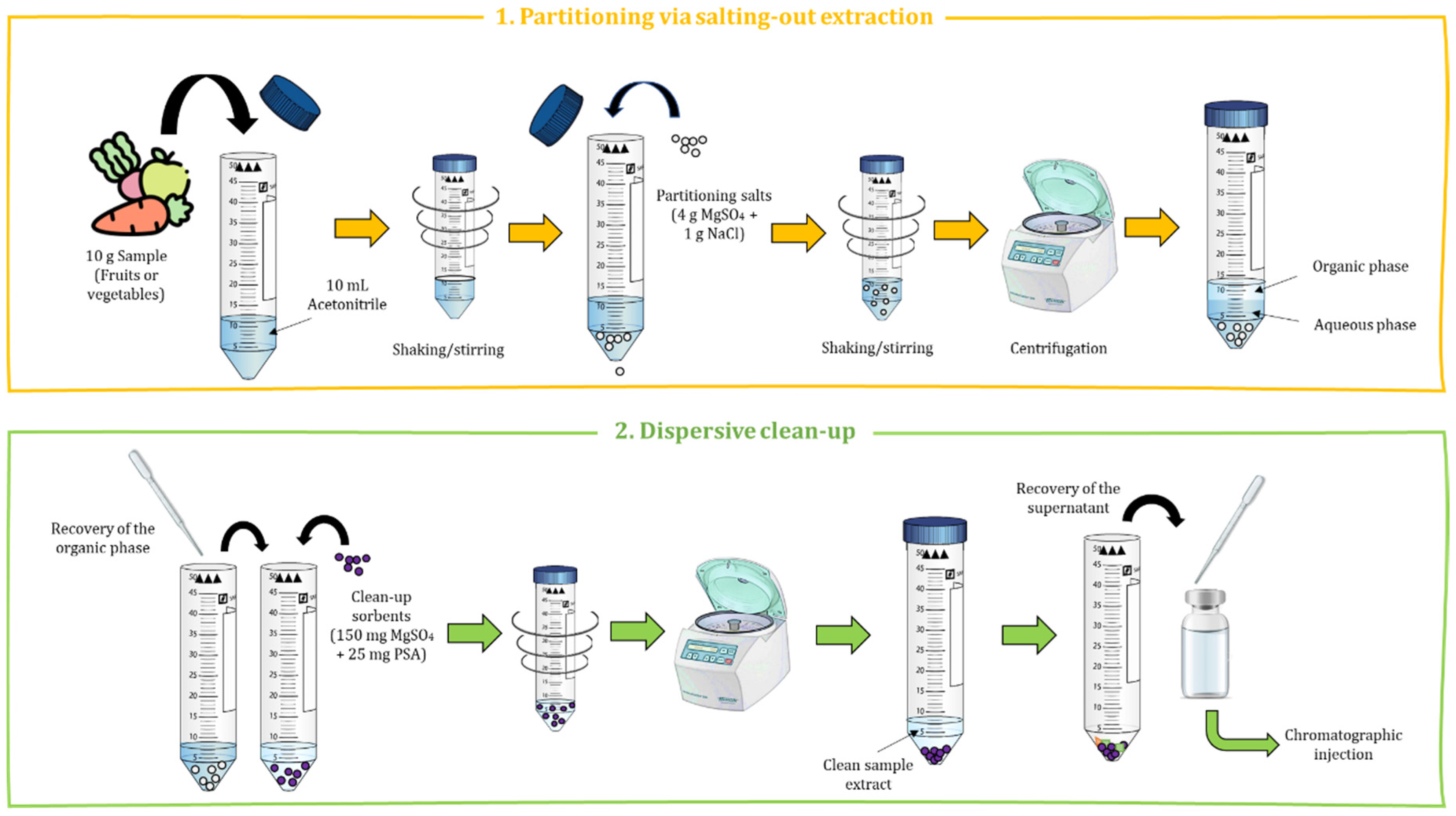
2. Basis of the QuEChERS Method
- (i)
-
Extraction step based on partitioning via salting-out extraction, achieving an equilibrium between an aqueous and an organic phase.
- (ii)
-
Clean-up step carried out by dispersive solid-phase extraction (dSPE) using different sorbent materials and salts to remove matrix interferences.
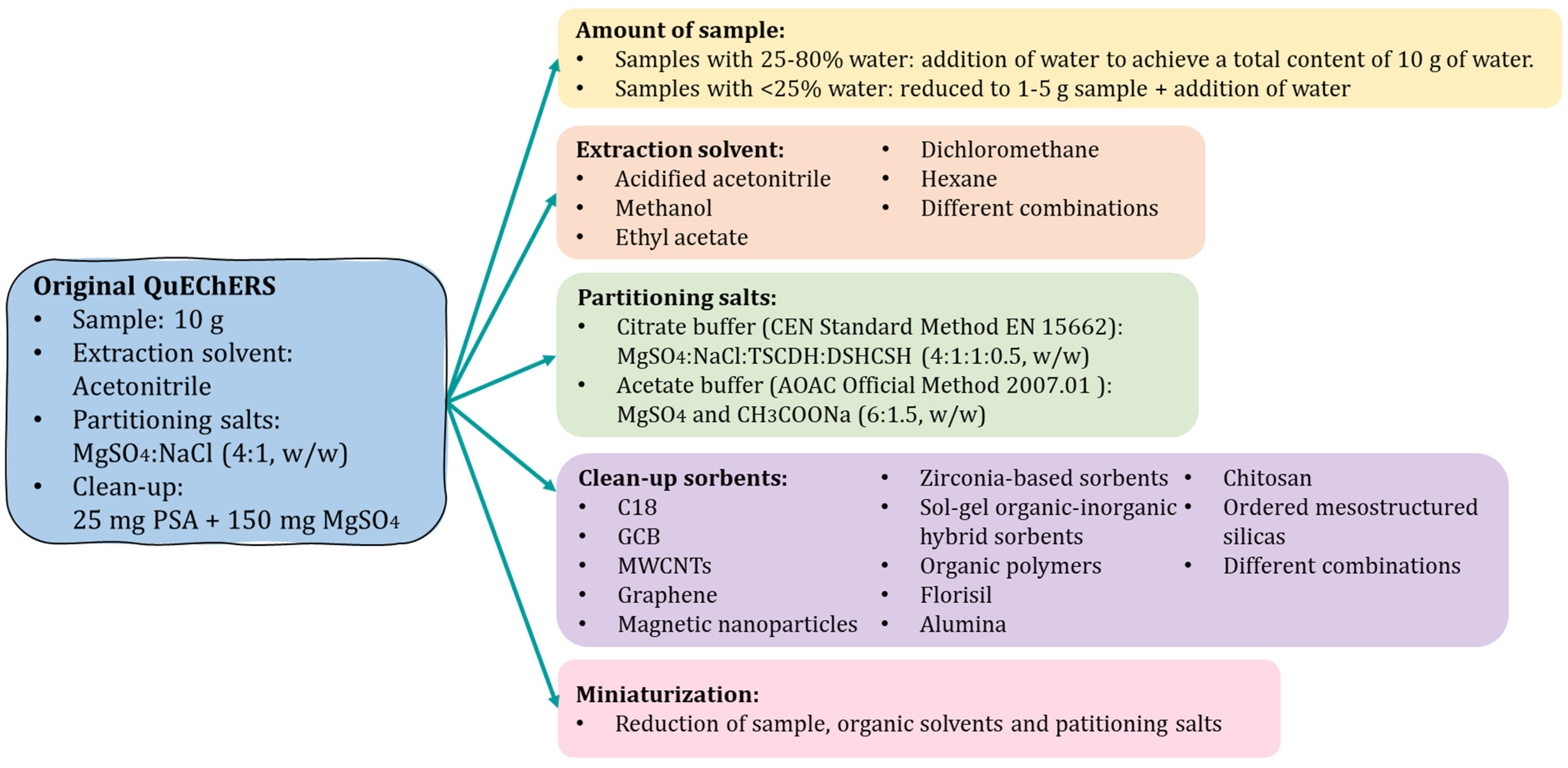
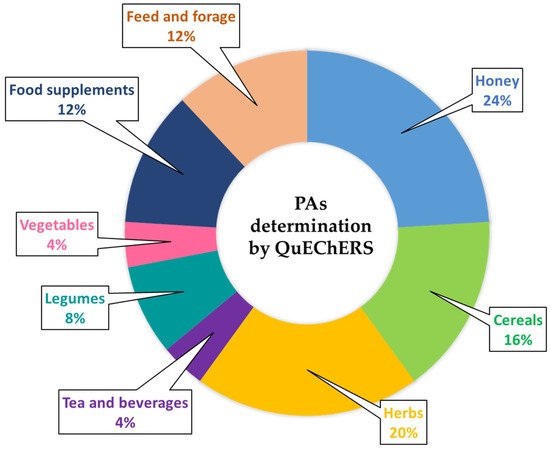
3. Evolution of the Original QuEChERS Method and Its Application to the Determination of Pyrrolizidine Alkaloids in Food and Feed Samples
| Sample (Amount) | Analytes | QuEChERs | Analysis | Recovery (%) | LOQ | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Extraction Solvent | Partition Salts | Clean-Up | ||||||
| Honey (1.5 g) | 9 PAs | LLE with 10 mL H2SO4 (0.1 M), addition of zinc dust. Supernatant with 10 mL ACN |
4 g MgSO4 1 g TSCDH 0.5 g DSHCSH 1 g NaCl |
150 mg PSA 900 mg MgSO4 |
HPLC-Q-Orbitrap-MS/MS ESI positive ion mode HRMS mode Column: C8 at 35 °C |
92–115 | 0.1–0.7 μg/Kg | [19] |
| Honey (2.5 g) | 9 PAs | LLE with 10 mL H2SO4 (0.05 M), addition of zinc dust. Supernatant with 10 mL ACN |
4 g MgSO4 1 g TSCDH 0.5 g DSHCSH 1 g NaCl |
150 mg PSA 45 mg C18 900 mg MgSO4 |
UHPLC-Q-MS ESI positive ion mode SIM mode Column: C8 at 34 °C |
67–122 | 0.081–4.35 μg/Kg | [20] |
| Honey (1 g) | 16 PAs/PANOs |
Dilution with 4 mL water, followed by 4 mL ACN | 0.8 g MgSO4 0.2 g TSCDH 0.1 g DSHCSH 0.2 g NaCl |
dSPE (500 mg MgSO4) | HPLC-TQ-MS/MS ESI positive ion mode Column: C18 |
97–105 | 1–50 μg/Kg | [21] |
| Honey (5 g) | 7 PAs/PANOs |
10 mL water followed by 10 mL ACN | 4 g MgSO4 1 g NaCl |
- | HPLC-QTRAP-MS/MS ESI Positive ion mode and MRM mode Column: C18 |
50–100 | - | [22] |
| Honey and herbal beverage (1 g) |
7 PAs | 1 mL water followed by 5 mL ACN | 1 g NaCl | 50 mg PSA | UPLC-IM-QTOF-MS/MS ESI Positive ion mode HDMSE mode Column: C18 at 50 °C |
61–120 | 1–20 μg/Kg | [23] |
| Bottled tea (4 mL) and tea leaves (2 g) |
27 PAs/PANOs |
Bottle tea: 4 mL of ACN with 1% formic acid Tea leaves: 12.5 mL water followed by 10 mL of ACN with 1% formic acid |
Bottle tea: approximately 2.2 g MgSO4, 0.6 g TSCDH, 0.2 g DSHCSH, 0.6 g NaCl Tea leaves: 4 g MgSO4, 1 g TSCDH, 0.5 g DSHCSH, 1 g NaCl |
Bottle tea: - Tea leaves: 150 mg PSA, 45 mg C18, 900 mg MgSO4 |
UHPLC-TQ-MS/MS ESI Positive ion mode MRM mode Column: C18 at 40 °C |
88–107 | 2–88 ng/L 0.10–1.61 μg/Kg |
[24] |
| Teas and herbs (1 g) | 28 PAs/PANOs |
30 mL ACN:water (75:25, v/v) with 0.5% formic acid | 6 g MgSO4 1.5 g CH3COONa |
400 mg PSA 400 mg C18 400 mg GCB 1200 mg MgSO4 |
HPLC-Q-Orbitrap-MS/MS ESI positive ion mode HRMS mode Column: C18 at 40 °C |
87–111 | 5 μg/Kg | [25] |
| Herbs (5 g) | 30 PAs/PANOs |
Addition of 10 mL water, followed by 10 mL ACN with 1% formic acid | 4 g MgSO4 1 g TSCDH 0.5 g DSHCSH 1 g NaCl |
200 mg graphene | HPLC-QTRAP-MS/MS ESI Positive ion mode and MRM mode Column: C18 at 40 °C |
61–128 | 1 μg/Kg | [26] |
| Oregano (0.2 g) | 21 PAs/PANOs | 1 mL water followed by 1 mL ACN | 0.4 g MgSO4 0.1 g TSCDH 0.05 g DSHCSH 0.1 g NaCl |
25 mg PSA 150 mg MgSO4 |
UHPLC-IT-MS/MS ESI positive ion mode MRM mode Column: Polar C18 at 25 °C |
77–96 | 0.5–25.0 μg/Kg | [27] |
| Aromatic herbs (0.2 g) |
21 PAs/PANOs |
1 mL water followed by 1 mL ACN. Re-extraction with 0.5 mL of ACN prior to clean-up step | 0.4 g MgSO4 0.1 g TSCDH 0.05 g DSHCSH 0.1 g NaCl |
25 mg LP-MS-NH2 150 mg MgSO4 |
UHPLC-IT-MS/MS ESI positive ion mode MRM mode Column: Polar C18 at 25 °C |
73–105 | 1.2-9.9 μg/Kg | [28] |
| Herbal dietary supplements (1 g tablets, capsules and softgels, 10 g for liquids) |
11 PAs/PANOs |
Tablets and capsules: 10 mL deionized water with 2% formic acid, afterward 10 mL ACN. Softgels: defatted with 4 mL hexane, addition of 10 mL deionized water with 2% formic acid, afterward 10 mL ACN. Liquids: 10 mL ACN with 2% formic acid |
4 g MgSO4 1 g NaCl |
Softgels: 100 mg C18 and 300 mg MgSO4 | UHPLC-Q-Orbitrap-MS/MS ESI positive ion mode HRMS mode Column: HSS T3 at 40 °C |
70–120 | ≤50–2500 μg/Kg | [29] |
| Food supplements, feed and honey (2.5 g) |
14 PAs/PANOs |
10 mL water followed by 10 mL ACN with 1% acetic acid | 4 g MgSO4 1 g CH3COONa |
- | HPLC-Orbitrap-MS ESI both positive and negative ion mode Column: C18 at 35 °C |
- | - | [30] |
| Leek, wheat, and tea (10 g, 2 g and 1 g, respectively) |
11 PAs/PANOs |
Acidification with 10 mL Milli-Q water with 0.2% formic acid, followed by 10 mL ACN | 4 g MgSO4 1 g NaCl |
100 mg C18 300 mg MgSO4 |
HPLC-Q-Orbitrap-MS/MS ESI both positive and negative ion mode HRMS mode Column: polar-reversed phase at 25 °C |
71–93 | ≤1–100 μg/Kg | [31] |
| Pea, soy, wheat flour and quinoa (1 g) |
56 PAs/PANOs |
10 mL water, followed by 10 mL ACN | 4 g MgSO4 1 g TSCDH 0.5 g DSHCSH 1 g NaCl |
- | UHPLC-Q-Orbitrap-MS/MS ESI both positive and negative ion mode HRMS mode Column: C18 at 50 °C |
31–132 | 1 μg/Kg | [32] |
| Sorghum, oregano, and mixed herbal tea (1 g) | 33 PAs/PANOs |
10 mL MeOH:water:formic acid (60:39.6:0.4, v/v/v) | - | 100 mg PSA C18 or zirconia-coated silica |
UHPLC-QTRAP-MS/MS ESI Positive ion mode MRM mode Column: C18 at 50 °C |
78–117 | 0.5–10 μg/Kg | [33] |
| Pollen (1 g) | 20 PAs | SLE with 10 mL H2SO4 (0.1 M), addition of zinc dust. Supernatant with 10 mL ACN |
4 g MgSO4 1 g TSCDH 0.5 g DSHCSH 1 g NaCl |
150 mg PSA 900 mg MgSO4 |
UHPLC-TQ-MS/MS ESI positive ion mode MRM mode Column: RP-MS at 40 °C |
73–106 | 4.0-9.0 μg/Kg | [34] |
| Forage grass (1 g) | 5 PAs | 10 mL of ACN/ammonium carbonate (200 mg/L), 84:16 (v/v, pH 8.5) solution. | - | 50 mg PSA | HPLC-TQ-MS/MS ESI positive ion mode MRM mode Column: C8 at 30 °C |
63–98 | 10 μg/Kg | [35] |
| Feed (2.5 g) | 5 PAs | 10 mL ACN followed by 10 mL 0.1% formic acid in water |
4 g MgSO41 g NaCl | - | UHPLC-TQ-MS/MS ESI Positive ion mode MRM mode Column: C18 at 40 °C |
72–98 | 5 μg/Kg | [36] |
References
- European Food Safety Authority. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 1–134.
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food. Sci. Technol. 2022, 120, 123–139.
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.; Peijnenburg, A.; Pfuhler, S.; Ivonne, M.C.M.; Rietjens, I.M.C.M.; Rutz, L.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment-A review. Food Chem. Toxicol. 2020, 136, 111107.
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461.
- RASFF Portal. Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff-food-and-feed-safety-alerts/rasff-portal_es (accessed on 11 March 2022).
- Mulder, P.P.; de Witte, S.L.; Stoopen, G.M.; van der Meulen, J.; van Wikselaar, P.G.; Gruys, E.; Groot, M.J.; Hoogenboom, R.L.A.P. Transfer of pyrrolizidine alkaloids from various herbs to eggs and meat in laying hens. Food Addit. Contam. Part A 2016, 33, 1826–1839.
- Mulder, P.P.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of pyrrolizidine alkaloids in food. EFSA Support. Publ. 2015, 12, 859E.
- Mulder, P.P.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal-and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133.
- Picron, J.F. Pyrrolizidine Alkaloids in Food…the Good, the Bad and the Ugly! Labinfo n° 18, RTF Étiqueté EndNote XML RIS. 2019. Available online: https://www.sciensano.be/fr/biblio/pyrrolizidine-alkaloids-food-good-bad-and-ugly (accessed on 4 April 2022).
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72.
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184.
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Beuerle, T. Toxic pyrrolizidine alkaloids in herbal medicines commonly used in Ghana. J. Ethnopharmacol. 2017, 202, 154–161.
- Rivera-Pérez, A.; Romero-González, R.; Garrido-Frenich, A. Determination and occurrence of alkenylbenzenes, pyrrolizidine and tropane alkaloids in spices, herbs, teas, and other plant-derived food products using chromatographic methods: Review from 2010–2020. Food Rev. Int. 2021, in press.
- COMMISSION REGULATION (EU) 2020/2040 of 11 December 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Pyrrolizidine Alkaloids in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R2040&from=ES (accessed on 11 March 2022).
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28.
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116.
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431.
- Lehotay, S.J.; Kok, A.D.; Hiemstra, M.; Bodegraven, P.V. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005, 88, 595–614.
- Martinello, M.; Borin, A.; Stella, R.; Bovo, D.; Biancotto, G.; Gallina, A.; Mutinelli, F. Development and validation of a QuEChERS method coupled to liquid chromatography and high resolution mass spectrometry to determine pyrrolizidine and tropane alkaloids in honey. Food Chem. 2017, 234, 295–302.
- Martinello, M.; Cristofoli, C.; Gallina, A.; Mutinelli, F. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: An evaluation in commercial honey. Food Control 2014, 37, 146–152.
- Kempf, M.; Wittig, M.; Reinhard, A.; Von der Ohe, K.; Blacquière, T.; Raezke, K.P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: Comparison of analytical methods. Food Addit. Contam. Part A 2011, 28, 332–347.
- Sixto, A.; Niell, S.; Heinzen, H. Straightforward determination of pyrrolizidine alkaloids in honey through simplified methanol extraction (QuPPE) and LC-MS/MS modes. ACS Omega 2019, 4, 22632–22637.
- Guo, Q.; Yang, Y.; Li, J.; Shao, B.; Zhang, J. Screening for plant toxins in honey and herbal beverage by ultrahigh-performance liquid chromatography-ion mobility-quadrupole time of flight mass spectrometry. Am. J. Anal. Chem. 2022, 13, 108–134.
- Takatsuji, Y.; Kakitani, A.; Nagatomi, Y.; Harayama, K.; Suzuki, K. A novel method for the detection of pyrrolizidine alkaloids in bottled tea and tea leaves by LC-MS/MS. Jpn. J. Food Chem. Saf. 2018, 25, 97–104.
- León, N.; Miralles, P.; Yusà, V.; Coscollà, C. A green analytical method for the simultaneous determination of 30 tropane and pyrrolizidine alkaloids and their N-oxides in teas and herbs for infusions by LC-Q-Orbitrap HRMS. J. Chromatogr. A 2022, 1666, 462835.
- Kaczyński, P.; Łozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351.
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A miniaturized QuEChERS method combined with ultrahigh liquid chromatography coupled to tandem mass spectrometry for the analysis of pyrrolizidine alkaloids in oregano samples. Foods 2020, 9, 1319.
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022, 380, 132189.
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal. Chim. Acta 2014, 810, 45–60.
- Mol, H.G.J.; Van Dam, R.C.J.; Zomer, P.; Mulder, P.P. Screening of plant toxins in food, feed and botanicals using full-scan high-resolution (Orbitrap) mass spectrometry. Food Addit. Contam. Part A 2011, 28, 1405–1423.
- Dzuman, Z.; Zachariasova, M.; Veprikova, Z.; Godula, M.; Hajslova, J. Multianalyte high performance liquid chromatography coupled to high resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta 2015, 863, 29–40.
- Bessaire, T.; Ernest, M.; Christinat, N.; Carrères, B.; Panchaud, A.; Badoud, F. High resolution mass spectrometry workflow for the analysis of food contaminants: Application to plant toxins, mycotoxins and phytoestrogens in plant-based ingredients. Food Addit. Contam. Part A 2021, 38, 978–996.
- Dzuman, Z.; Jonatova, P.; Stranska-Zachariasova, M.; Prusova, N.; Brabenec, O.; Novakova, A.; Fenclova, M.; Hajslova, J. Development of a new LC-MS method for accurate and sensitive determination of 33 pyrrolizidine and 21 tropane alkaloids in plant-based food matrices. Anal. Bioanal. Chem. 2020, 412, 7155–7167.
- Martinello, M.; Manzinello, C.; Gallina, A.; Mutinelli, F. In-house validation and application of UHPLC-MS/MS method for the quantification of pyrrolizidine and tropane alkaloids in commercial honey bee-collected pollen, teas and herbal infusions purchased on Italian market in 2019–2020 referring to recent European Union regulations. Int. J. Food Sci. Technol. 2022, in press.
- Qie, M.; Li, S.; Guo, C.; Yang, S.; Zhao, Y. Study of the occurrence of toxic alkaloids in forage grass by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1654, 462463.
- Bolechová, M.; Čáslavský, J.; Pospíchalová, M.; Kosubová, P. UPLC–MS/MS method for determination of selected pyrrolizidine alkaloids in feed. Food Chem. 2015, 170, 265–270.
- Mandić, B.M.; Vlajić, M.D.; Trifunović, S.S.; Simić, M.R.; Vujisić, L.V.; VuČković, I.M.; Novaković, M.M.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; et al. Optimisation of isolation procedure for pyrrolizidine alkaloids from Rindera umbellata Bunge. Nat. Prod. Res. 2015, 29, 887–890.
- Ma, C.; Liu, Y.; Zhu, L.; Ji, H.; Song, X.; Guo, H.; Yi, T. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem. Toxicol. 2018, 119, 50–60.





