Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ravinder Nagpal | -- | 3689 | 2022-04-30 04:07:54 | | | |
| 2 | Vivi Li | -20 word(s) | 3669 | 2022-05-05 04:18:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nagpal, R.; Kadyan, S.; , .; Arjmandi, B.; Singh, P. Prebiotic Potential of Dietary Beans and Pulses. Encyclopedia. Available online: https://encyclopedia.pub/entry/22528 (accessed on 03 March 2026).
Nagpal R, Kadyan S, , Arjmandi B, Singh P. Prebiotic Potential of Dietary Beans and Pulses. Encyclopedia. Available at: https://encyclopedia.pub/entry/22528. Accessed March 03, 2026.
Nagpal, Ravinder, Saurabh Kadyan, , Bahram Arjmandi, Prashant Singh. "Prebiotic Potential of Dietary Beans and Pulses" Encyclopedia, https://encyclopedia.pub/entry/22528 (accessed March 03, 2026).
Nagpal, R., Kadyan, S., , ., Arjmandi, B., & Singh, P. (2022, April 30). Prebiotic Potential of Dietary Beans and Pulses. In Encyclopedia. https://encyclopedia.pub/entry/22528
Nagpal, Ravinder, et al. "Prebiotic Potential of Dietary Beans and Pulses." Encyclopedia. Web. 30 April, 2022.
Copy Citation
Dietary pulses, including dry beans, lentils, chickpeas, and dry peas, have the highest proportion of fiber among different legume cultivars and are inexpensive, easily accessible, and have a long shelf-life. The inclusion of pulses in regular dietary patterns is an easy and effective solution for achieving recommended fiber intake and maintaining a healthier gut and overall health. Dietary pulses-derived resistant starch (RS) is a relatively less explored prebiotic ingredient. Several in vitro and preclinical studies have elucidated the crucial role of RS in fostering and shaping the gut microbiota composition towards homeostasis thereby improving host metabolic health.
aging
beans
fiber
gut health
lentils
microbiota
microbiome
prebiotic
pulses
resistant starch
1. Introduction
Pulses are valuable dry grains from leguminous crops. Domesticated around 10,000 years ago, pulses have been consumed as a key staple food crop, especially in developing nations, thus providing a primary means of protein and energy [1]. However, the past century has witnessed a change in the eating habits of the population, especially the decline of the pulse consumption in the daily diet and a surge in the chronic disease rates [2]. Based on a posteriori and a priori dietary patterns, consumption of whole grains and legumes/pulses are linked with longevity and better cardiovascular, metabolic, and cognitive health [3]. On the contrary, diets rich in refined grains, red meat, and sugar have been associated with an increased risk of mortality and adverse cardiometabolic outcomes [3].
Although there are numerous pulse varieties available worldwide, Food and Agriculture Organization (FAO) has listed 11 main types, namely beans, broad beans, Bambara beans, chickpeas, lentils, cowpeas, peas, pigeon peas, vetches, lupins, and other “minor” pulses [4]. Among them, lentils (Lens culinaris L.), beans (Paseolus vulgaris L.), chickpeas (Cicer arietinum L.), and peas (Pisum sativum L.) are the most frequently consumed pulses worldwide [5]. Pulses possess superior nutritional properties and harbor various bioactive compounds, viz., fermentable fibers, bioactive peptides, and phytochemicals [6]. The high nutritional value of pulses is attributed to their high-quality protein and soluble and insoluble dietary fiber [7]. The daily intake of dietary fiber at a level of 14 g/1000 kcal or above has been proposed to confer health benefits in human cohorts [8]. Still, a developed nation like the United States is far from achieving this level, and the magnitude of the gap is nearly 50–70% shortfall [9]. To address this shortfall, supplementation of diets with pulses could be one promising strategy as the total fiber content in pulses can range up to 30 g/100 g dry weight (peas: 14–26 g; lentils: 18–20 g; chickpeas: 18–22 g; beans: 23–32 g), with insoluble fiber being the major sub-component (peas: 10–15 g; lentils: 11–17 g; chick-peas: 10–18 g; beans: 20–28 g) [4].
Starch is the major carbohydrate in pulses accounting for nearly 50% portion of carbohydrates [10]. Certain starches present in the raw and/or cooked pulses exist in the form of dietary fiber instead of available carbohydrates. This is due to the partial or complete modification in the starch structure during heat processing of foods leading to the formation of resistant starch (RS). RS remains un-digested in the upper-gastrointestinal tract and reaches the large intestine, where it is metabolized by intestinal microbes into a wide range of metabolites, which helps in the maintenance of optimal human health [11]. Past studies have also proven the prebiotic potential of RS in improving the post-prandial glycemic and insulinemic responses, increasing satiety, reducing cholesterol and stored fat, and promoting weight loss, making it an apt ingredient, especially for the management of gut-associated metabolic disorders [12][13][14][15]. Hitherto, studies assessing the human health benefits of RS were confined to RS derived from cereals and tubers, with little to no focus given on RS derived from pulses. Recently, efforts were made in researchers' lab to isolate and purify starches from 18 pulses which were evaluated for their functional properties in order to promote their use as superior food ingredients in industry [16]. Owing to the superior sensory property of selected pulse RS compared to traditional fibers like whole cereals, fruit fibers, etc., the supplementation of this functional ingredient in diet could act as a beneficial nutritional intervention for the control of metabolic diseases [17].
Nowadays, it has been widely popularized that the human gastrointestinal (GI) tract is a frontline mediator system wherein the intestinal bacteria aid in the digestion of dietary constituents of consumed foods and synthesizes low molecular weight bioactive molecules, which ultimately exerts a crucial role on human health and well-being [18]. The human gastrointestinal tract contains nearly 1014 microorganisms belonging to over 1000 species and has a bacterial genomic content of approximately 100 times over compared to the human genome [19]. About 95% of the total microbes present in the human body are colonized in the GI tract. The GI tract is the home of bacteria, eukaryotes, and archaea and is collectively known as gut microbiota [20]. Several factors such as the morphology of the gut, nutrient availability, pH, and presence or absence of oxygen are responsible for the variation in gut microbiota composition and growth of certain microbial taxa specific to different regions of the gut. The most common gut bacteria are associated with the four major phyla, with the most abundant being Firmicutes (65%), followed by Bacteroidetes (25%), Proteobacteria (8%), and Actinobacteria (5%). Moving down the taxonomic hierarchy, the GI tract harbors three main groups of extremophile anaerobes Clostridium coccoides group (or Clostridium cluster XIVa), Clostridium leptum group (or Clostridium cluster IV), and Bacteroides [21]. The gut microbes, together with their metabolites produced as a result of the degradation of different substrates, provide a range of immune, metabolic and neurobehavioral functions to host health.
Gut microbiota is dynamic in nature and changes continuously during the lifespan of an individual [22]. During the aging process of an individual, dynamic changes occur in behavioral, environmental, biological, and social processes. Genomic instability, epigenetic alterations, and telomere attrition are primary indicators of aging, resulting in cellular senescence, problems in nutrient sensing, and mitochondrial-related dysfunctions, which further negatively impact intercellular communication and exhaustion of stem cells [23]. Thus, the aging-associated decline in the cellular functions and immune system responses leads to chronic low-grade inflammation and increased gut permeability, thereby marking the onset of various gastrointestinal disorders, cardiometabolic disease, muscle frailty, cognitive decline, and gut dysbiosis [24]. Aging-associated problems are further aggravated by the ill effects of western diets rich in fat and sugars, which may increase the propensity towards gut dysbiosis [25]. The maintenance of a healthy and diverse gut microbiota that coevolves with our lifespan is a principal factor in the amelioration of various age-related diseases. Earlier studies by researchers' group indicated that the severity of gut dysbiosis is higher in older cohorts than the young ones [26].
2. Resistant Starch and Human Health
Starch is a dietary carbohydrate that is commonly found in everyday food. It is the second most abundant chemical compound in the plants after cellulose. Chemically, starch is composed of two monosaccharide molecules that are amylose (linear chain) and amylopectin (branched chain). These molecules are linked together with alpha 1-4 and/or alpha 1-6 glycosidic bonds. Based on physical and physiological properties, starch can be classified into three categories, namely rapidly digestible starch, slowly digestible starch, and resistant starch (RS) [27]. Englyst and coworkers (1982), in an in vitro study, found that some portion of the starch remained undigested even after enzymatic treatment. Further studies confirmed that these starches were undigested by the amylases in the small intestine and enter the colon, where it is utilized by gut microbial communities. They named this starch fragment “resistant starch” [28]. The digestibility of the starch in the small intestine is primarily affected by the structure of the starch molecule and the ratio of amylose to amylopectin. Chemically, RS has a relatively low molecular weight (12 KDa) and has a linear structure made up of α-1,4-D-glucan moieties obtained from the retrograded amylose fraction [17].
Resistant starch is further subdivided into five types depending upon its structural features. RS type 1 (RS1) is physically inaccessible starch and has the most complex structures as it is frequently found entrapped within protein matrix or non-starch components of the plant cell wall (e.g., whole grains or pulses) [11]. Compared to RS1, the cellular structure is absent in RS type 2 (RS2). The RS type 2 possesses native, uncooked, and semi-crystalline starch granules having a B- or C-type polymorph (e.g., high-amylose starch, raw potato starch) [11]. The RS type 3 (RS3) is obtained by retrogradation process upon cooking and cooling of starch-containing foods. Its resistance to digestion could be due to lower activity of pancreatic α-amylases toward starch double helices as against fully gelatinized starch molecules (e.g., retrograded high amylose maize starch) [29]. The RS type 4 (RS4) is the starch-modified through chemical processes such as esterification, crosslinking, hydroxypropylation, acetylation, and phosphorylation [30]. The functional groups block the site of action of starch digestive enzymes, which confers resistance of RS4 to digestion. The RS type 5 (RS5) is defined as the starch obtained by complex formation between high amylose starch with the lipids, which further increases the enzyme resistance of high amylose by preventing granule swelling during cooking [17].
Resistant starch possesses many desirable functional and health-promoting properties [27]. An overview of the effect of resistant starch derived from the pulses on the health outcome of humans and rodents is summarized in Figure 1. RS fermentation in the lower GI tract produces different starch oligomers and SCFAs. SCFAs are actively involved in reducing the risk of diabetes, cancer, obesity, and other cardiovascular diseases [8][9][25]. Among them, acetate, propionate, and butyrate have been extensively studied for their health benefits. Acetate is the major SCFA that is produced to the tune of 65% in the colon resulting in significant drops in pH. Thus, it helps in the inhibition of various pathogenic microorganisms and indirectly aids in the absorption of minerals such as calcium, iron, and sodium Butyrate, on the other hand, provides energy to colonocytes, possesses anti-inflammatory properties, protects against colon cancer, and plays a key role in gut homeostasis as well as maintaining the integrity of epithelium [31]. Butyrate is also responsible for lower levels of glycolysis and glycogenolysis (Ashwar et al., 2017). Propionate is another important metabolite that is partially absorbed via portal veins and reaches the liver. It is then metabolized as a glucogenic substrate resulting in inhibition of pathways leading to reduced 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA) activity and suppression of acetyl-CoA reductase, thereby imparting reduction in blood plasma cholesterol levels [32]. The serum cholesterol-lowering effect of RS was demonstrated in rats when they were fed a cholesterol-free diet [33].
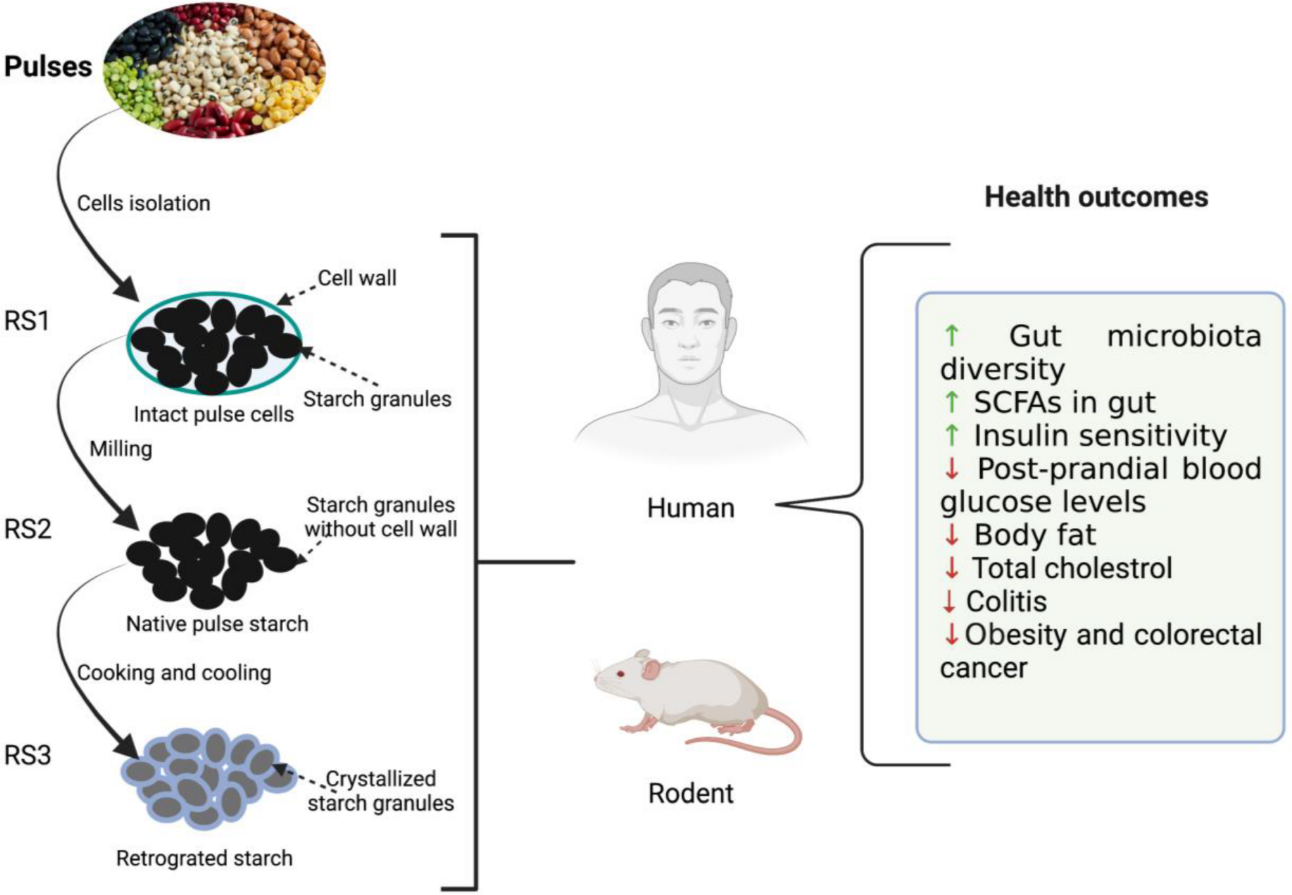
Figure 1. Illustration depicting the effects of resistant starches (RS) derived from dietary beans and pulses on host (rodent and human) health. ↑: increased; ↓: decreased.
3. Benefits of Dietary Beans and Pulses on Gut Health
The recent advances linking the role of dietary fibers in ameliorating different disease states have led to increased interest in pulse-based foods. Various types of fibers present in pulses include long-chain soluble and insoluble polysaccharides, resistant starch, and galactooligosaccharides. In addition, these components can act as prebiotic precursors, which are digested by beneficial microorganisms in the gut. The consumption of pulses in the diet has been linked to the reduction in serum cholesterol, increased satiety, and low post-prandial blood glucose levels, thus mitigating the risk of different metabolic diseases like cardiovascular diseases, obesity, diabetes, etc. [34][35]. In fact, several meta-analyses concluded that daily pulse intake of approximately 2/3 cups could significantly lower total and LDL cholesterol [36]. The low glycemic response of pulse is associated to the physical barrier between the starch and digestive enzymes by the intact cell wall of whole pulses after cooking. Furthermore, pulse consumption is closely associated with reducing blood pressure and providing protection against reactive oxygen species due to the presence of high levels of polyphenols [37].
In the last few years, more research has been directed towards pulses which could be a sustainable source of plant protein compared to animal protein to feed the growing population and to simultaneously address the food insecurity problems [4]. Additionally, whole pulses being rich in plant-based protein and dietary fibers underpins the hypothesis of their positive effects on the gut microbiota. Table 1 summarizes the influence of consumption of pulses in various forms—cooked, flour, meals, or supplemented in the diet, on the gut microbiota changes in rodents and humans. A study on pulse flour exhibited improved growth of genera Bifidobacterium, Faecalibacterium, Clostridium, Eubacterium, and Roseburia along with enhanced butyrate and acetate production [38]. Several studies have reported that the incorporation of pulses in the diet increases the abundance of Prevotella, Dorea, and Ruminococcus flavefaciens, and decreased abundance of Ruminococcus gnavus in mice models [18][39][40][41][42]. Prevotella is a genus possessing a large spectrum of glycoside hydrolases and is known for its ability to produce SCFAs following the carbohydrates fermentation [39]. The species Ruminococcus flavefaciens had been found to decrease in overweight (BMI: 25.0–29.9) and obese (BMI: >30.0) subjects [43]. The abundance of Ruminococcus gnavus, a mucolytic species, has been linked to an increase in gut-barrier pathologies in subjects with obesity and inflammatory bowel disease [41]. Another positive effect of pulse intake is the increased prevalence of Akkermansia muciniphila in the gut, which is often categorized as next-generation probiotics [8][44]. Interestingly, this bacterium is also mucolytic but has an inverse correlation with R. gnavus [45]. Majority of the studies reported herein demonstrated a decrease in the ratio of Firmicutes to Bacteroidetes. This reduction in the ratio of two major phyla has been associated with the amelioration of obesity, possibly due to altered energy extraction from carbohydrates metabolism in the colon [46]. Among the Bacteroidales, the members representative of the pulse-based diets includes Muribaculaceae (S24-7), Rikenellaceae and B. acidifaciens [18]. Lentil consumption was found to be associated with increased prevalence of Roseburia in mouse feces [40]. Roseburia is involved in butyrate production and has negative correlation with several diseases such as colitis and Crohn’s disease [47]. Although these studies revealed beneficial effects of pulses in positively modulating the gut microbiome, the impact on different gut genera is complex, which may be dependent upon many variables, such as pulse type, dose, age, status of cohorts, duration of the study and the sequencing methodology adopted.
Table 1. Effect of dietary pulses on gut microbiota-related changes in rodents and humans.
| Pulse-Type | Cohort | State of Cohort | Age | Dose | Duration of Study | Key Shifts in Gut Microbiota | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Cooked chickpeas | Human | Healthy | 18–65 years | 200 g/d | 3 weeks |
|
|
[48] |
| Cooked pinto beans | Human | Healthy; Pre-metabolic syndrome | 18–51 years | 130 g/d | 12 weeks + 4 weeks run-in |
|
|
[49] |
| Cooked navy bean powder | Human | Colorectal cancer survivors (overweight and obese) | 47–81 years | 35 g/d | 28 days |
|
|
[50] |
| Cooked navy beans (incorporated in meals and snacks) | Human | Colorectal cancer survivors (overweight and obese) | NB: 60.9 ± 11.0 years Control: 65.50 ± 3.07 years |
35 g/d | 4 weeks |
|
[51] | |
| Beans, chickpeas, peas, or lentils-based foods | Human | Healthy | 57 ± 6.3 | 150 g/d | 4 months |
|
[52] | |
| Dolichos lablab L. (standardized extract) | Mice (C57BL/6 male) |
IBS model | 7 weeks | 100–400 mg/kg | 15 days |
|
[53] | |
| Chickpea supplemented diet | Mice (C57BL/6 male) | Healthy | 5 weeks | 200 g/kg diet | 3 weeks |
|
|
[41] |
| Cooked white and dark red kidney beans | Mice (C57BL/6 male) | DSS induced colitis | 5 weeks | BD + 20% beans | 3 weeks |
|
[42] | |
| Cooked Navy bean or black bean | Mice (C57Bl/6 male) |
Healthy | 4 weeks | Supplementation @20% to the basal diet | 3 weeks |
|
|
[39] |
| Cranberry beans | Mice (C57BL/6 male) |
Healthy and DSS induced colitis | 5 weeks | BD + 20% beans | 3 weeks |
|
In healthy cohorts:
In diseased cohorts:
|
[54] |
| Lentil, chickpea, bean, and dry pea | Mice (C57BL/6NCrl mice) |
Healthy | 3–4 weeks | 40 g/100 g obesogenic diet (by replacing 35% protein) | 17 weeks |
|
|
[18] |
| Cooked red lentils | Mice (C57Bl/6 male) |
Healthy | 5 weeks | 20% w/w basal diet | 3 weeks |
|
|
[40] |
| Chickpea, lentil, dry peas, and bean | Mice (C57BL/6 male) |
Obese | 3–4 weeks | 40% w/w diet | 17 weeks |
|
|
[8] |
| Whole mung bean | Mice (C57BL/6 male) |
Diet-induced obesity (1 w HFD feeding) |
4 weeks | HFD + 30% bean | 12 weeks |
|
|
[44] |
| Lentil (Lens culinaris Medikus) | Rats (Sprague−Dawley) |
Healthy | 8 weeks | 70.8% red lentil diet | 6 weeks |
|
|
[55] |
| Yellow pea flour | Rats | Diet-induced obesity (5 w HFD feeding) | 5 weeks | 30% w/w diet | 42 days |
|
|
[46] |
| Whole yellow pea flour | Hamster (Golden Syrian) | Hypercholesterolemic diet (28 days) | 2 weeks | 10% replacement of corn starch with pea flour in the diet | 28 days |
|
|
[56] |
NB: navy bean; BB: black bean; DSS: dextran sodium sulphate; IBS: irritable bowel syndrome; BD: basal diet; ↑: increased; ↓: decreased.
Common beans, chickpea, and lentils have been shown to exert positive effects in the modulation of the colonic microenvironment in animal models [18][39][40][42]. These include enhancement of (i) crypt mucus content and mucin mRNA expression; (ii) expression of epithelial tight junction proteins; (iii) crypt length, epithelial cell proliferation, and goblet cell number; (iv) SCFAs levels (acetate, propionate, and butyrate); (v) expression of G protein-coupled receptors in the intestine; (vi) reduced pro-inflammatory cytokines in the serum. Increased expression of G protein-coupled receptors in the colon is related to sensing high SCFA production by gut microbes which are implicated in adipose tissue metabolism and appetite regulation [57]. The benign role of whole pulse consumption in the modulation of human gut microbiota and metabolite profile have also been explored in the past by researchers using clinical trials [48][49][50][51][52]. Some of these include reduction in pathogenic and putrefactive gut bacteria species; increase in Bacteroidetes and Faecalibacterium prausnitzii; decreased total serum cholesterol, LDL- and HDL-cholesterol; boost in microbial richness, and significant change in metabolite profile (e.g., ophthalmate) in colorectal cancer survivors.
4. Prebiotic Potential of Pulses-Derived Resistant Starch for Gut Health
The concentration of SCFAs in the lower GI tract normally reduces from the proximal to the distal colon. The amount of SCFAs production is majorly dependent upon the amount of fiber reaching the distal colon. Therefore, one way of increasing the SCFAs in the distal gut is the selection of dietary fibers, which are minimally digested prior to reaching the distal colon. Increasing the consumption of resistant starches in the diet is a promising strategy to modulate gut health and benefit the host.
Native RS is present in varying proportions in cereals, tubers, and legumes. In addition, the RS content can be altered using cooking and cooling operations. Interestingly, the comparison of RS content among cooked cereals, legumes, and tubers samples showed legumes with the highest RS content [58]. Brummer, Kaviani and Tosh [6] reported that cooked pulses have a relatively high proportion of resistant starch (3.75–4.66% of pulse dry weight basis) than many other cooked foods. Similarly, Garcia-Alonso et al. [59] reported a marginal increase in the RS content of chickpeas, lentils, and common beans upon boiling, cooling, and reheating. Retrogradation of the gelatinized starch post-cooking and cooling is usually associated with the increased content of resistant starch in the cooked pulses [60]. Still, the amount of RS in raw, baked, and boiled pulses differ significantly, and it is a function of its intrinsic factors (e.g., amylose to amylopectin ratio, crystallinity, granular structure) and external factors (e.g., processing methods employed, storage period and conditions [13]. In brown lentils (Lens culinaris, Medikus), RS content was further increased by the addition of lipids, resulting in the formation of amylose-lipid complexes (RS5 type) [61].
References
- Henry J. Thompson; Dietary Bean Consumption and Human Health. Nutrients 2019, 11, 3074, 10.3390/nu11123074.
- Effie Viguiliouk; Sonia Blanco Mejia; Cyril W.C. Kendall; John L. Sievenpiper; Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Annals of the New York Academy of Sciences 2017, 1392, 43-57, 10.1111/nyas.13312.
- Jessica C. Kiefte-De Jong; John C. Mathers; Oscar Franco; Nutrition and healthy ageing: the key ingredients. Proceedings of the Nutrition Society 2014, 73, 249-259, 10.1017/s0029665113003881.
- Helena Ferreira; Marta Vasconcelos; Ana M. Gil; Elisabete Pinto; Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Critical Reviews in Food Science and Nutrition 2020, 61, 85-96, 10.1080/10408398.2020.1716680.
- C.P.F. Marinangeli; S.V. Harding; M. Zafron; T.C. Rideout; A systematic review of the effect of dietary pulses on microbial populations inhabiting the human gut. Beneficial Microbes 2020, 11, 1-12, 10.3920/bm2020.0028.
- Yolanda Brummer; Mina Kaviani; Susan M. Tosh; Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Research International 2014, 67, 117-125, 10.1016/j.foodres.2014.11.009.
- Joyce Boye; Fatemeh Zare; Alison Pletch; Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Research International 2010, 43, 414-431, 10.1016/j.foodres.2009.09.003.
- John McGinley; Vanessa Fitzgerald; Elizabeth Neil; Heather Omerigic; Adam Heuberger; Tiffany Weir; Rebecca McGee; George Vandemark; Henry Thompson; Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593, 10.3390/nu12030593.
- Henry J Thompson; Mark A Brick; Perspective: Closing the Dietary Fiber Gap: An Ancient Solution for a 21st Century Problem.. Advances in Nutrition: An International Review Journal 2016, 7, 623-6, 10.3945/an.115.009696.
- Ratnajothi Hoover; Stewart C. Rorke; Antonio M. Martin; ISOLATION AND CHARACTERIZATION OF LIMA BEAN (PHASEOLUS LUNATUS) STARCH. Journal of Food Biochemistry 1991, 15, 117-136, 10.1111/j.1745-4514.1991.tb00149.x.
- Li, C.; Hu, Y. Align resistant starch structures from plant-based foods with human gut microbiome for personalized health promotion. Crit Rev Food Sci Nutr 2021, 1-12, doi:10.1080/10408398.2021.1976722
- Janine A. Higgins; Resistant Starch and Energy Balance: Impact on Weight Loss and Maintenance. Critical Reviews in Food Science and Nutrition 2013, 54, 1158-1166, 10.1080/10408398.2011.629352.
- Diane F. Birt; Terri Boylston; Suzanne Hendrich; Jay-Lin Jane; James Hollis; Li Li; John McClelland; Samuel Moore; Gregory J. Phillips; Matthew Rowling; et al.Kevin SchalinskeM. Paul ScottElizabeth M. Whitley Resistant Starch: Promise for Improving Human Health. Advances in Nutrition: An International Review Journal 2013, 4, 587-601, 10.3945/an.113.004325.
- Yong Wang; Jing Chen; Ying-Han Song; Rui Zhao; Lin Xia; Yi Chen; Ya-Ping Cui; Zhi-Yong Rao; Yong Zhou; Wen Zhuang; et al.Xiao-Ting Wu Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: a systematic review and meta-analysis. Nutrition & Diabetes 2019, 9, 1-11, 10.1038/s41387-019-0086-9.
- Janine A Higgins; Resistant Starch: Metabolic Effects and Potential Health Benefits. Journal of AOAC INTERNATIONAL 2004, 87, 761-768, 10.1093/jaoac/87.3.761.
- Oluwatoyin O. Sangokunle; Shridhar K. Sathe; Prashant Singh; Purified Starches from 18 Pulses Have Markedly Different Morphology, Oil Absorption and Water Absorption Capacities, Swelling Power, and Turbidity. Starch - Stärke 2020, 72, 2000022, 10.1002/star.202000022.
- Aswir Abd Rashed; Fatin Saparuddin; Devi-Nair Gunasegavan Rathi; Nur Najihah Mohd Nasir; Ezarul Faradianna Lokman; Effects of Resistant Starch Interventions on Metabolic Biomarkers in Pre-Diabetes and Diabetes Adults. Frontiers in Nutrition 2022, 8, 793414, 10.3389/fnut.2021.793414.
- Tymofiy Lutsiv; Tiffany L. Weir; John N. McGinley; Elizabeth S. Neil; Yuren Wei; Henry J. Thompson; Compositional Changes of the High-Fat Diet-Induced Gut Microbiota upon Consumption of Common Pulses. Nutrients 2021, 13, 3992, 10.3390/nu13113992.
- Nannan Guan; Xiaowei He; Shaokang Wang; Feitong Liu; Qiang Huang; Xiong Fu; Tingting Chen; Bin Zhang; Cell Wall Integrity of Pulse Modulates the in Vitro Fecal Fermentation Rate and Microbiota Composition. Journal of Agricultural and Food Chemistry 2020, 68, 1091-1100, 10.1021/acs.jafc.9b06094.
- Fredrik Bäckhed; Ruth E. Ley; Justin L. Sonnenburg; Daniel A. Peterson; Jeffrey I. Gordon; Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915-1920, 10.1126/science.1104816.
- Loris R Lopetuso; Franco Scaldaferri; Valentina Petito; Antonio Gasbarrini; Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathogens 2012, 5, 1-8, 10.1186/1757-4749-5-23.
- Elizabeth Thursby; Nathalie Juge; Introduction to the human gut microbiota. Biochemical Journal 2017, 474, 1823-1836, 10.1042/bcj20160510.
- Carlos López-Otín; Maria A. Blasco; Linda Partridge; Manuel Serrano; Guido Kroemer; The Hallmarks of Aging. Cell 2013, 153, 1194-1217, 10.1016/j.cell.2013.05.039.
- Dwina Juliana Warman; Huijuan Jia; Hisanori Kato; The Potential Roles of Probiotics, Resistant Starch, and Resistant Proteins in Ameliorating Inflammation during Aging (Inflammaging). Nutrients 2022, 14, 747, 10.3390/nu14040747.
- Yawen Zhang; Luyi Chen; Mengjia Hu; John J. Kim; RenBin Lin; Jilei Xu; Lina Fan; Yadong Qi; Lan Wang; Weili Liu; et al.Yanyong DengJianmin SiShujie Chen Dietary type 2 resistant starch improves systemic inflammation and intestinal permeability by modulating microbiota and metabolites in aged mice on high-fat diet. Aging 2020, 12, 9173-9187, 10.18632/aging.103187.
- Shokouh Ahmadi; Ravinder Nagpal; Shaohua Wang; Jason Gagliano; Dalane W Kitzman; Sabihe Soleimanian-Zad; Mahmoud Sheikh-Zeinoddin; Russel Read; Hariom Yadav; Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome–gut–brain axis modulation. The Journal of Nutritional Biochemistry 2019, 67, 1-13, 10.1016/j.jnutbio.2019.01.011.
- Pinky Raigond; Rajarathnam Ezekiel; Baswaraj Raigond; Resistant starch in food: a review. Journal of the Science of Food and Agriculture 2014, 95, 1968-1978, 10.1002/jsfa.6966.
- H. Englyst; H. S. Wiggins; J. H. Cummings; Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. The Analyst 1981, 107, 307-318, 10.1039/an9820700307.
- Hamung Patel; Paul Royall; Simon Gaisford; Gareth R. Williams; Cathrina Edwards; Frederick Warren; Bernadine M. Flanagan; Peter R. Ellis; Peter J. Butterworth; Structural and enzyme kinetic studies of retrograded starch: Inhibition of α-amylase and consequences for intestinal digestion of starch. Carbohydrate Polymers 2017, 164, 154-161, 10.1016/j.carbpol.2017.01.040.
- Bilal Ahmad Ashwar; Adil Gani; Asima Shah; Farooq Ahmad Masoodi; Physicochemical properties, in-vitro digestibility and structural elucidation of RS4 from rice starch. International Journal of Biological Macromolecules 2017, 105, 471-477, 10.1016/j.ijbiomac.2017.07.057.
- Junyi Yang; Devin J. Rose; The impact of long-term dietary pattern of fecal donor on in vitro fecal fermentation properties of inulin. Food & Function 2015, 7, 1805-1813, 10.1039/c5fo00987a.
- Jiangbin Xu; Zhen Ma; Xiaoping Li; Liu Liu; Xinzhong Hu; A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food & Function 2020, 11, 1982-1995, 10.1039/c9fo02025j.
- Naoto Hashimoto; Yusaku Ito; Kyu-Ho Han; Ken-Ichiro Shimada; Mitsuo Sekikawa; David L. Topping; Anthony R. Bird; Takahiro Noda; Hideyuki Chiji; Michihiro Fukushima; et al. Potato Pulps Lowered the Serum Cholesterol and Triglyceride Levels in Rats. Journal of Nutritional Science and Vitaminology 2005, 52, 445-450, 10.3177/jnsv.52.445.
- J. L. Sievenpiper; C. W. C. Kendall; A. Esfahani; J. M. W. Wong; A. J. Carleton; H. Y. Jiang; R. P. Bazinet; E. Vidgen; D. J. A. Jenkins; Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479-1495, 10.1007/s00125-009-1395-7.
- Vanessa Ha; John L. Sievenpiper; Russell J. de Souza; Viranda H. Jayalath; Arash Mirrahimi; Arnav Agarwal; Laura Chiavaroli; Sonia Blanco Mejia; Frank M. Sacks; Marco Di Buono; et al.Adam M. BernsteinLawrence A. LeiterPenny M. Kris-EthertonVladimir VuksanRichard P. BazinetRobert G. JosseJoseph BeyeneCyril W.C. KendallDavid J.A. Jenkins Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. Canadian Medical Association Journal 2014, 186, E252-E262, 10.1503/cmaj.131727.
- Emily M.T. Padhi; D. Dan Ramdath; A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. Journal of Functional Foods 2017, 38, 635-643, 10.1016/j.jff.2017.03.043.
- V. H. Jayalath; R. J. De Souza; J. L. Sievenpiper; V. Ha; L. Chiavaroli; A. Mirrahimi; M. Di Buono; A. M. Bernstein; L. A. Leiter; Penny Kris-Etherton; et al.Vladimir VuksanJ. BeyeneC. W. C. KendallD. J. A. Jenkins Effect of Dietary Pulses on Blood Pressure: A Systematic Review and Meta-analysis of Controlled Feeding Trials. American Journal of Hypertension 2013, 27, 56-64, 10.1093/ajh/hpt155.
- Patricia Gullón; Beatriz Gullón; Freni Tavaria; Marta Vasconcelos; Ana Maria Gomes; In vitro fermentation of lupin seeds (Lupinus albus) and broad beans (Vicia faba): dynamic modulation of the intestinal microbiota and metabolomic output. Food & Function 2015, 6, 3316-3322, 10.1039/c5fo00675a.
- Jennifer M. Monk; Dion Lepp; Wenqing Wu; K. Peter Pauls; Lindsay E. Robinson; Krista A. Power; Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. The Journal of Nutritional Biochemistry 2017, 49, 89-100, 10.1016/j.jnutbio.2017.08.002.
- Daniela Graf; Jennifer M. Monk; Dion Lepp; Wenqing Wu; Laurel McGillis; Kyle Roberton; Yolanda Brummer; Susan M. Tosh; Krista A. Power; Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice. Nutrients 2019, 11, 1853, 10.3390/nu11081853.
- Jennifer M. Monk; Dion Lepp; Wenqing Wu; Daniela Graf; Laurel H. McGillis; Adeel Hussain; Christine Carey; Lindsay E. Robinson; Ronghua Liu; Rong Tsao; et al.Yolanda BrummerSusan M. ToshKrista Power Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. Journal of Functional Foods 2017, 38, 663-674, 10.1016/j.jff.2017.02.002.
- Jennifer M. Monk; Claire P. Zhang; Wenqing Wu; Leila Zarepoor; Jenifer T. Lu; Ronghua Liu; K. Peter Pauls; Geoffrey Wood; Rong Tsao; Lindsay E. Robinson; et al.Krista Power White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. The Journal of Nutritional Biochemistry 2015, 26, 752-760, 10.1016/j.jnutbio.2015.02.003.
- Andreas Schwiertz; David Taras; Klaus Schäfer; Silvia Beijer; Nicolaas A. Bos; Christiane Donus; Philip D. Hardt; Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2009, 18, 190-195, 10.1038/oby.2009.167.
- Dianzhi Hou; Qingyu Zhao; Laraib Yousaf; Yong Xue; Qun Shen; Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. European Journal of Nutrition 2020, 59, 3617-3634, 10.1007/s00394-020-02196-2.
- Chin Wen Png; Sara K Lindén; Kristen S Gilshenan; Erwin G Zoetendal; Chris S McSweeney; Lindsay I Sly; Michael McGuckin; Timothy H J Florin; Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. American Journal of Gastroenterology 2010, 105, 2420-2428, 10.1038/ajg.2010.281.
- Amanda J. Eslinger; Lindsay K. Eller; Raylene A. Reimer; Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats. Nutrition Research 2014, 34, 714-722, 10.1016/j.nutres.2014.07.016.
- Zohreh Tamanai-Shacoori; Imen Smida; Latifa Bousarghin; Olivier Loreal; Vincent Meuric; Shao Bing Fong; Martine Bonnaure-Mallet; Anne Jolivet-Gougeon; Roseburia spp.: a marker of health?. Future Microbiology 2017, 12, 157-170, 10.2217/fmb-2016-0130.
- W. M. U. Fernando; Janet Hill; G. A. Zello; R. T. Tyler; W. J. Dahl; A. G. Van Kessel; Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Beneficial Microbes 2010, 1, 197-207, 10.3920/bm2009.0027.
- John W. Finley; James B. Burrell; Philip G. Reeves; Pinto Bean Consumption Changes SCFA Profiles in Fecal Fermentations, Bacterial Populations of the Lower Bowel, and Lipid Profiles in Blood of Humans. The Journal of Nutrition 2007, 137, 2391-2398, 10.1093/jn/137.11.2391.
- Amy Sheflin; Erica C. Borresen; Jay S. Kirkwood; Claudia M. Boot; Alyssa Whitney; Shen Lu; Regina J. Brown; Corey Broeckling; Elizabeth P. Ryan; Tiffany L. Weir; et al. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors.. Molecular Nutrition & Food Research 2016, 61, 1500905, 10.1002/mnfr.201500905.
- Bridget A. Baxter; Renee C. Oppel; Elizabeth P. Ryan; Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients 2018, 11, 28, 10.3390/nu11010028.
- Saman Abeysekara; Philip D. Chilibeck; Hassanali Vatanparast; Gordon A. Zello; A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. British Journal of Nutrition 2012, 108, S103-S110, 10.1017/s0007114512000748.
- Eunho Chun; Soojung Yoon; Amna Parveen; Mirim Jin; Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model. Nutrients 2018, 10, 1475, 10.3390/nu10101475.
- Jennifer M. Monk; Dion Lepp; Claire P. Zhang; Wenqing Wu; Leila Zarepoor; Jenifer T. Lu; K. Peter Pauls; Rong Tsao; Geoffrey A. Wood; Lindsay E. Robinson; et al.Krista A. Power Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. The Journal of Nutritional Biochemistry 2015, 28, 129-139, 10.1016/j.jnutbio.2015.10.014.
- Niroshan Siva; Casey R. Johnson; Vincent Richard; Elliot D. Jesch; William Whiteside; Abdullah A. Abood; PushparajaH Thavarajah; Susan Duckett; Dil Thavarajah; Lentil (Lens culinaris Medikus) Diet Affects the Gut Microbiome and Obesity Markers in Rat. Journal of Agricultural and Food Chemistry 2018, 66, 8805-8813, 10.1021/acs.jafc.8b03254.
- Christopher P.F. Marinangeli; Peter J.H. Jones; Chronic Intake of Fractionated Yellow Pea Flour Reduces Postprandial Energy Expenditure and Carbohydrate Oxidation. Journal of Medicinal Food 2011, 14, 1654-1662, 10.1089/jmf.2010.0255.
- Jessica Soldavini; Jonathan D. Kaunitz; Pathobiology and Potential Therapeutic Value of Intestinal Short-Chain Fatty Acids in Gut Inflammation and Obesity. Digestive Diseases and Sciences 2013, 58, 2756-2766, 10.1007/s10620-013-2744-4.
- B. S. Yadav; A. Sharma; R. B. Yadav; Resistant starch content of conventionally boiled and pressure-cooked cereals, legumes and tubers. Journal of Food Science and Technology 2009, 47, 84-88, 10.1007/s13197-010-0020-6.
- Alejandra García-Alonso; Isabel Goñi; F. Saura-Calixto; Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Zeitschrift für Lebensmitteluntersuchung und -Forschung A 1998, 206, 284-287, 10.1007/s002170050258.
- Kai Wang; Jovin Hasjim; Alex Chi Wu; Robert J. Henry; Robert G. Gilbert; Variation in Amylose Fine Structure of Starches from Different Botanical Sources. Journal of Agricultural and Food Chemistry 2014, 62, 4443-4453, 10.1021/jf5011676.
- Bahar Nur Okumus; Zeynep Tacer-Caba; Kevser Kahraman; Dilara Nilufer-Erdil; Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chemistry 2018, 240, 550-558, 10.1016/j.foodchem.2017.07.157.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





