
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elizabeta A. Rojas | -- | 2439 | 2022-05-10 12:20:46 | | | |
| 2 | Lindsay Dong | -8 word(s) | 2431 | 2022-05-11 08:46:24 | | |
Video Upload Options
Plasma cell leukemia (PCL) is a rare and highly aggressive plasma cell dyscrasia characterized by the presence of clonal circulating plasma cells in peripheral blood. PCL accounts for approximately 2–4% of all multiple myeloma (MM) cases. PCL can be classified in primary PCL (pPCL) when it appears de novo and in secondary PCL (sPCL) when it arises from a pre-existing relapsed/refractory MM. The development of new high-throughput technologies, such as microarrays and new generation sequencing (NGS), has contributed to a better understanding of the peculiar biological and clinical features of this disease. Relevant information is now available on cytogenetic alterations, genetic variants, transcriptome, methylation patterns, and non-coding RNA profiles. Additionally, attempts have been made to integrate genomic alterations with gene expression data.
1. Introduction
2. Cytogenetic Abnormalities
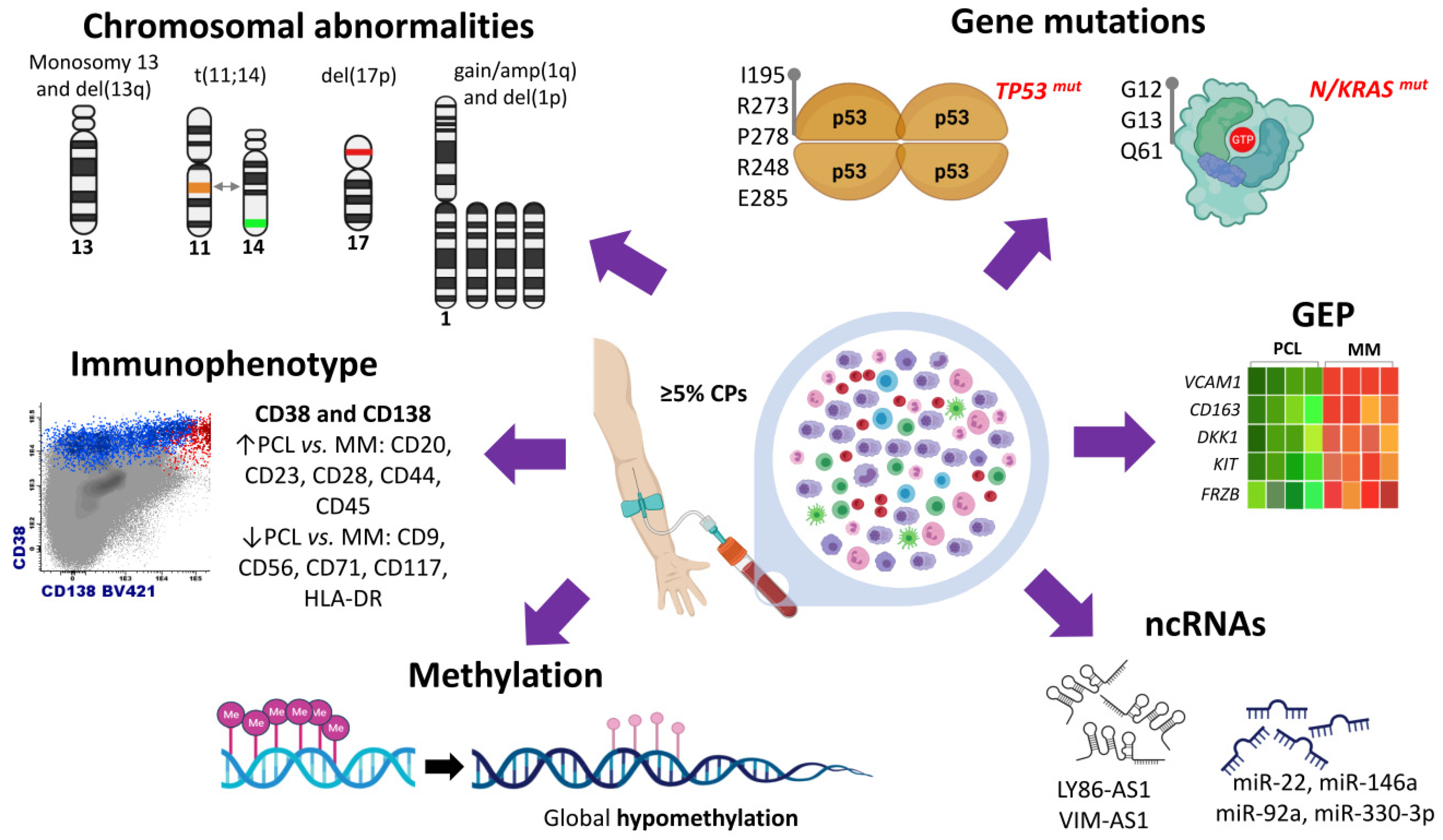
3. Gene Mutations
4. Transcriptome Characterization
5. Non-Coding RNA Profile
6. Methylation Patterns
References
- Gundesen, M.T.; Lund, T.; Moeller, H.E.H.; Abildgaard, N. Plasma Cell Leukemia: Definition, Presentation, and Treatment. Curr. Oncol. Rep. 2019, 21, 8.
- Gonsalves, W.I.; Rajkumar, S.V.; Go, R.S.; Dispenzieri, A.; Gupta, V.; Singh, P.P.; Buadi, F.K.; Lacy, M.Q.; Kapoor, P.; Dingli, D.; et al. Trends in Survival of Patients with Primary Plasma Cell Leukemia: A Population-Based Analysis. Blood 2014, 124, 907–912.
- Fernández de Larrea, C.; Kyle, R.A.; Durie, B.G.M.; Ludwig, H.; Usmani, S.; Vesole, D.H.; Hajek, R.; San Miguel, J.F.; Sezer, O.; Sonneveld, P.; et al. Plasma Cell Leukemia: Consensus Statement on Diagnostic Requirements, Response Criteria and Treatment Recommendations by the International Myeloma Working Group. Leukemia 2013, 27, 780–791.
- Fernández de Larrea, C.; Kyle, R.; Rosiñol, L.; Paiva, B.; Engelhardt, M.; Usmani, S.; Caers, J.; Gonsalves, W.; Schjesvold, F.; Merlini, G.; et al. Primary Plasma Cell Leukemia: Consensus Definition by the International Myeloma Working Group According to Peripheral Blood Plasma Cell Percentage. Blood Cancer J. 2021, 11, 192.
- Tiedemann, R.E.; Gonzalez-Paz, N.; Kyle, R.A.; Santana-Davila, R.; Price-Troska, T.; Van Wier, S.A.; Chng, W.J.; Ketterling, R.P.; Gertz, M.A.; Henderson, K.; et al. Genetic Aberrations and Survival in Plasma Cell Leukemia. Leukemia 2008, 22, 1044–1052.
- Chaulagain, C.P.; Diacovo, M.-J.; Van, A.; Martinez, F.; Fu, C.-L.; Jimenez Jimenez, A.M.; Ahmed, W.; Anwer, F. Management of Primary Plasma Cell Leukemia Remains Challenging Even in the Era of Novel Agents. Clin. Med. Insights Blood Disord. 2021, 14.
- Mina, R.; D’Agostino, M.; Cerrato, C.; Gay, F.; Palumbo, A. Plasma Cell Leukemia: Update on Biology and Therapy. Leuk. Lymphoma 2017, 58, 1538–1547.
- Jimenez-Zepeda, V.H.; Dominguez-Martinez, V.J. Plasma Cell Leukemia: A Highly Aggressive Monoclonal Gammopathy with a Very Poor Prognosis. Int. J. Hematol. 2009, 89, 259–268.
- Jurczyszyn, A.; Radocha, J.; Davila, J.; Fiala, M.A.; Gozzetti, A.; Grząśko, N.; Robak, P.; Hus, I.; Waszczuk-Gajda, A.; Guzicka-Kazimierczak, R.; et al. Prognostic Indicators in Primary Plasma Cell Leukaemia: A Multicentre Retrospective Study of 117 Patients. Br. J. Haematol. 2018, 180, 831–839.
- Ramsingh, G.; Mehan, P.; Luo, J.; Vij, R.; Morgensztern, D. Primary Plasma Cell Leukemia: A Surveillance, Epidemiology, and End Results Database Analysis between 1973 and 2004. Cancer 2009, 115, 5734–5739.
- García-Sanz, R.; Orfão, A.; González, M.; Tabernero, M.D.; Bladé, J.; Moro, M.J.; Fernández-Calvo, J.; Sanz, M.A.; Pérez-Simón, J.A.; Rasillo, A.; et al. Primary Plasma Cell Leukemia: Clinical, Immunophenotypic, DNA Ploidy, and Cytogenetic Characteristics. Blood 1999, 93, 1032–1037.
- Kraj, M.; Pogłód, R.; Kopeć-Szlezak, J.; Sokołowska, U.; Woźniak, J.; Kruk, B. C-Kit Receptor (CD117) Expression on Plasma Cells in Monoclonal Gammopathies. Leuk. Lymphoma 2004, 45, 2281–2289.
- Kraj, M.; Kopeć-Szlęzak, J.; Pogłód, R.; Kruk, B. Flow Cytometric Immunophenotypic Characteristics of 36 Cases of Plasma Cell Leukemia. Leuk. Res. 2011, 35, 169–176.
- Avet-Loiseau, H.; Daviet, A.; Brigaudeau, C.; Callet-Bauchu, E.; Terré, C.; Lafage-Pochitaloff, M.; Désangles, F.; Ramond, S.; Talmant, P.; Bataille, R. Cytogenetic, Interphase, and Multicolor Fluorescence in Situ Hybridization Analyses in Primary Plasma Cell Leukemia: A Study of 40 Patients at Diagnosis, on Behalf of the Intergroupe Francophone Du Myélome and the Groupe Français de Cytogénétique Hématologique. Blood 2001, 97, 822–825.
- Gutiérrez, N.C.; Hernández, J.M.; García, J.L.; Cañizo, M.C.; González, M.; Hernández, J.; González, M.B.; Garciá-Marcos, M.A.; San Miguel, J.F. Differences in Genetic Changes between Multiple Myeloma and Plasma Cell Leukemia Demonstrated by Comparative Genomic Hybridization. Leukemia 2001, 15, 840–845.
- Mosca, L.; Musto, P.; Todoerti, K.; Barbieri, M.; Agnelli, L.; Fabris, S.; Tuana, G.; Lionetti, M.; Bonaparte, E.; Sirchia, S.M.; et al. Genome-Wide Analysis of Primary Plasma Cell Leukemia Identifies Recurrent Imbalances Associated with Changes in Transcriptional Profiles. Am. J. Hematol. 2013, 88, 16–23.
- Chang, H.; Qi, X.; Yeung, J.; Reece, D.; Xu, W.; Patterson, B. Genetic Aberrations Including Chromosome 1 Abnormalities and Clinical Features of Plasma Cell Leukemia. Leuk. Res. 2009, 33, 259–262.
- Yu, T.; Xu, Y.; An, G.; Tai, Y.-T.; Ho, M.; Li, Z.; Deng, S.; Zou, D.; Yu, Z.; Hao, M.; et al. Primary Plasma Cell Leukemia: Real-World Retrospective Study of 46 Patients From a Single-Center Study in China. Clin. Lymphoma Myeloma Leuk. 2020, 20, e652–e659.
- Schinke, C.; Boyle, E.M.; Ashby, C.; Wang, Y.; Lyzogubov, V.; Wardell, C.; Qu, P.; Hoering, A.; Deshpande, S.; Ryan, K.; et al. Genomic Analysis of Primary Plasma Cell Leukemia Reveals Complex Structural Alterations and High-Risk Mutational Patterns. Blood Cancer J. 2020, 10, 70.
- Hanamura, I.; Stewart, J.P.; Huang, Y.; Zhan, F.; Santra, M.; Sawyer, J.R.; Hollmig, K.; Zangarri, M.; Pineda-Roman, M.; van Rhee, F.; et al. Frequent Gain of Chromosome Band 1q21 in Plasma-Cell Dyscrasias Detected by Fluorescence in Situ Hybridization: Incidence Increases from MGUS to Relapsed Myeloma and Is Related to Prognosis and Disease Progression Following Tandem Stem-Cell Transplantation. Blood 2006, 108, 1724–1732.
- Hanamura, I. Gain/Amplification of Chromosome Arm 1q21 in Multiple Myeloma. Cancers (Basel) 2021, 13, 256.
- Cazaubiel, T.; Buisson, L.; Maheo, S.; Do Souto Ferreira, L.; Lannes, R.; Perrot, A.; Hulin, C.; Avet-Loiseau, H.; Corre, J. The Genomic and Transcriptomic Landscape of Plasma Cell Leukemia. Blood 2020, 136, 48–49.
- Chang, H.; Qi, X.; Jiang, A.; Xu, W.; Young, T.; Reece, D. 1p21 Deletions Are Strongly Associated with 1q21 Gains and Are an Independent Adverse Prognostic Factor for the Outcome of High-Dose Chemotherapy in Patients with Multiple Myeloma. Bone Marrow Transpl. 2010, 45, 117–121.
- Chang, H.; Qi, C.; Jiang, A.; Xu, W.; Trieu, Y.; Reece, D.E. Loss of Chromosome 1p21 Band Is Associated with Disease Progression and Poor Survival in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation. Blood 2008, 112, 1702.
- Lionetti, M.; Barbieri, M.; Manzoni, M.; Fabris, S.; Bandini, C.; Todoerti, K.; Nozza, F.; Rossi, D.; Musto, P.; Baldini, L.; et al. Molecular Spectrum of TP53 Mutations in Plasma Cell Dyscrasias by next Generation Sequencing: An Italian Cohort Study and Overview of the Literature. Oncotarget 2016, 7, 21353–21361.
- Nandakumar, B.; Kumar, S.K.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; Leung, N.; Fonder, A.; et al. Clinical Characteristics and Outcomes of Patients With Primary Plasma Cell Leukemia in the Era of Novel Agent Therapy. Mayo Clin. Proc. 2021, 96, 677–687.
- Avet-Loiseau, H.; Li, J.Y.; Facon, T.; Brigaudeau, C.; Morineau, N.; Maloisel, F.; Rapp, M.J.; Talmant, P.; Trimoreau, F.; Jaccard, A.; et al. High Incidence of Translocations t(11;14)(Q13;Q32) and t(4;14)(P16;Q32) in Patients with Plasma Cell Malignancies. Cancer Res. 1998, 58, 5640–5645.
- Avet-Loiseau, H.; Facon, T.; Grosbois, B.; Magrangeas, F.; Rapp, M.-J.; Harousseau, J.-L.; Minvielle, S.; Bataille, R. Intergroupe Francophone du Myélome Oncogenesis of Multiple Myeloma: 14q32 and 13q Chromosomal Abnormalities Are Not Randomly Distributed, but Correlate with Natural History, Immunological Features, and Clinical Presentation. Blood 2002, 99, 2185–2191.
- Chiecchio, L.; Dagrada, G.P.; White, H.E.; Towsend, M.R.; Protheroe, R.K.M.; Cheung, K.L.; Stockley, D.M.; Orchard, K.H.; Cross, N.C.P.; Harrison, C.J.; et al. Frequent Upregulation of MYC in Plasma Cell Leukemia. Genes Chromosomes Cancer 2009, 48, 624–636.
- Pagano, L.; Valentini, C.G.; De Stefano, V.; Venditti, A.; Visani, G.; Petrucci, M.T.; Candoni, A.; Specchia, G.; Visco, C.; Pogliani, E.M.; et al. Primary Plasma Cell Leukemia: A Retrospective Multicenter Study of 73 Patients. Ann. Oncol. 2011, 22, 1628–1635.
- Papadhimitriou, S.I.; Terpos, E.; Liapis, K.; Pavlidis, D.; Marinakis, T.; Kastritis, E.; Dimopoulos, M.-A.; Tsitsilonis, O.E.; Kostopoulos, I.V. The Cytogenetic Profile of Primary and Secondary Plasma Cell Leukemia: Etiopathogenetic Perspectives, Prognostic Impact and Clinical Relevance to Newly Diagnosed Multiple Myeloma with Differential Circulating Clonal Plasma Cells. Biomedicines 2022, 10, 209.
- Todoerti, K.; Taiana, E.; Puccio, N.; Favasuli, V.; Lionetti, M.; Silvestris, I.; Gentile, M.; Musto, P.; Morabito, F.; Gianelli, U.; et al. Transcriptomic Analysis in Multiple Myeloma and Primary Plasma Cell Leukemia with t(11;14) Reveals Different Expression Patterns with Biological Implications in Venetoclax Sensitivity. Cancers (Basel) 2021, 13, 4898.
- Avet-Loiseau, H.; Gerson, F.; Magrangeas, F.; Minvielle, S.; Harousseau, J.L.; Bataille, R. Intergroupe Francophone du Myélome Rearrangements of the C-Myc Oncogene Are Present in 15% of Primary Human Multiple Myeloma Tumors. Blood 2001, 98, 3082–3086.
- Jonveaux, P.; Berger, R. Chromosome Studies in Plasma Cell Leukemia and Multiple Myeloma in Transformation. Genes Chromosomes Cancer 1992, 4, 321–325.
- Dimopoulos, M.A.; Palumbo, A.; Delasalle, K.B.; Alexanian, R. Primary Plasma Cell Leukaemia. Br. J. Haematol. 1994, 88, 754–759.
- Bezieau, S.; Devilder, M.C.; Avet-Loiseau, H.; Mellerin, M.P.; Puthier, D.; Pennarun, E.; Rapp, M.J.; Harousseau, J.L.; Moisan, J.P.; Bataille, R. High Incidence of N and K-Ras Activating Mutations in Multiple Myeloma and Primary Plasma Cell Leukemia at Diagnosis. Hum. Mutat. 2001, 18, 212–224.
- Cifola, I.; Lionetti, M.; Pinatel, E.; Todoerti, K.; Mangano, E.; Pietrelli, A.; Fabris, S.; Mosca, L.; Simeon, V.; Petrucci, M.T.; et al. Whole-Exome Sequencing of Primary Plasma Cell Leukemia Discloses Heterogeneous Mutational Patterns. Oncotarget 2015, 6, 17543–17558.
- Lionetti, M.; Barbieri, M.; Todoerti, K.; Agnelli, L.; Marzorati, S.; Fabris, S.; Ciceri, G.; Galletti, S.; Milesi, G.; Manzoni, M.; et al. Molecular Spectrum of BRAF, NRAS and KRAS Gene Mutations in Plasma Cell Dyscrasias: Implication for MEK-ERK Pathway Activation. Oncotarget 2015, 6, 24205–24217.
- Andersen, M.N.; Abildgaard, N.; Maniecki, M.B.; Møller, H.J.; Andersen, N.F. Monocyte/Macrophage-Derived Soluble CD163: A Novel Biomarker in Multiple Myeloma. Eur. J. Haematol. 2014, 93, 41–47.
- Terpos, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Iakovaki, M.; Panagiotidis, I.; Ziogas, D.C.; Fotiou, D.; et al. Increased Circulating VCAM-1 Correlates with Advanced Disease and Poor Survival in Patients with Multiple Myeloma: Reduction by Post-Bortezomib and Lenalidomide Treatment. Blood Cancer J. 2016, 6, e428.
- Kryukov, F.; Dementyeva, E.; Kubiczkova, L.; Jarkovsky, J.; Brozova, L.; Petrik, J.; Nemec, P.; Sevcikova, S.; Minarik, J.; Stefanikova, Z.; et al. Cell Cycle Genes Co-Expression in Multiple Myeloma and Plasma Cell Leukemia. Genomics 2013, 102, 243–249.
- Rojas, E.A.; Corchete, L.A.; Mateos, M.V.; García-Sanz, R.; Misiewicz-Krzeminska, I.; Gutiérrez, N.C. Transcriptome Analysis Reveals Significant Differences between Primary Plasma Cell Leukemia and Multiple Myeloma Even When Sharing a Similar Genetic Background. Blood Cancer J. 2019, 9, 90.
- Chen, D.; Yang, X.; Liu, M.; Zhang, Z.; Xing, E. Roles of MiRNA Dysregulation in the Pathogenesis of Multiple Myeloma. Cancer Gene Ther. 2021, 28, 1256–1268.
- Carrasco-Leon, A.; Ezponda, T.; Meydan, C.; Valcárcel, L.V.; Ordoñez, R.; Kulis, M.; Garate, L.; Miranda, E.; Segura, V.; Guruceaga, E.; et al. Characterization of Complete LncRNAs Transcriptome Reveals the Functional and Clinical Impact of LncRNAs in Multiple Myeloma. Leukemia 2021, 35, 1438–1450.
- Misiewicz-Krzeminska, I.; Krzeminski, P.; Corchete, L.A.; Quwaider, D.; Rojas, E.A.; Herrero, A.B.; Gutiérrez, N.C. Factors Regulating MicroRNA Expression and Function in Multiple Myeloma. Noncoding RNA 2019, 5, 9.
- Lionetti, M.; Musto, P.; Di Martino, M.T.; Fabris, S.; Agnelli, L.; Todoerti, K.; Tuana, G.; Mosca, L.; Gallo Cantafio, M.E.; Grieco, V.; et al. Biological and Clinical Relevance of MiRNA Expression Signatures in Primary Plasma Cell Leukemia. Clin. Cancer Res. 2013, 19, 3130–3142.
- De Veirman, K.; Wang, J.; Xu, S.; Leleu, X.; Himpe, E.; Maes, K.; De Bruyne, E.; Van Valckenborgh, E.; Vanderkerken, K.; Menu, E.; et al. Induction of MiR-146a by Multiple Myeloma Cells in Mesenchymal Stromal Cells Stimulates Their pro-Tumoral Activity. Cancer Lett. 2016, 377, 17–24.
- Ronchetti, D.; Agnelli, L.; Taiana, E.; Galletti, S.; Manzoni, M.; Todoerti, K.; Musto, P.; Strozzi, F.; Neri, A. Distinct LncRNA Transcriptional Fingerprints Characterize Progressive Stages of Multiple Myeloma. Oncotarget 2016, 7, 14814–14830.
- Todoerti, K.; Calice, G.; Trino, S.; Simeon, V.; Lionetti, M.; Manzoni, M.; Fabris, S.; Barbieri, M.; Pompa, A.; Baldini, L.; et al. Global Methylation Patterns in Primary Plasma Cell Leukemia. Leuk. Res. 2018, 73, 95–102.
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant Global Methylation Patterns Affect the Molecular Pathogenesis and Prognosis of Multiple Myeloma. Blood 2011, 117, 553–562.




