Dietary pulses, including dry beans, lentils, chickpeas, and dry peas, have the highest proportion of fiber among different legume cultivars and are inexpensive, easily accessible, and have a long shelf-life. The inclusion of pulses in regular dietary patterns is an easy and effective solution for achieving recommended fiber intake and maintaining a healthier gut and overall health. Dietary pulses-derived resistant starch (RS) is a relatively less explored prebiotic ingredient. Several in vitro and preclinical studies have elucidated the crucial role of RS in fostering and shaping the gut microbiota composition towards homeostasis thereby improving host metabolic health. However, in humans and aged animal models, the effect of only the cereals and tubers derived RS has been studied.
- aging
- beans
- fiber
- gut health
- lentils
- microbiota
- microbiome
- prebiotic
- pulses
- resistant starch
1. Introduction
2. Resistant Starch and Human Health
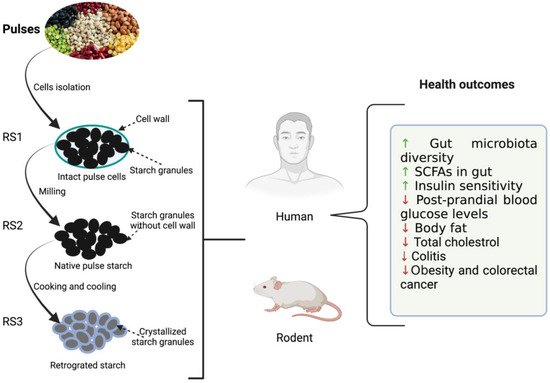
3. Benefits of Dietary Beans and Pulses on Gut Health
| Pulse-Type | Cohort | State of Cohort | Age | Dose | Duration of Study | Key Shifts in Gut Microbiota | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Cooked chickpeas | Human | Healthy | 18–65 years | 200 g/d | 3 weeks |
|
|
[48] |
| Cooked pinto beans | Human | Healthy; Pre-metabolic syndrome | 18–51 years | 130 g/d | 12 weeks + 4 weeks run-in |
|
|
[49] |
| Cooked navy bean powder | Human | Colorectal cancer survivors (overweight and obese) | 47–81 years | 35 g/d | 28 days |
|
|
[50] |
| Cooked navy beans (incorporated in meals and snacks) | Human | Colorectal cancer survivors (overweight and obese) | NB: 60.9 ± 11.0 years Control: 65.50 ± 3.07 years |
35 g/d | 4 weeks |
|
[51] | |
| Beans, chickpeas, peas, or lentils-based foods | Human | Healthy | 57 ± 6.3 | 150 g/d | 4 months |
|
[52] | |
| Dolichos lablab L. (standardized extract) | Mice (C57BL/6 male) |
IBS model | 7 weeks | 100–400 mg/kg | 15 days |
|
[53] | |
| Chickpea supplemented diet | Mice (C57BL/6 male) | Healthy | 5 weeks | 200 g/kg diet | 3 weeks |
|
|
[41] |
| Cooked white and dark red kidney beans | Mice (C57BL/6 male) | DSS induced colitis | 5 weeks | BD + 20% beans | 3 weeks |
|
[42] | |
| Cooked Navy bean or black bean | Mice (C57Bl/6 male) |
Healthy | 4 weeks | Supplementation @20% to the basal diet | 3 weeks |
|
|
[39] |
| Cranberry beans | Mice (C57BL/6 male) |
Healthy and DSS induced colitis | 5 weeks | BD + 20% beans | 3 weeks |
|
In healthy cohorts:
In diseased cohorts:
|
[54] |
| Lentil, chickpea, bean, and dry pea | Mice (C57BL/6NCrl mice) |
Healthy | 3–4 weeks | 40 g/100 g obesogenic diet (by replacing 35% protein) | 17 weeks |
|
|
[18] |
| Cooked red lentils | Mice (C57Bl/6 male) |
Healthy | 5 weeks | 20% w/w basal diet | 3 weeks |
|
|
[40] |
| Chickpea, lentil, dry peas, and bean | Mice (C57BL/6 male) |
Obese | 3–4 weeks | 40% w/w diet | 17 weeks |
|
|
[8] |
| Whole mung bean | Mice (C57BL/6 male) |
Diet-induced obesity (1 w HFD feeding) |
4 weeks | HFD + 30% bean | 12 weeks |
|
|
[44] |
| Lentil (Lens culinaris Medikus) | Rats (Sprague−Dawley) |
Healthy | 8 weeks | 70.8% red lentil diet | 6 weeks |
|
|
[55] |
| Yellow pea flour | Rats | Diet-induced obesity (5 w HFD feeding) | 5 weeks | 30% w/w diet | 42 days |
|
|
[46] |
| Whole yellow pea flour | Hamster (Golden Syrian) | Hypercholesterolemic diet (28 days) | 2 weeks | 10% replacement of corn starch with pea flour in the diet | 28 days |
|
|
[56] |
4. Prebiotic Potential of Pulses-Derived Resistant Starch for Gut Health
This entry is adapted from the peer-reviewed paper 10.3390/nu14091726
References
- Henry J. Thompson; Dietary Bean Consumption and Human Health. Nutrients 2019, 11, 3074, 10.3390/nu11123074.
- Effie Viguiliouk; Sonia Blanco Mejia; Cyril W.C. Kendall; John L. Sievenpiper; Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Annals of the New York Academy of Sciences 2017, 1392, 43-57, 10.1111/nyas.13312.
- Jessica C. Kiefte-De Jong; John C. Mathers; Oscar Franco; Nutrition and healthy ageing: the key ingredients. Proceedings of the Nutrition Society 2014, 73, 249-259, 10.1017/s0029665113003881.
- Helena Ferreira; Marta Vasconcelos; Ana M. Gil; Elisabete Pinto; Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Critical Reviews in Food Science and Nutrition 2020, 61, 85-96, 10.1080/10408398.2020.1716680.
- C.P.F. Marinangeli; S.V. Harding; M. Zafron; T.C. Rideout; A systematic review of the effect of dietary pulses on microbial populations inhabiting the human gut. Beneficial Microbes 2020, 11, 1-12, 10.3920/bm2020.0028.
- Yolanda Brummer; Mina Kaviani; Susan M. Tosh; Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Research International 2014, 67, 117-125, 10.1016/j.foodres.2014.11.009.
- Joyce Boye; Fatemeh Zare; Alison Pletch; Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Research International 2010, 43, 414-431, 10.1016/j.foodres.2009.09.003.
- John McGinley; Vanessa Fitzgerald; Elizabeth Neil; Heather Omerigic; Adam Heuberger; Tiffany Weir; Rebecca McGee; George Vandemark; Henry Thompson; Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593, 10.3390/nu12030593.
- Henry J Thompson; Mark A Brick; Perspective: Closing the Dietary Fiber Gap: An Ancient Solution for a 21st Century Problem.. Advances in Nutrition: An International Review Journal 2016, 7, 623-6, 10.3945/an.115.009696.
- Ratnajothi Hoover; Stewart C. Rorke; Antonio M. Martin; ISOLATION AND CHARACTERIZATION OF LIMA BEAN (PHASEOLUS LUNATUS) STARCH. Journal of Food Biochemistry 1991, 15, 117-136, 10.1111/j.1745-4514.1991.tb00149.x.
- Li, C.; Hu, Y. Align resistant starch structures from plant-based foods with human gut microbiome for personalized health promotion. Crit Rev Food Sci Nutr 2021, 1-12, doi:10.1080/10408398.2021.1976722
- Janine A. Higgins; Resistant Starch and Energy Balance: Impact on Weight Loss and Maintenance. Critical Reviews in Food Science and Nutrition 2013, 54, 1158-1166, 10.1080/10408398.2011.629352.
- Diane F. Birt; Terri Boylston; Suzanne Hendrich; Jay-Lin Jane; James Hollis; Li Li; John McClelland; Samuel Moore; Gregory J. Phillips; Matthew Rowling; et al. Resistant Starch: Promise for Improving Human Health. Advances in Nutrition: An International Review Journal 2013, 4, 587-601, 10.3945/an.113.004325.
- Yong Wang; Jing Chen; Ying-Han Song; Rui Zhao; Lin Xia; Yi Chen; Ya-Ping Cui; Zhi-Yong Rao; Yong Zhou; Wen Zhuang; et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: a systematic review and meta-analysis. Nutrition & Diabetes 2019, 9, 1-11, 10.1038/s41387-019-0086-9.
- Janine A Higgins; Resistant Starch: Metabolic Effects and Potential Health Benefits. Journal of AOAC INTERNATIONAL 2004, 87, 761-768, 10.1093/jaoac/87.3.761.
- Oluwatoyin O. Sangokunle; Shridhar K. Sathe; Prashant Singh; Purified Starches from 18 Pulses Have Markedly Different Morphology, Oil Absorption and Water Absorption Capacities, Swelling Power, and Turbidity. Starch - Stärke 2020, 72, 2000022, 10.1002/star.202000022.
- Aswir Abd Rashed; Fatin Saparuddin; Devi-Nair Gunasegavan Rathi; Nur Najihah Mohd Nasir; Ezarul Faradianna Lokman; Effects of Resistant Starch Interventions on Metabolic Biomarkers in Pre-Diabetes and Diabetes Adults. Frontiers in Nutrition 2022, 8, 793414, 10.3389/fnut.2021.793414.
- Tymofiy Lutsiv; Tiffany L. Weir; John N. McGinley; Elizabeth S. Neil; Yuren Wei; Henry J. Thompson; Compositional Changes of the High-Fat Diet-Induced Gut Microbiota upon Consumption of Common Pulses. Nutrients 2021, 13, 3992, 10.3390/nu13113992.
- Nannan Guan; Xiaowei He; Shaokang Wang; Feitong Liu; Qiang Huang; Xiong Fu; Tingting Chen; Bin Zhang; Cell Wall Integrity of Pulse Modulates the in Vitro Fecal Fermentation Rate and Microbiota Composition. Journal of Agricultural and Food Chemistry 2020, 68, 1091-1100, 10.1021/acs.jafc.9b06094.
- Fredrik Bäckhed; Ruth E. Ley; Justin L. Sonnenburg; Daniel A. Peterson; Jeffrey I. Gordon; Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915-1920, 10.1126/science.1104816.
- Loris R Lopetuso; Franco Scaldaferri; Valentina Petito; Antonio Gasbarrini; Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathogens 2012, 5, 1-8, 10.1186/1757-4749-5-23.
- Elizabeth Thursby; Nathalie Juge; Introduction to the human gut microbiota. Biochemical Journal 2017, 474, 1823-1836, 10.1042/bcj20160510.
- Carlos López-Otín; Maria A. Blasco; Linda Partridge; Manuel Serrano; Guido Kroemer; The Hallmarks of Aging. Cell 2013, 153, 1194-1217, 10.1016/j.cell.2013.05.039.
- Dwina Juliana Warman; Huijuan Jia; Hisanori Kato; The Potential Roles of Probiotics, Resistant Starch, and Resistant Proteins in Ameliorating Inflammation during Aging (Inflammaging). Nutrients 2022, 14, 747, 10.3390/nu14040747.
- Yawen Zhang; Luyi Chen; Mengjia Hu; John J. Kim; RenBin Lin; Jilei Xu; Lina Fan; Yadong Qi; Lan Wang; Weili Liu; et al. Dietary type 2 resistant starch improves systemic inflammation and intestinal permeability by modulating microbiota and metabolites in aged mice on high-fat diet. Aging 2020, 12, 9173-9187, 10.18632/aging.103187.
- Shokouh Ahmadi; Ravinder Nagpal; Shaohua Wang; Jason Gagliano; Dalane W Kitzman; Sabihe Soleimanian-Zad; Mahmoud Sheikh-Zeinoddin; Russel Read; Hariom Yadav; Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome–gut–brain axis modulation. The Journal of Nutritional Biochemistry 2019, 67, 1-13, 10.1016/j.jnutbio.2019.01.011.
- Pinky Raigond; Rajarathnam Ezekiel; Baswaraj Raigond; Resistant starch in food: a review. Journal of the Science of Food and Agriculture 2014, 95, 1968-1978, 10.1002/jsfa.6966.
- H. Englyst; H. S. Wiggins; J. H. Cummings; Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. The Analyst 1981, 107, 307-318, 10.1039/an9820700307.
- Hamung Patel; Paul Royall; Simon Gaisford; Gareth R. Williams; Cathrina Edwards; Frederick Warren; Bernadine M. Flanagan; Peter R. Ellis; Peter J. Butterworth; Structural and enzyme kinetic studies of retrograded starch: Inhibition of α-amylase and consequences for intestinal digestion of starch. Carbohydrate Polymers 2017, 164, 154-161, 10.1016/j.carbpol.2017.01.040.
- Bilal Ahmad Ashwar; Adil Gani; Asima Shah; Farooq Ahmad Masoodi; Physicochemical properties, in-vitro digestibility and structural elucidation of RS4 from rice starch. International Journal of Biological Macromolecules 2017, 105, 471-477, 10.1016/j.ijbiomac.2017.07.057.
- Junyi Yang; Devin J. Rose; The impact of long-term dietary pattern of fecal donor on in vitro fecal fermentation properties of inulin. Food & Function 2015, 7, 1805-1813, 10.1039/c5fo00987a.
- Jiangbin Xu; Zhen Ma; Xiaoping Li; Liu Liu; Xinzhong Hu; A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food & Function 2020, 11, 1982-1995, 10.1039/c9fo02025j.
- Naoto Hashimoto; Yusaku Ito; Kyu-Ho Han; Ken-Ichiro Shimada; Mitsuo Sekikawa; David L. Topping; Anthony R. Bird; Takahiro Noda; Hideyuki Chiji; Michihiro Fukushima; et al. Potato Pulps Lowered the Serum Cholesterol and Triglyceride Levels in Rats. Journal of Nutritional Science and Vitaminology 2005, 52, 445-450, 10.3177/jnsv.52.445.
- J. L. Sievenpiper; C. W. C. Kendall; A. Esfahani; J. M. W. Wong; A. J. Carleton; H. Y. Jiang; R. P. Bazinet; E. Vidgen; D. J. A. Jenkins; Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479-1495, 10.1007/s00125-009-1395-7.
- Vanessa Ha; John L. Sievenpiper; Russell J. de Souza; Viranda H. Jayalath; Arash Mirrahimi; Arnav Agarwal; Laura Chiavaroli; Sonia Blanco Mejia; Frank M. Sacks; Marco Di Buono; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. Canadian Medical Association Journal 2014, 186, E252-E262, 10.1503/cmaj.131727.
- Emily M.T. Padhi; D. Dan Ramdath; A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. Journal of Functional Foods 2017, 38, 635-643, 10.1016/j.jff.2017.03.043.
- V. H. Jayalath; R. J. De Souza; J. L. Sievenpiper; V. Ha; L. Chiavaroli; A. Mirrahimi; M. Di Buono; A. M. Bernstein; L. A. Leiter; Penny Kris-Etherton; et al. Effect of Dietary Pulses on Blood Pressure: A Systematic Review and Meta-analysis of Controlled Feeding Trials. American Journal of Hypertension 2013, 27, 56-64, 10.1093/ajh/hpt155.
- Patricia Gullón; Beatriz Gullón; Freni Tavaria; Marta Vasconcelos; Ana Maria Gomes; In vitro fermentation of lupin seeds (Lupinus albus) and broad beans (Vicia faba): dynamic modulation of the intestinal microbiota and metabolomic output. Food & Function 2015, 6, 3316-3322, 10.1039/c5fo00675a.
- Jennifer M. Monk; Dion Lepp; Wenqing Wu; K. Peter Pauls; Lindsay E. Robinson; Krista A. Power; Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. The Journal of Nutritional Biochemistry 2017, 49, 89-100, 10.1016/j.jnutbio.2017.08.002.
- Daniela Graf; Jennifer M. Monk; Dion Lepp; Wenqing Wu; Laurel McGillis; Kyle Roberton; Yolanda Brummer; Susan M. Tosh; Krista A. Power; Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice. Nutrients 2019, 11, 1853, 10.3390/nu11081853.
- Jennifer M. Monk; Dion Lepp; Wenqing Wu; Daniela Graf; Laurel H. McGillis; Adeel Hussain; Christine Carey; Lindsay E. Robinson; Ronghua Liu; Rong Tsao; et al. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. Journal of Functional Foods 2017, 38, 663-674, 10.1016/j.jff.2017.02.002.
- Jennifer M. Monk; Claire P. Zhang; Wenqing Wu; Leila Zarepoor; Jenifer T. Lu; Ronghua Liu; K. Peter Pauls; Geoffrey Wood; Rong Tsao; Lindsay E. Robinson; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. The Journal of Nutritional Biochemistry 2015, 26, 752-760, 10.1016/j.jnutbio.2015.02.003.
- Andreas Schwiertz; David Taras; Klaus Schäfer; Silvia Beijer; Nicolaas A. Bos; Christiane Donus; Philip D. Hardt; Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2009, 18, 190-195, 10.1038/oby.2009.167.
- Dianzhi Hou; Qingyu Zhao; Laraib Yousaf; Yong Xue; Qun Shen; Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. European Journal of Nutrition 2020, 59, 3617-3634, 10.1007/s00394-020-02196-2.
- Chin Wen Png; Sara K Lindén; Kristen S Gilshenan; Erwin G Zoetendal; Chris S McSweeney; Lindsay I Sly; Michael McGuckin; Timothy H J Florin; Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. American Journal of Gastroenterology 2010, 105, 2420-2428, 10.1038/ajg.2010.281.
- Amanda J. Eslinger; Lindsay K. Eller; Raylene A. Reimer; Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats. Nutrition Research 2014, 34, 714-722, 10.1016/j.nutres.2014.07.016.
- Zohreh Tamanai-Shacoori; Imen Smida; Latifa Bousarghin; Olivier Loreal; Vincent Meuric; Shao Bing Fong; Martine Bonnaure-Mallet; Anne Jolivet-Gougeon; Roseburia spp.: a marker of health?. Future Microbiology 2017, 12, 157-170, 10.2217/fmb-2016-0130.
- W. M. U. Fernando; Janet Hill; G. A. Zello; R. T. Tyler; W. J. Dahl; A. G. Van Kessel; Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Beneficial Microbes 2010, 1, 197-207, 10.3920/bm2009.0027.
- John W. Finley; James B. Burrell; Philip G. Reeves; Pinto Bean Consumption Changes SCFA Profiles in Fecal Fermentations, Bacterial Populations of the Lower Bowel, and Lipid Profiles in Blood of Humans. The Journal of Nutrition 2007, 137, 2391-2398, 10.1093/jn/137.11.2391.
- Amy Sheflin; Erica C. Borresen; Jay S. Kirkwood; Claudia M. Boot; Alyssa Whitney; Shen Lu; Regina J. Brown; Corey Broeckling; Elizabeth P. Ryan; Tiffany L. Weir; et al. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors.. Molecular Nutrition & Food Research 2016, 61, 1500905, 10.1002/mnfr.201500905.
- Bridget A. Baxter; Renee C. Oppel; Elizabeth P. Ryan; Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients 2018, 11, 28, 10.3390/nu11010028.
- Saman Abeysekara; Philip D. Chilibeck; Hassanali Vatanparast; Gordon A. Zello; A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. British Journal of Nutrition 2012, 108, S103-S110, 10.1017/s0007114512000748.
- Eunho Chun; Soojung Yoon; Amna Parveen; Mirim Jin; Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model. Nutrients 2018, 10, 1475, 10.3390/nu10101475.
- Jennifer M. Monk; Dion Lepp; Claire P. Zhang; Wenqing Wu; Leila Zarepoor; Jenifer T. Lu; K. Peter Pauls; Rong Tsao; Geoffrey A. Wood; Lindsay E. Robinson; et al. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. The Journal of Nutritional Biochemistry 2015, 28, 129-139, 10.1016/j.jnutbio.2015.10.014.
- Niroshan Siva; Casey R. Johnson; Vincent Richard; Elliot D. Jesch; William Whiteside; Abdullah A. Abood; PushparajaH Thavarajah; Susan Duckett; Dil Thavarajah; Lentil (Lens culinaris Medikus) Diet Affects the Gut Microbiome and Obesity Markers in Rat. Journal of Agricultural and Food Chemistry 2018, 66, 8805-8813, 10.1021/acs.jafc.8b03254.
- Christopher P.F. Marinangeli; Peter J.H. Jones; Chronic Intake of Fractionated Yellow Pea Flour Reduces Postprandial Energy Expenditure and Carbohydrate Oxidation. Journal of Medicinal Food 2011, 14, 1654-1662, 10.1089/jmf.2010.0255.
- Jessica Soldavini; Jonathan D. Kaunitz; Pathobiology and Potential Therapeutic Value of Intestinal Short-Chain Fatty Acids in Gut Inflammation and Obesity. Digestive Diseases and Sciences 2013, 58, 2756-2766, 10.1007/s10620-013-2744-4.
- B. S. Yadav; A. Sharma; R. B. Yadav; Resistant starch content of conventionally boiled and pressure-cooked cereals, legumes and tubers. Journal of Food Science and Technology 2009, 47, 84-88, 10.1007/s13197-010-0020-6.
- Alejandra García-Alonso; Isabel Goñi; F. Saura-Calixto; Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Zeitschrift für Lebensmitteluntersuchung und -Forschung A 1998, 206, 284-287, 10.1007/s002170050258.
- Kai Wang; Jovin Hasjim; Alex Chi Wu; Robert J. Henry; Robert G. Gilbert; Variation in Amylose Fine Structure of Starches from Different Botanical Sources. Journal of Agricultural and Food Chemistry 2014, 62, 4443-4453, 10.1021/jf5011676.
- Bahar Nur Okumus; Zeynep Tacer-Caba; Kevser Kahraman; Dilara Nilufer-Erdil; Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chemistry 2018, 240, 550-558, 10.1016/j.foodchem.2017.07.157.
