Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zaneta Kimber-Trojnar | -- | 3092 | 2022-05-10 12:04:16 | | | |
| 2 | Jessie Wu | Meta information modification | 3092 | 2022-05-11 04:49:02 | | | | |
| 3 | Jason Zhu | + 4 word(s) | 3092 | 2022-05-11 05:00:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kimber-Trojnar, Z.; Dłuski, D.; , .; Kopeć, M.; Bożena, L. Treatment of Metformin for Endometriosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/22759 (accessed on 07 February 2026).
Kimber-Trojnar Z, Dłuski D, , Kopeć M, Bożena L. Treatment of Metformin for Endometriosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/22759. Accessed February 07, 2026.
Kimber-Trojnar, Zaneta, Dominik Dłuski, , Monika Kopeć, Leszczyńska-Gorzelak Bożena. "Treatment of Metformin for Endometriosis" Encyclopedia, https://encyclopedia.pub/entry/22759 (accessed February 07, 2026).
Kimber-Trojnar, Z., Dłuski, D., , ., Kopeć, M., & Bożena, L. (2022, May 10). Treatment of Metformin for Endometriosis. In Encyclopedia. https://encyclopedia.pub/entry/22759
Kimber-Trojnar, Zaneta, et al. "Treatment of Metformin for Endometriosis." Encyclopedia. Web. 10 May, 2022.
Copy Citation
Endometriosis is a common disease in women of reproductive age, and its pathogenesis seems to be largely affected by hormone imbalance, inflammation, oxidative stress, and autophagy dysregulation. Metformin is an insulin sensitizer widely used for the treatment of type 2 diabetes mellitus. In endometriosis, metformin might modify the stroma–epithelium communication via Wnt2/β-catenin. With its unique therapeutic mechanisms and no serious side effects, metformin seems to be a helpful anti-inflammatory and anti-proliferative agent in the treatment of endometriosis.
Metformin
Endometriosis
Mechanisms
1. Metformin
Metformin is an antidiabetic medicament currently used as the first-choice treatment for type 2 diabetes mellitus (T2DM), which does not cause excessive hypoglycaemia [1]. Metformin leads to a significant reduction in plasma fasting insulin levels and a reduction in insulin resistance. Therefore, it is considered an insulin sensitizer, which seems to be associated with its beneficial effects on tyrosine kinase activity and insulin receptor expression [2]. Metformin, by regulation of multiple components of the incretin axis, may have a positive effect on metabolism. Maida et al. [3] presented that this agent stimulates gene expression of islet incretin receptor by a mechanism that is dependent on peroxisome proliferator-activated receptor (PPAR)-α and intensely raises levels of glucagon-like peptide 1 (GLP-1) in plasma.
Nevertheless, a great deal of argumentation from clinical researchers confirms that the fundamental action of metformin is to reduce intrahepatic glucose production, mostly by inhibiting gluconeogenesis through a mild and temporary inhibition of the mitochondrial respiratory chain complex 1 [4][5][6]. Furthermore, the decline in hepatic energy status initiates the AMP-activated protein kinase (AMPK), which provides a mechanism for metformin function on the hepatic gluconeogenic program.
Metformin also decreases obesity-associated inflammation and other inflammatory reactions and affects the steroidogenesis in ovarian granulosa and thecal cells [7][8][9][10][11][12][13]. Metformin inhibits plasminogen activator inhibitor-1 (PAI-1) levels, endometrial androgens receptor expression, and plasmatic endothelin-I (ET-1), which are factors that increase the risk of miscarriage.
The therapeutic potential of this medicament, which is most likely induced by the improvement in insulin sensitivity, is employed in different conditions, including cardiovascular diseases, diabetic nephropathy, polycystic ovary syndrome (PCOS), and the prevention or treatment of cancer [1][14][15] (Figure 1). Hyperinsulinemia caused by insulin resistance might, in fact, induce carcinogenesis indirectly by raising the levels of steroid sex hormones and directly through the insulin receptor or insulin-like growth factors (IGF), inflammatory processes, and interrupting adipokines homeostasis [16].
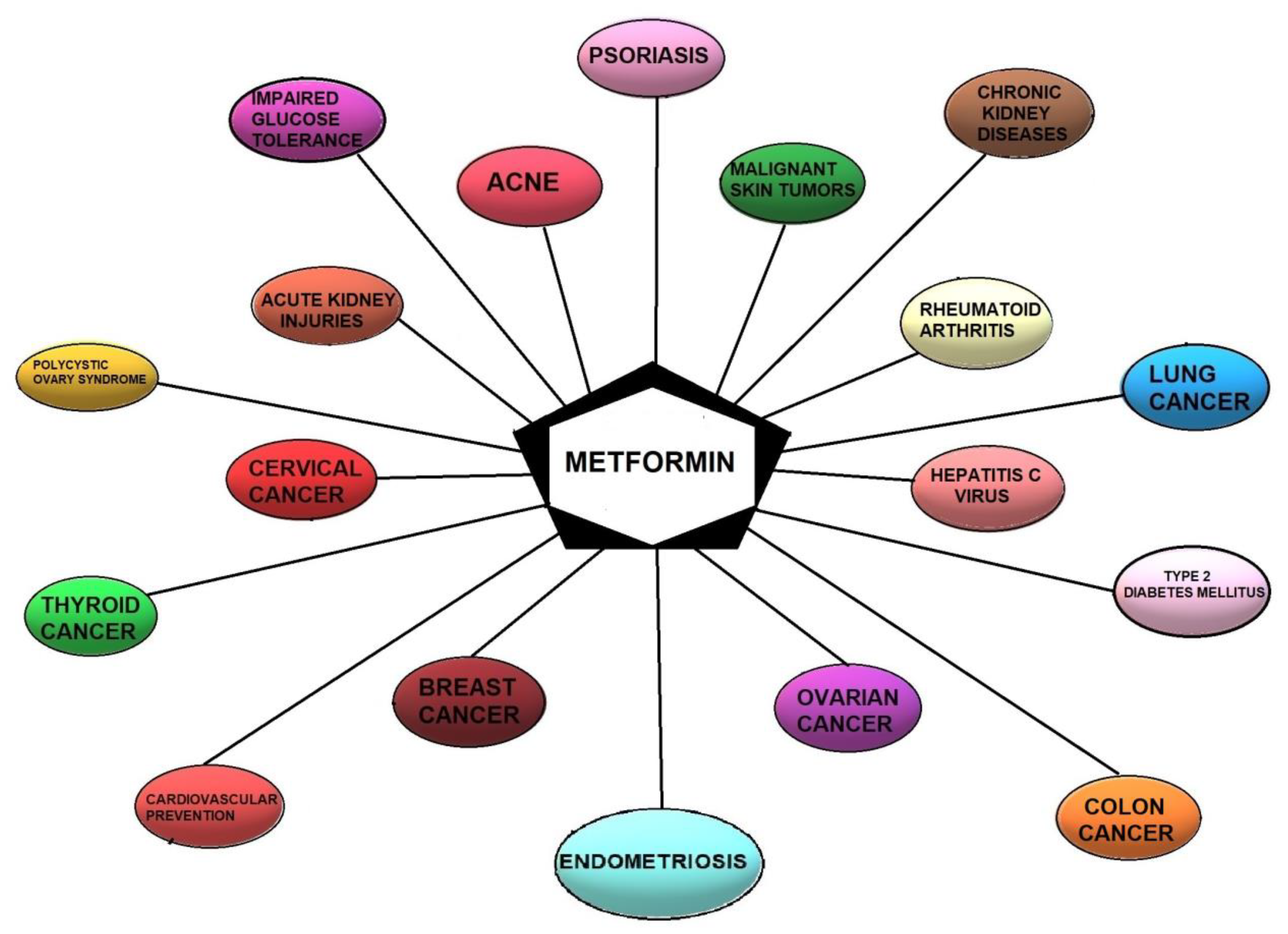
Figure 1. Current and potential therapeutic indications for metformin.
2. Metformin as a Potential Treatment Option for Endometriosis—Mechanisms
Metformin has a pleiotropic effect by stimulating AMPK, which is the major regulator of cellular energy homeostasis that inhibits mTOR, the main suppressor of autophagy [17][18][19]. The activation of AMPK is of particular importance for understanding the mechanism of metformin’s action in women with endometriosis. The AMPK, as a serine/threonine-protein kinase, is a principal enzyme that regulates energy homeostasis in cells [7]. Furthermore, mitogen-activated protein kinase (MEK)/extracellular-signal -regulated kinases (ERK) phosphorylation is triggered by metformin [20][21].
Zhou et al. [7] reported that the mechanism of metformin action is the inhibition of prostaglandin E2 (PGE2)-induced cytochrome P450 19A1 (CYP19A1) mRNA expression and aromatase activity in endometriotic stromal cells (ESC) by inhibiting the binding of the cAMP-Response Element Binding protein (CREB) to the Proximal Promoter (PII). This process was associated with the activation of AMPK. It should be emphasized that profuse CYP19A1 mRNA expression and elevated local oestrogen production have been found in endometriotic tissues, implying that P450 aromatase is involved in the local oestrogen production [7]. Changes in the expression of CYP19A1 mRNA may result in a significant decrease in aromatase activity.
Additionally, it has been confirmed that metformin inhibits the mRNA expression of insulin-stimulated CYP19A1 and follicle-stimulating hormone (FSH) in granulosa cells [10][22] and also significantly decreases the forskolin/phorbol ester (FSK/PMA) -dependent upregulation of CYP19A1 mRNA expression in primary stromal cells of human breast adipose tissue [23]. The intracellular actions related to these various effects of metformin in ESCs rely on the fact that the AMPK pathway is the main mechanism of metformin action [7][23][24][25][26][27][28]. This means that metformin can reduce CYP19A1 mRNA expression and aromatase activity to some extent independently of AMPK [7].
Nevertheless, metformin regulates the expression of aromatase using other signalling pathways and unknown mechanisms. Furthermore, the AMPK pathway is gradually phosphorylated upon metformin stimulation in ESC [20]. A similar effect on the gene expression of CYP19A1 in human ESCs might have the AMPK inhibitor and PGE2 [7]. In addition, adiponectin, whose concentrations are reduced in serum and peritoneal fluid in endometriotic patients, stimulates AMPK and inhibits the production of inflammatory cytokines in endometriosis [29][30][31]. Moreover, AMPK participates in the anti-inflammatory action of metformin shown by ESCs [21]. These observations confirm that induction of AMPK in endometriosis may be a protective factor. Steroidogenesis and CYP19A1 gene expression have been shown to be regulated by the MEK/ERK signalling cascade; however, conflicting findings regarding the mechanism in various steroidogenic cells have been reported. For instance, it has been reported that inhibition of MEK activity with PD98059 and U0126 is associated with stimulation, inhibition, or no effect on the steroidogenic response [20][21][32][33][34][35]. Zhou et al. [7] demonstrated the inhibition of CYP19A1 mRNA expression by metformin as autonomous of the MEK/ERK pathway.
Stimulation of the MEK/ERK pathway may be relevant in other metformin mechanisms. Metformin may reduce PGE2-activated CREB binding to CYP19A1 PII in human ESCs [7]. Notably, metformin decreases CYP19A1 expression at the transcriptional level. It is noteworthy that metformin interacts with the FSH-stimulated cAMP/PKA/CREB pathway, being the major signalling pathway regulating the expression of the CYP19A1 gene in the ovaries [36]. The above-mentioned observations indicate that the benefits of metformin may also be mediated by the inhibition of oestrogen production in the ovaries by the same actions as outlined in ESCs.
In stromal cells of human breast adipose tissue, metformin suppresses the nuclear translocation of CREB-regulated transcription co-activator 2 (CRTC2), which elevates aromatase expression by binding to CYP19A1 PII. It is also the main purpose of AMPK [23][24]. In granulosa cells, metformin decreases the FSH-induced phosphorylation of CREB, thereby reducing the CREB action, which may cause interference of the CREB-binding protein (CBP)-CRTC2 co-activator complex that connects to CRE in PII of the CYP19A1 gene. Metformin is able to disturb the CREB-CRTC2 complex in human ESCs [20]. The above results confirm that metformin attenuates the PGE2-stimulated binding of CREB to CYP19A1 PII, possibly by interfering with the CREB-CRTC2 complex [7].
It has been confirmed that metformin interferes with the pathophysiology of endometriosis by reducing matrix MMPs expression, although it is not the major mechanism of its action [37]. MMPs are proteolytic enzymes that participate in the reduction and reconstruction of the extracellular matrix. The disturbance of the regulation of MMPs is considered crucial in the progress of pathological disorders such as endometriosis [38][39][40]. Accordingly, MMP inhibitors may impair the progression of the disease.
It has been proven that oxidative status balance may affect lipid and glucose metabolism [41]. It has also been confirmed that patients affected by endometriosis have abnormalities in both types of these metabolisms. For example, McKinnon et al. [42] reported that in endometrial lesions, the expression of the glucose-4 transporter (GLUT4) protein is increased, possibly due to elevated glucose availability in the growing lesions. Melo et al. [43] found that patients with endometriosis often have an unfavorable lipid profile (decrease in high-density lipoprotein and increase in low-density), potentially conducive to oxidative stress (by lipid peroxidation), which tends to increase in this group of women.
The unique therapeutic action of metformin in endometriosis is associated with its impact on inflammation, angiogenesis, invasion, and adhesion, as well as apoptosis. The mechanisms of action of metformin in the above-mentioned processes require a more detailed description.
2.1. The Impact of Metformin on Inflammation
Inflammation plays an important role in the process of endometriosis [44][45] and is associated with progesterone resistance. Many studies have revealed that metformin suppresses inflammation. In endometrial stromal cells, metformin may limit the inflammatory process by affecting the secretion of IL-6 and IL-8. Metformin inhibits the production of IL-1β and suppresses the production of IL-8. Moreover, human ESCs cultures incubated with metformin show statistically significantly reduced IL-1β-induced IL-8 production in a dose-dependent fashion [46]. The same phenomenon was found in the ability of stromal cells to transform androstenedione into estrone (reaction dependent on aromatase activity) as well as in deoxyribonucleic acid (DNA) synthesis (a marker of cell proliferation). The anti-proliferative effect of metformin on IL-8 depends on the cell type because it does not suppress the secretion of IL-8 from eutopic (normal) endometrial stromal cells.
Metformin may reduce serum levels of TNF-α in women with endometriosis [47]. Treatment with metformin can intensify the autophagy process by inhibiting mTOR [48][49]. Co-therapy of quercetin and metformin significantly inhibits mTOR messenger ribonucleic acid (mRNA) expression and increases the gene expression of autophagy factors in ectopic endometrial tissues. This combined treatment regressed endometrial implants in rodents mostly through antiestrogenic and anti-inflammatory functions.
2.2. The Impact of Metformin on Angiogenesis
Angiogenesis is a physiological phenomenon of blood vessel formation on the basis of the existing ones. This process requires an equilibrium between stimulatory and inhibitory signals. Once the balance is disturbed, the vascularization could be activated. Angiogenesis plays an important role in the neoplastic development and metastases [50][51]. In vitro and in vivo studies revealed that metformin could inhibit tumour angiogenesis in different mechanisms, such as the AMPK/mTOR pathway activation, downregulation of platelet-derived growth factor B (PDGF-B), and inhibition of several angiogenic-related proteins, such as vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1 (HIF-1), insulin-like growth factor binding protein-2 (IGFBP-2), platelet-derived growth factor-AA (PDGF-AA), VEGF, angiogenin, matrix metalloprotein-9 (MMP-9), and endostatin [52][53][54][55][56]. In vivo, metformin limited the microvascular tumour density and modified the perivascular/endothelial cell ratio [57].
Although endometriosis is a benign condition, it is linked to various cancer-related processes including angiogenesis [58][59]. It has been reported that angiogenesis resulting in the dysfunction of the vascular basal membranes, as well as the surrounding extracellular matrix (ECM) [60], is a hallmark of endometriosis. Unfortunately, precise angiogenesis mechanisms in this disorder are still unknown [58][61]. The use of metformin prevents the progression of endometrial lesions. Metformin suppresses the proliferation of endothelial cells and reduces vasculature lesions by inhibiting the expression of VEGF. Yilmaz et al. noticed that in rats treated with metformin, the concentrations of superoxide dismutase (SOD) and MMP-2 were increased, whereas the levels of VEGF and MMP-9 were decreased in the endometrial lesions [62].
It is worth noticing that metformin decreased the risk of endometrial hyperplasia by reducing the expression levels of urothelial cancer associated 1 (UCA1), transforming growth factor-β (TGF-β) and protein kinase B, while increasing the levels of microRNA-144 and active Caspase-3 [63].
2.3. The Impact of Metformin on Adhesion and Invasion
Metformin may inhibit the vascular adhesion molecule expression in endometriosis. Metformin may affect the binding of monocytes to endothelial cells by reducing vascular cell adhesion molecule-1 (VCAM-1) expression on activated endothelial cells. Studies confirmed that metformin in rat abdominal endometriosis induced reduction in weight, mean area, and volume of lesions compared to no reduction in the control group [11][64]. Histological assessment of the lesions proved that metformin therapy was related to a significant decrease in the number of epithelial cells. The risk and severity of adhesions were also lower.
Lipolysis-stimulated lipoprotein receptor (LSR) revealed itself as a recent molecular constituent of tricellular contacts that have a barrier mechanism for the cellular sheet [65]. LSR recruits tricellulin (TRIC), which is the first molecular element of tricellular tight junctions. The knockdown of LSR enhances invasion and motility of some cancer cells [66]. In endometriosis, LSR is noticed in the secretory phase of normal endometrial epithelial cells. Furthermore, LSR in cancer is reduced in association with the malignancy. The downregulation of LSR by siRNA provokes cell migration, proliferation and invasion, while TRIC transfers from the tricellular region to the bicellular region of the membrane [67]. Metformin increases LSR expression and prevents the migration and invasion of the cells activated by knockdown of LSR in those treated with siRNA or leptin in endometrial cancer cells. Shimada et al. noticed upregulation of LSR by metformin via MAPK and upregulation of LSR via MAPK, PI3K and JAK2/STAT [67].
2.4. The Impact of Metformin on Apoptosis
Homeostasis of tissues is modulated by apoptosis. A balance between cell proliferation and apoptosis preserves this homeostasis against cellular disturbances. In endometriosis, the reduction in cell death can lead to the progression of this disease [68][69]. The ratio of cell apoptosis is suppressed in endometrial cells [70]. Moreover, in endometriosis, the induction of the NF-κB pathway is associated with both apoptosis and proliferation [71][72].
Hsieh Li et al. [73] observed that metformin reduces p53 expression levels and significantly induces apoptotic cell death. Contrary to this, Xiao et al. [74] noticed that metformin increases AMPK stimulation without modifying the expression levels of p53 and liver kinase B1 (LKB1). Metformin is able to increase LKB1 phosphorylation, promote p53 activation and AMPK, and suppress cell cycle progression [75]. Furthermore, Chen et al. [76] demonstrated that cytotoxicity induced by metformin occurs through the induction of the caspase-dependent apoptotic signalling pathway.
Metformin has inhibitory actions on cell proliferation, metastasis, apoptosis, angiogenesis, and chemoresistance in diverse malignancies in vivo and in vitro, such as ovarian, hepatocellular, and endometrial cancers [77][78][79]. The above inhibitory properties of metformin are associated with the induction of the PI3K/AKT/mTOR signalling pathway [77][78][79]. Metformin also suppresses tumour growth by stimulating the ATM serine/threonine kinase/AMPK/p53 signalling pathway and reducing the AKT/mTOR/eukaryotic translation initiation factor 4E-binding protein 1 signalling pathway. It leads to an increased response to radiation [80]. Moreover, clinical studies confirmed that metformin use is associated with higher overall survival in the group of patients with various cancers [81][82]. Metformin, by acting on the AMPK/p53 and PI3K/AKT/mTOR signalling pathways, may induce apoptosis and cell cycle arrest.
To summarize, metformin can reduce angiogenesis, inflammation, invasion, and adhesion and may cause regression of endometrial lesions. Insulin sensitizers are reported to diminish cytokine and chemokine expression in endometriotic stromal cells [83], regulate angiogenesis [84], and provoke apoptosis in endometrial lesions [85].
3. The Use of Metformin as Pharmacological Therapy in Endometriosis
Metformin is a multipotential medicament with a rich history and encouraging prospects for the future. The biguanide derivative was used for the first time in the 1960s and registered as a hypoglycaemic substance in the pharmacotherapy of diabetes mellitus type 2. Metformin looks similar to a drug with one-off therapeutic potential. Clinical and experimental studies show that metformin might also be beneficial in other diseases such as polycystic ovary syndrome, cancer, or endometriosis.
In 2010, Yilmaz et al. presented the action of metformin in reducing endometrial changes in their work. First, endometriosis was induced in rats by surgery. Next, the 2 groups (group A and B) were given metformin in doses of 25 and 50 mg/kg/day, respectively, for 28 days. Group C, the control group, was given a placebo (saline). Histologic score, weight, and mean volume of implants in groups A and B were significantly lower in comparison to the control group. The activity of MMP-2 and SOD were significantly higher than in the control group. Additionally, the levels of VEGF and MMP-9 in endometrial implants were significantly decreased [66].
Xu et al. analysed and described the molecular and cellular mechanism by which metformin modulates steroidogenic acute regulatory protein (StAR) expression in human ESCs. They showed highlighted data of CREB-regulated transcription co-activator 2 (CRTC2) role in the mechanism by which metformin downregulates StAR expression. The nuclear translocation of CRTC2 is achieved by increasing AMPK phosphorylation, which inhibits transcription of StAR by blemishing the formation of CREB-CRTC2 complex involving the activation of the StAR promoter cyclic adenosine monophosphate (cAMP) response element [20].
In another study, Takemura et al. assessed the effect of metformin on the proliferation of ESCs, oestradiol production, and inflammatory response. They decided to measure IL-8 production, mRNA expression, 5-bromo-2′-deoxyuridine incorporation, and aromatase activity in ESCs using 10 μm-, 100 μm-, and 1000 μm- doses of metformin. They obtained endometriotic tissues from women who underwent surgery for ovarian endometriomas. This research revealed that metformin significantly decreased aromatase activity in ESCs, cAMP-induced mRNA expression, 5-bromo-2′-deoxyuridine incorporation, and the interleukin 1β (IL-1β)-induced IL-8 production in a dose-dependent manner with maximal effect at 1000 μm [46].
Metformin may also play a role as an anti-angiogenic agent. In 2021, Yari et al. used three types of human cells: normal ESCs (N-ESCs) from healthy endometrial tissue, eutopic ESCs (EU-ESCs), and ectopic ESCs (ECT-ESCs) from women with endometriosis. All types of cells were cultured and treated with different metformin concentrations for 72 h. The scientists assessed metformin’s effect on cell migration, proliferation, and viability. In addition, the expression of inflammatory and angiogenic genes was also checked. The results showed that metformin inhibited the proliferation and migration of cells in a concentration-dependent manner in ESCs. Moreover, metformin significantly lowered VEGF-A expression in EU-ESCs and ECT-ESCs and hypoxia-inducible factor 1α (HIF-1α) in ECT-ESCs. EU-ESCs and N-ESCs presented a significantly decreased macrophage migration inhibitory factor (MIF) expression after metformin treatment. Although EU-ESCS showed decreased gene expression of MMP-2 and MMP-9 after metformin use, in ECT-ESCs and N-ESCs, the expression of tissue inhibitor of MMPs was significantly increased after drug treatment [86].
Jamali et al. analysed the effects of quercin, metformin, and their combination on experimental endometriosis in a rat model. A total of 60 female rats were divided into six groups. Five of them underwent surgery to induce endometriosis; after 4 weeks, daily treatment lasting 4 weeks began. Afterward, histoarchitecture and size of the endometrial implants, serum levels of progesterone, TNF-α and 17β-estradiol, markers of autophagy, and oxidative stress were assessed using gene expression analysis and enzyme-linked immunosorbent assay (ELISA). The results showed that serum levels of TNF-α and 17β-estradiol were higher in rats with endometriosis. Additionally, quinone oxidoreductase (NQO1) enzyme reduced nicotinamide adenine dinucleotide phosphate (NADPH) activity and gene expression levels of autophagy markers, and nuclear factor erythroid 2-related factor 2 (Nrf2) were significantly decreased. Moreover, mTOR gene expression was higher in the ectopic endometrial tissue in comparison to the eutopic one. The combination of quercin and metformin reversed the alterations and had a marked effect on the size of endometrial implants and gene expression levels of autophagy markers and mTOR in ectopic endometrial cells [17].
Oner et al. investigated the effects of letrozole and metformin on experimentally induced endometriosis in rats. A total of 38 female rats were divided into four groups: a control group, two metformin groups, and a letrozole group. Rats were treated for 4 weeks; next, they were sacrificed, and the size of endometrial implants and scores of adhesions were assessed. The surface area of endometrial implants was significantly reduced in treatment groups, and the effect was comparable in metformin groups and letrozole groups. The histopathologic assessment indicated that the histopathologic score was lowest after 100 mg/kg/day dose of metformin. Moreover, metformin decreased the severity of adhesions [11].
References
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270.
- Gunton, J.E.; Delhanty, P.J.; Takahashi, S.; Baxter, R.C. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J. Clin. Endocrinol. Metab. 2003, 88, 1323–1332.
- Maida, A.; Lamont, B.J.; Cao, X.; Drucker, D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia 2011, 54, 339–349.
- Cusi, K.; Consoli, A.; DeFronzo, R.A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067.
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069.
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441.
- Zhou, Y.; Xu, J.N.; Zeng, C.; Li, X.; Zhou, Y.F.; Qi, Y.; Xue, Q. Metformin Suppresses Prostaglandin E2-Induced Cytochrome P450 Aromatase Gene Expression and Activity via Stimulation of AMP-Activated Protein Kinase in Human Endometriotic Stromal Cells. Reprod. Sci. 2015, 22, 1162–1170.
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 611–617.
- Attia, G.R.; Rainey, W.E.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524.
- Mansfield, R.; Galea, R.; Brincat, M.; Hole, D.; Mason, H. Metformin has direct effects on human ovarian steroidogenesis. Fertil. Steril. 2003, 79, 956–962.
- Oner, G.; Ozcelik, B.; Ozgun, M.T.; Serin, I.S.; Ozturk, F.; Basbug, M. The effects of metformin and letrozole on endometriosis and comparison of the two treatment agents in a rat model. Hum. Reprod. 2010, 25, 932–937.
- Bergheim, I.; Luyendyk, J.P.; Steele, C.; Russell, G.K.; Guo, L.; Roth, R.A.; Arteel, G.E. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J. Pharmacol. Exp. Ther. 2006, 316, 1053–1061.
- Lin, H.Z.; Yang, S.Q.; Chuckaree, C.; Kuhajda, F.; Ronnet, G.; Diehl, A.M. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000, 6, 998–1003.
- Lord, J.; Wilkin, T. Metformin in polycystic ovary syndrome. Curr. Opin. Obstet. Gynecol. 2004, 16, 481–486.
- Rotermund, C.; Machetanz, G.; Fitzgerald, J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front. Endocrinol. 2018, 9, 400.
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.; Gans, R.O.; de Vries, E.G. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380.
- Jamali, N.; Zal, F.; Mostafavi-Pour, Z.; Samare-Najaf, M.; Poordast, T.; Dehghanian, A. Ameliorative Effects of Quercetin and Metformin and Their Combination Against Experimental Endometriosis in Rats. Reprod. Sci. 2021, 28, 683–692.
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236.
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32.
- Xu, J.N.; Zeng, C.; Zhou, Y.; Peng, C.; Zhou, Y.F.; Xue, Q. Metformin inhibits StAR expression in human endometriotic stromal cells via AMPK-mediated disruption of CREB-CRTC2 complex formation. J. Clin. Endocrinol. Metab. 2014, 99, 2795–2803.
- Rice, S.; Pellatt, L.; Ramanathan, K.; Whitehead, S.A.; Mason, H.D. Metformin inhibits aromatase via an extracellular signal regulated kinase-mediated pathway. Endocrinology 2009, 150, 4794–4801.
- Rice, S.; Elia, A.; Jawad, Z.; Pellatt, L.; Mason, H.D. Metformin inhibits follicle-stimulating hormone (FSH) action in human granulosa cells: Relevance to polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1491–E1500.
- Brown, K.A.; McInnes, K.J.; Hunger, N.I.; Oakhill, J.S.; Steinberg, G.R.; Simpson, E.R. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009, 69, 5392–5399.
- Brown, K.A.; Hunger, N.I.; Docanto, M.; Simpson, E.R. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res. Treat. 2010, 123, 591–596.
- Kola, B.; Boscaro, M.; Rutter, G.A.; Grossman, A.B.; Korbonits, M. Expanding role of AMPK in endocrinology. Trends Endocrinol. Metab. 2006, 17, 205–215.
- Tosca, L.; Solnais, P.; Ferre, P.; Foufelle, F.; Dupont, J. Metformininduced stimulation of adenosine 50-monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006, 75, 342–351.
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 50-monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378.
- Tosca, L.; Uzbekova, S.; Chabrolle, C.; Dupont, J. Possible role of 50AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol. Reprod. 2007, 77, 452–465.
- Takemura, Y.; Osuga, Y.; Harada, M.; Hirata, T.; Koga, K.; Morimoto, C.; Hirota, Y.; Yoshino, O.; Yano, T.; Taketani, Y. Serum adiponectin concentrations are decreased in women with endometriosis. Hum. Reprod. 2005, 20, 3510–3513.
- Takemura, Y.; Osuga, Y.; Harada, M.; Hirata, T.; Koga, K.; Yoshino, O.; Hirota, Y.; Morimoto, C.; Yano, T.; Taketani, Y. Concentration of adiponectin in peritoneal fluid is decreased in women with endometriosis. Am. J. Reprod. Immunol. 2005, 54, 217–221.
- Takemura, Y.; Osuga, Y.; Yamauchi, T.; Kobayashi, M.; Harada, M.; Hirata, T.; Morimoto, C.; Hirota, Y.; Yoshino, O.; Koga, K.; et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 2006, 147, 3203–3210.
- Manna, P.R.; Chandrala, S.P.; King, S.R.; Jo, Y.; Counis, R.; Huhtaniemi, I.T.; Stocco, D.M. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol. Endocrinol. 2006, 20, 362–378.
- Martinelle, N.; Holst, M.; Soder, O.; Svechnikov, K. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology 2004, 145, 4629–4634.
- Eaton, J.L.; Unno, K.; Caraveo, M.; Lu, Z.; Kim, J.J. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, E1871–E1879.
- Sirianni, R.; Chimento, A.; Malivindi, R.; Mazzitelli, I.; Ando, S.; Pezzi, V. Insulin-like growth factor-I, regulating aromatase expression through steroidogenic factor 1, supports estrogen-dependent tumor Leydig cell proliferation. Cancer Res. 2007, 67, 8368–8377.
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell. Signal. 2006, 18, 1351–1359.
- Soares, S.R.; Martínez-Varea, A.; Hidalgo-Mora, J.J.; Pellicer, A. Pharmacologic therapies in endometriosis: A systematic review. Fertil. Steril. 2012, 98, 529–555.
- Paul, S.; Sharma, A.V.; Mahapatra, P.D.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal. Res. 2008, 44, 439–449.
- Mori, T.; Yamasaki, S.; Masui, F.; Matsuda, M.; Sasabe, H.; Zhou, Y.F. Suppression of the development of experimentally induced uterine adenomyosis by a novel matrix metalloproteinase inhibitor, ONO-4817, in mice. Exp. Biol. Med. 2001, 226, 429–433.
- Mori, T.; Nakahashi, K.; Kyokuwa, M.; Yamasaki, S.; Nagasawa, H. A matrix metalloproteinase inhibitor, ONO-4817, retards the development of mammary tumor and the progression of uterine adenomyosis in mice. Anticancer. Res. 2002, 22, 3985–3988.
- Vitagliano, A.; Noventa, M.; Gizzo, S. Is it time to consider patients suffering from endometriosis-related infertility as “novel candidates” for targeted peri-conceptional D-chiro inositol supplementation? Hypothesis, rationale and some considerations. J. Assist. Reprod. Genet. 2015, 32, 407–408.
- McKinnon, B.; Bertschi, D.; Wotzkow, C.; Bersinger, N.A.; Evers, J.; Mueller, M.D. Glucose transporter expression in eutopic endometrial tissue and ectopic endometriotic lesions. J. Mol. Endocrinol. 2014, 52, 169–179.
- Melo, A.S.; Rosa-e-Silva, J.C.; Rosa-e-Silva, A.C.; Poli-Neto, O.B.; Ferriani, R.A.; Vieira, C.S. Unfavorable lipid profile in women with endometriosis. Fertil. Steril. 2010, 93, 2433–2436.
- Vallée, A.; Vallée, J.N.; Le Blanche, A.; Lecarpentier, Y. PPARγ Agonists: Emergent Therapy in Endometriosis. Pharmaceuticals 2021, 14, 543.
- Santulli, P.; Marcellin, L.; Noël, J.-C.; Borghese, B.; Fayt, I.; Vaiman, D.; Chapron, C.; Méhats, C. Sphingosine Pathway Deregulation in Endometriotic Tissues. Fertil. Steril. 2012, 97, 904–911.
- Takemura, Y.; Osuga, Y.; Yoshino, O.; Hasegawa, A.; Hirata, T.; Hirota, Y.; Nose, E.; Morimoto, C.; Harada, M.; Koga, K.; et al. Metformin suppresses interleukin (IL)-1beta-induced IL-8 production, aromatase activation, and proliferation of endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2007, 92, 3213–3218.
- Omer, N.A.; Taher, M.A.; Aljebory, H.D.S. Effect ofmetformin treatment on some blood biomarkers in women with endometriosis. Iraq. J. Pharm. Sci. 2016, 25, 28–36.
- Klappan, A.K.; Hones, S.; Mylonas, I.; Brüning, A. Proteasome inhibition by quercetin triggers macroautophagy and blocks mTOR activity. Histochem. Cell. Biol. 2012, 137, 25–36.
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130.
- Ren, Y.; Luo, H. Metformin: The next angiogenesis panacea? SAGE Open Med. 2021, 9, 20503121211001641.
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.M.S.A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8.
- Qu, H.; Yang, X. Metformin inhibits angiogenesis induced by interaction of hepatocellular carcinoma with hepatic stellate cells. Cell Biochem. Biophys. 2015, 71, 931–936.
- Rattan, R.; Graham, R.P.; Maguire, J.L.; Giri, S.; Shridhar, V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 2011, 13, 483–491.
- Wang, J.C.; Li, G.Y.; Wang, B.; Han, S.X.; Sun, X.; Jiang, Y.N.; Shen, Y.W.; Zhou, C.; Feng, J.; Lu, S.Y.; et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J. Exp. Clin. Cancer Res. 2019, 38, 235.
- Moschetta, M.G.; Leonel, C.; Maschio-Signorini, L.B.; Borin, T.F.; Gelaleti, G.B.; Jardim-Perassi, B.V.; Ferreira, L.C.; Sonehara, N.M.; Carvalho, L.G.S.; Hellmén, E.; et al. Evaluation of Angiogenesis Process after Metformin and LY294002 Treatment in Mammary Tumor. Anticancer Agents Med. Chem. 2019, 19, 655–666.
- Orecchioni, S.; Reggiani, F.; Talarico, G.; Mancuso, P.; Calleri, A.; Gregato, G.; Labanca, V.; Noonan, D.M.; Dallaglio, K.; Albini, A.; et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int. J. Cancer 2015, 136, E534–E544.
- Qian, W.; Li, J.; Chen, K.; Jiang, Z.; Cheng, L.; Zhou, C.; Yan, B.; Cao, J.; Ma, Q.; Duan, W. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 2018, 208, 253–261.
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell Physiol. 2019, 234, 19384–19392.
- Matsuzaki, S.; Darcha, C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum. Reprod. 2015, 30, 1606–1616.
- Rooprai, H.K.; McCormick, D. Proteases and Their Inhibitors in Human Brain Tumours: A Review. Anticancer. Res. 1997, 17, 4151–4162.
- Wang, N.; Liu, B.; Liang, L.; Wu, Y.; Xie, H.; Huang, J.; Guo, X.; Tan, J.; Zhan, X.; Liu, Y.; et al. Antiangiogenesis therapy of endometriosis using PAMAM as a gene vector in a noninvasive animal model. Biomed. Res. Int. 2014, 2014, 546479.
- Yilmaz, B.; Sucak, A.; Kilic, S.; Aksakal, O.; Aksoy, Y.; Lortlar, N.; Sut, N.; Gungor, T. Metformin regresses endometriotic implants in rats by improving implant levels of superoxide dismutase, vascular endothelial growth factor, tissue inhibitor of metalloproteinase-2, and matrix metalloproteinase-9. Am. J. Obstet. Gynecol. 2010, 202, 368.
- Guo, M.; Zhou, J.J.; Huang, W. Metformin alleviates endometrial hyperplasia through the UCA1/miR-144/TGF-β1/AKT signaling pathway. Int. J. Mol. Med. 2020, 45, 623–633.
- Kyama, C.M.; Overbergh, L.; Mihalyi, A.; Meuleman, C.; Mwenda, J.M.; Mathieu, C.; D’Hooghe, T.M. Endometrial and Peritoneal Expression of Aromatase, Cytokines, and Adhesion Factors in Women with Endometriosis. Fertil. Steril. 2008, 89, 301–310.
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J. Cell. Sci. 2011, 124, 548–555.
- Herbsleb, M.; Birkenkamp-Demtroder, K.; Thykjaer, T.; Wiuf, C.; Hein, A.M.; Orntoft, T.F.; Dyrskjøt, L. Increased cell motility and invasion upon knockdown of lipolysis stimulated lipoprotein receptor (LSR) in SW780 bladder cancer cells. BMC Med. Genomics 2008, 1, 31.
- Shimada, H.; Satohisa, S.; Kohno, T.; Takahashi, S.; Hatakeyama, T.; Konno, T.; Tsujiwaki, M.; Saito, T.; Kojima, T. The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells. Oncotarget 2016, 7, 27735–27752.
- Gebel, H.M.; Braun, D.P.; Tambur, A.; Frame, D.; Rana, N.; Dmowski, W.P. Spontaneous Apoptosis of Endometrial Tissue Is Impaired in Women with Endometriosis. Fertil. Steril. 1998, 69, 1042–1047.
- Harada, A.; Kimura, Y.; Kojima, C.; Kono, K. Effective Tolerance to Serum Proteins of Head-Tail Type Polycation Vectors by PEGylation at the Periphery of the Head Block. Biomacromolecules 2010, 11, 1036–1042.
- Vaskivuo, T.E.; Stenbäck, F.; Karhumaa, P.; Risteli, J.; Dunkel, L.; Tapanainen, J.S. Apoptosis and Apoptosis-Related Proteins in Human Endometrium. Mol. Cell. Endocrinol. 2000, 165, 75–83.
- Iba, Y.; Harada, T.; Horie, S.; Deura, I.; Iwabe, T.; Terakawa, N. Lipopolysaccharide-Promoted Proliferation of Endometriotic Stromal Cells via Induction of Tumor Necrosis Factor Alpha and Interleukin-8 Expression. Fertil. Steril. 2004, 82 (Suppl. S3), 1036–1042.
- Khan, K.N.; Masuzaki, H.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Sekine, I.; Matsuyama, T.; Ishimaru, T. Interleukin-6- and Tumour Necrosis Factor Alpha-Mediated Expression of Hepatocyte Growth Factor by Stromal Cells and Its Involvement in the Growth of Endometriosis. Hum. Reprod. 2005, 20, 2715–2723.
- Hsieh Li, S.M.; Liu, S.T.; Chang, Y.L.; Ho, C.L.; Huang, S.M. Metformin causes cancer cell death through downregulation of p53-dependent differentiated embryo chondrocyte 1. J. Biomed. Sci. 2018, 25, 81.
- Xiao, X.; He, Q.; Lu, C.; Werle, K.D.; Zhao, R.X.; Chen, J.; Davis, B.C.; Cui, R.; Liang, J.; Xu, Z.X. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol. Oncol. 2012, 127, 249–255.
- Irie, H.; Banno, K.; Yanokura, M.; Iida, M.; Adachi, M.; Nakamura, K.; Umene, K.; Nogami, Y.; Masuda, K.; Kobayashi, Y.; et al. Metformin: A candidate for the treatment of gynecological tumors based on drug repositioning. Oncol. Lett. 2016, 11, 1287–1293.
- Chen, Y.H.; Yang, S.F.; Yang, C.K.; Tsai, H.D.; Chen, T.H.; Chou, M.C.; Hsiao, Y.H. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol. Med. Rep. 2021, 23, 88.
- Fu, Y.L.; Zhang, Q.H.; Wang, X.W.; He, H. Antidiabetic drug metformin mitigates ovarian cancer SKOV3 cell growth by triggering G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1169–1175.
- Zhao, Y.; Sun, H.; Feng, M.; Zhao, J.; Zhao, X.; Wan, Q.; Cai, D. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol. Endocrinol. 2018, 34, 428–432.
- Zhang, H.H.; Zhang, Y.; Cheng, Y.N.; Gong, F.L.; Cao, Z.Q.; Yu, L.G.; Guo, X.L. Metformin in combination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol. Carcinog. 2018, 57, 44–56.
- Storozhuk, Y.; Hopmans, S.N.; Sanli, T.; Barron, C.; Tsiani, E.; Cutz, J.C.; Pond, G.; Wright, J.; Singh, G.; Tsakiridis, T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br. J. Cancer 2013, 108, 2021–2032.
- Dhillon, S.S.; Groman, A.; Meagher, A.; Demmy, T.; Warren, G.W.; Yendamuri, S. Metformin and not diabetes influences the survival of resected early stage NSCLC patients. J. Cancer Sci. Ther. 2014, 6, 217–222.
- Sayed, R.; Saad, A.S.; ElWakeel, L.; Elkholy, E.; Badary, O. Metformin addition to chemotherapy in stage IV non-small cell lung cancer: An open label randomized controlled study. Asian Pac. J. Cancer Prev. 2015, 16, 6621–6626.
- Falcone, T.; Lebovic, D.I. Clinical management of endometriosis. Obstet. Gynecol. 2011, 118, 691–705.
- Collinet, P.; Fritel, X.; Revel-Delhom, C.; Ballester, M.; Bolze, P.A.; Borghese, B.; Bornsztein, N.; Boujenah, J.; Brillac, T.; Chabbert-Buffet, N.; et al. Management of endometriosis: CNGOF/HAS clinical practice guidelines—Short version. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 265–274.
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565.
- Yari, S.; Khoei, H.H.; Saber, M.; Esfandiari, F.; Moini, A.; Shahhoseini, M. Metformin attenuates expression of angiogenic and inflammatory genes in human endometriotic stromal cells. Exp. Cell Res. 2021, 404, 112659.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
3 times
(View History)
Update Date:
11 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




