Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Renata Siedlecka | -- | 2187 | 2022-04-28 08:06:12 | | | |
| 2 | Catherine Yang | -5 word(s) | 2182 | 2022-04-28 09:37:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Siedlecka, R.; , . Heteroaromatic N-Oxides in Asymmetric Catalysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/22418 (accessed on 03 March 2026).
Siedlecka R, . Heteroaromatic N-Oxides in Asymmetric Catalysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/22418. Accessed March 03, 2026.
Siedlecka, Renata, . "Heteroaromatic N-Oxides in Asymmetric Catalysis" Encyclopedia, https://encyclopedia.pub/entry/22418 (accessed March 03, 2026).
Siedlecka, R., & , . (2022, April 28). Heteroaromatic N-Oxides in Asymmetric Catalysis. In Encyclopedia. https://encyclopedia.pub/entry/22418
Siedlecka, Renata and . "Heteroaromatic N-Oxides in Asymmetric Catalysis." Encyclopedia. Web. 28 April, 2022.
Copy Citation
Chiral heteroaromatic N-oxides can work as powerful electron-pair donors, providing suitable electronic environments in the transition state formed within the reaction. The nucleophilicity of the oxygen atom in N-oxides, coupled with a high affinity of silicon to oxygen, represent ideal properties for the development of synthetic methodology based on nucleophilic activation of organosilicon reagents.
chiral heteroaromatic N-oxides
organocatalysis
asymmetric transformations stereoselectivity
1. Chiral Heteroaromatic N-Oxides as Organocatalysts
Properties of heteroaromatic N-oxides can be classified as strong Lewis bases because of N-O bond polarization [1]. They are able to activate the C-Si bond in halosilane compounds (Lewis acids), which makes them perfect mediators for allylation and crotylation of aldehydes with allyltrichlorosilanes, which are called Sakurai–Hosomi–Denmark-type reactions (see Scheme 1). This explains why allylation is the most popular testing reaction and is very well examined. It has also become a standard testing ground for new chiral Lewis basic organocatalysts [2][3][4][5]. The good selectivity is obtained when the catalyst allows the reaction to proceed via a closed cyclic chair-like transition state involving hypervalent silicates, as shown in Scheme 1. Additionally, the resulting homoallylic alcohols are considered the cornerstones of organic synthesis. Mostly, they are used in the synthesis of numerous natural products as building blocks but also in the synthesis of drug candidates and other functional molecules [5][6][7][8][9][10][11]. Apart from allylation there were also reported examples of using N-oxides in the catalytic ring-opening of meso-epoxides [12][13][14][15], propargylation [16], allenylation [17], aldol reaction [18][19] and reduction of ketoimines [20][21].
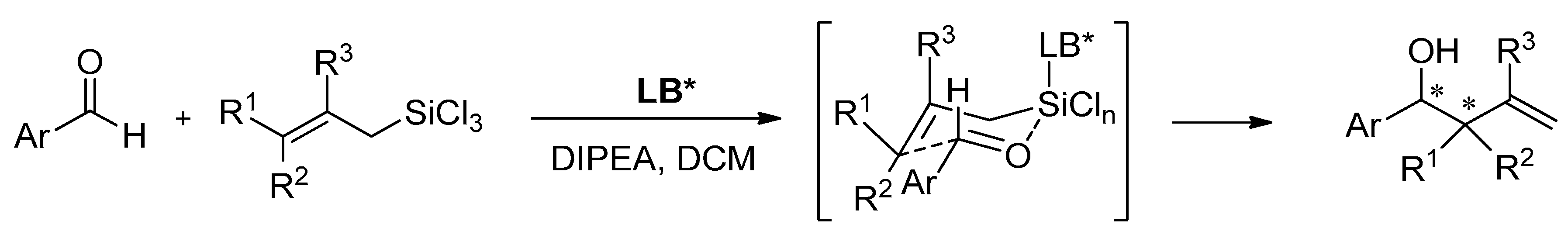
Scheme 1. Lewis base (LB) catalyzed allylation of aldehydes with substituted allyltrichlorosilanes.
1.1. Axially Chiral N-Oxides and N,N′-Dioxides
The first mention of N-oxides and their catalytic asymmetric applications were related to those, containing axial chirality and were developed by Nakajima et al. [22]. Initially, compounds were based on 4,4’-biquinoline and 2,2’-bipyridine N,N′-dioxide backbones (1 and 2, see Figure 1) which, apart from Nakajima’s work, were also investigated by Feng and coworkers [23]. A little later, Kotora et al. synthesized N-oxides with tetrahydroisoquinoline [24] and bis(tetrahydroisoquinoline) frameworks [25][26]. It is worth to highlight that organocatalysts 1 and 2 give, so far, one of the highest results (both in terms of yield and enantioselectivity) in the allylation of benzaldehyde with allyltrichlorosilane (85%, 88% ee (R) and 95%, 84% ee (S) respectively). Chang et al. received an analog of 1, containing ethyl instead of methyl groups (3, see Figure 1) and describe their application as organocatalyst (10 mol%) in allylation of 4- metoxybenzaldehyde with allyltrichlorosilane. The chiral product was obtained with high enantiomeric purity (92% ee) with satisfactory yield 66% [27], which slightly exceeds the result obtained with the application of 1.
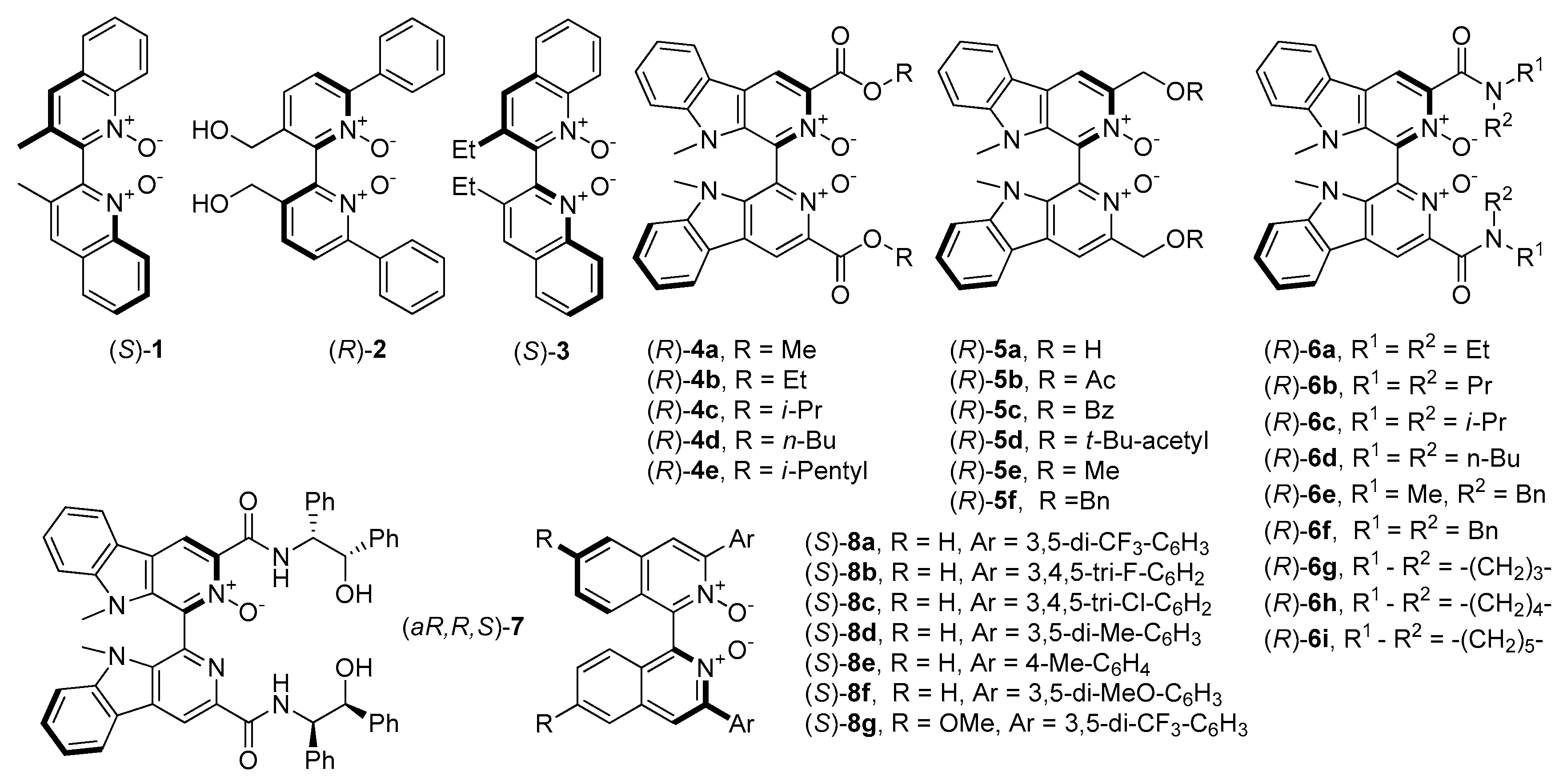
Figure 1. N-oxides possessing an axial element of chirality ((aR,R,S)-7, which possess also central chirality is placed here due to the similarity of the structure).
1.2. N-Oxides Possessing Central Chirality
Much more attention through the past decade has been given to compounds with central chirality. The report of Mlostoń and Jurczak from 2009 deserves to be mentioned [28]. Authors presented novel chiral C2-symmetric bisimidazole-N-oxides 9 (see Figure 2), derived from trans-1,2-diaminocyclohexane, thereby breaking the tendency of catalysts based on pyridine or bipyridine N-oxides. Screening of catalysts in the reaction of benzaldehyde with allyltrichlorosilane showed that the presence of phenyl group in the imidazole ring (R2 in 9b) has a positive effect on the reaction efficiency (3 examples, 84–86%). Unfortunately, the introduction of two phenyl substituents (9d), only slightly increased enantioselectivity (from 43% ee to 53% ee). However, the presence of the second phenyl substituent in 9d caused the inversion of the absolute configuration of obtained homoallyl alcohol. To improve the catalytic efficiency, different options of catalyst loading for (R,R)-9d, as well as reaction temperature was checked. The best result (90%, 64% ee) has been received for a reaction carried out at 0 °C with 10 mol% of (R,R)-9d. Also, estimation for a scope of aldehyde substrates was done. Higher enantioselectivity for m-substituted, than for o-substituted benzaldehydes was noticed. The highest asymmetric induction was observed for heteroaromatic aldehydes—furfural (76% ee) and thiophene-2-carboxyaldehyde (80% ee).
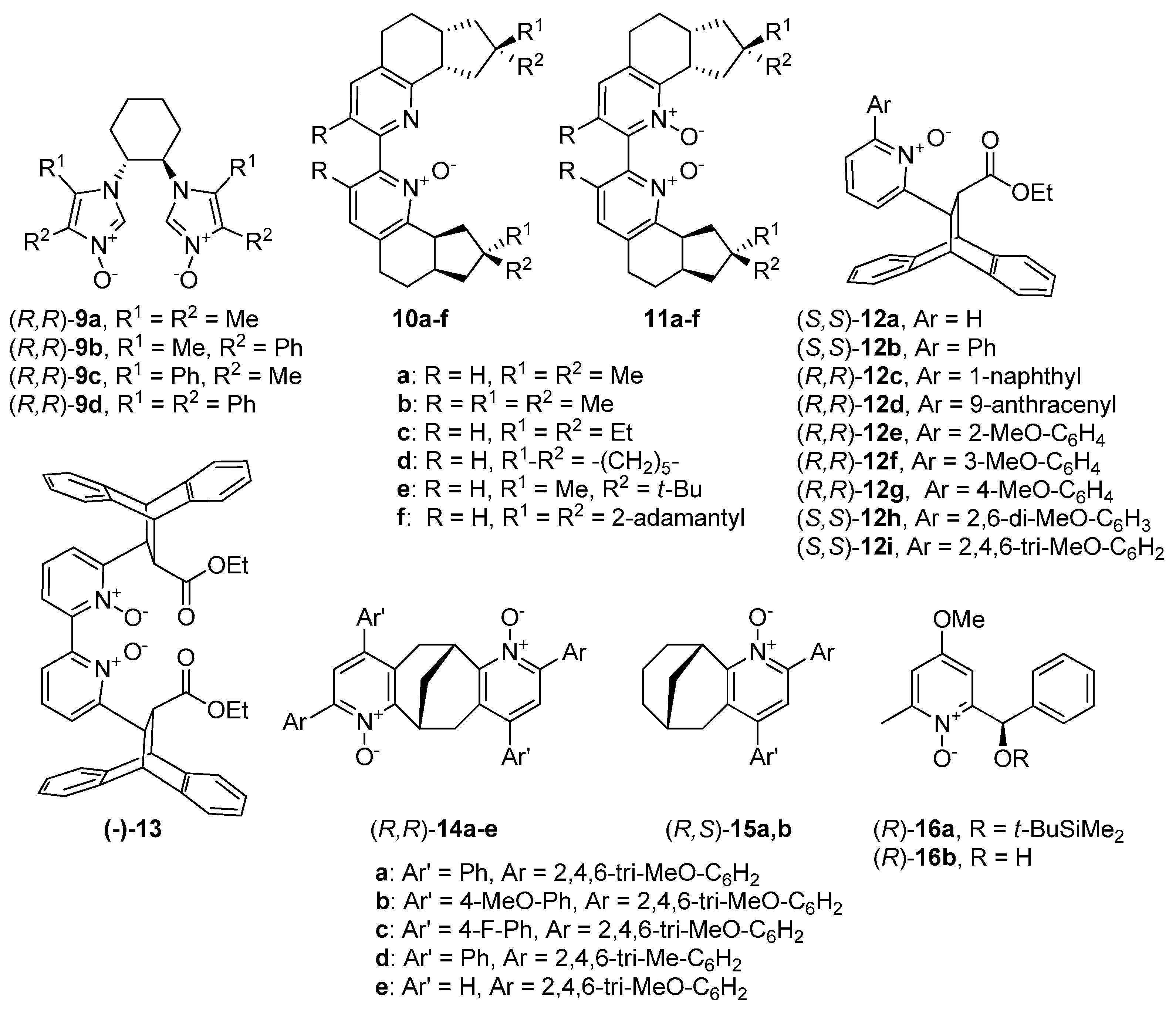
Figure 2. N-oxides possessing a central element of chirality.
Boyd presented the synthetic pathway to obtain bipyridine N-oxide derivatives 10 and corresponding N,N’-dioxides 11 [29], showed in Figure 2. It was found that the allylation reaction is slower when using the mono N-oxides, and thus, the reactions applying them were carried out at higher temperatures (0 °C or −40 °C, 24 h), compared with those using the corresponding N,N’- dioxides (−78 °C, 12 h). In that case, although the mono- and dioxides were applied to allylation of the same aldehydes with allyltrichlorosilane, it is difficult to compare the results unambiguously. The optimal enantioselectivity was observed in the allylation of 4-methoxybenzaldehyde as a substrate, using either N-oxides 10 (56–86% ee) or corresponding N,N’-dioxides 11 (59–80% ee), compared to allylation of benzaldehyde (24–35% ee and 14–26% ee). The highest enantioselectivity (86% ee) was observed in allylation of 4-methoxybenzaldehyde using 10b as a catalyst. Despite the clear difference in the enantioselectivity of these reactions, their yields did not differ much and were mostly in the range of 30–40% (except 10a–60–72%). Better yields were observed when using 11 as the catalyst, and the compound 11a gave the highest induction (80% ee).
1.3. N-Oxides with Central and Axial Chirality
An interesting direction in the exploration of catalytically useful N-oxides is the combination of two types of chirality. Researches mainly concentrate on symmetrically or unsymmetrically substituted chiral bis(tetrahydroisoquinoline) N,N’-dioxides (Figure 3), which with their structure resemble compound 8. In 2008 Kotora et al. synthesized symmetrically substituted by (R)-tetrahydrofuran-2-yl dioxides 32 (see Figure 3), which were tested in the allylation of aromatic [30] as well as aliphatic aldehydes [25]. Catalysts synthesis was based on cyclotrimerization of tetrayne with (R)-tetrahydrofuran-2-carbonitrile, followed by oxidation of received bipyridines by m-CPBA. At the end separation of a resulted mixture of diastereoisomers was necessary. A simple column chromatography on alumina, gave isolated yields 48% for (aR,R,R)-32 and 28% for (aS,R,R)-32. The configuration was assigned by X-ray crystallographic analysis. In allylation of benzaldehyde, 4-trifluoromethylbenzaldehyde or 4-methoxybenzaldehyde performed in MeCN, at −40 °C for 1 h, with 1 mol% catalyst loading, both diastereomeric catalysts were effective [30]. Aldehydes were converted into corresponding homoallylic alcohols almost quantitatively (entries 1, 4, 10). Only for allylation of 4-trifluoromethylbenzaldehyde using (aR,R,R)-32 the yield was slightly lower (82%, entry 10). Applying the same catalyst, the enantioselectivity was lower for aldehyde with an electron-withdrawing group (15% ee in comparison to 48% ee for benzaldehyde, entry 10) and higher for aldehyde substituted with an electron-donating group (60% ee, entry 4). When (aS,R,R)-32 was used decrease of asymmetric induction for 4-trifluoromethylbenzaldehyde was observed again (entry 10), but for 4-methoxybenzaldehyde obtained alcohol was a racemate (entry 4).
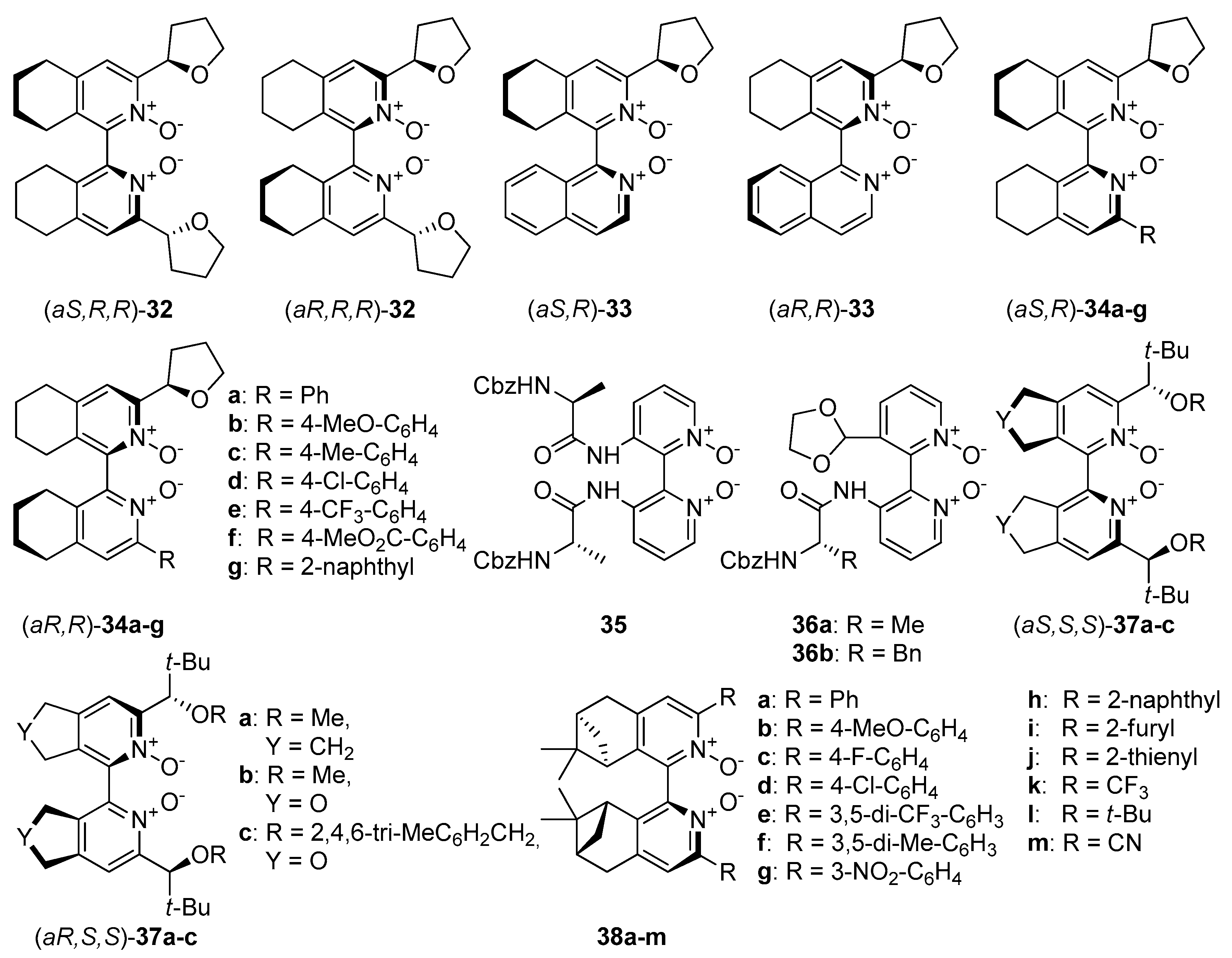
Figure 3. N-Oxides possessing two types of chirality—central and axial.
1.4. Helical-Chiral N-Oxides
A completely different group of N-oxide catalysts introduced Takenaka [15][16][17]. Seminal report from 2008, presented synthetic route to helical-chiral pyridine N-oxides 39–41 (see Figure 4) and results of their application in desymmetrization of meso-epoxides [15]. The catalytic reaction was performed for two epoxides possessing aromatic groups and two alkyl epoxides. In all cases the efficiency of the reaction was good (68–80%). Desymmetrization of aromatic meso-epoxides was characterized by higher enantioselectivity than for aliphatic ones (73–94% ee versus 22–65% ee). Introduction of two additional aromatic rings to 39, creating branched structure 41, allowed to obtain in all cases the highest ee values. For 1,5-cyclooctadiene oxide, the growth of enantioselectivity was the most visible–33% ee with 41, in comparison to racemate obtained using 39. Epoxide ring opening of several variously substituted cis-stilbene derivatives catalyzed by 41 was also examined. It could be concluded, that the presence of the electron-withdrawing group in cis-stilbene has no effect on enantioselectivity (2 examples, 92–94% ee) but the presence of electron-donating group slightly decreases the enantioselectivity (87% ee).
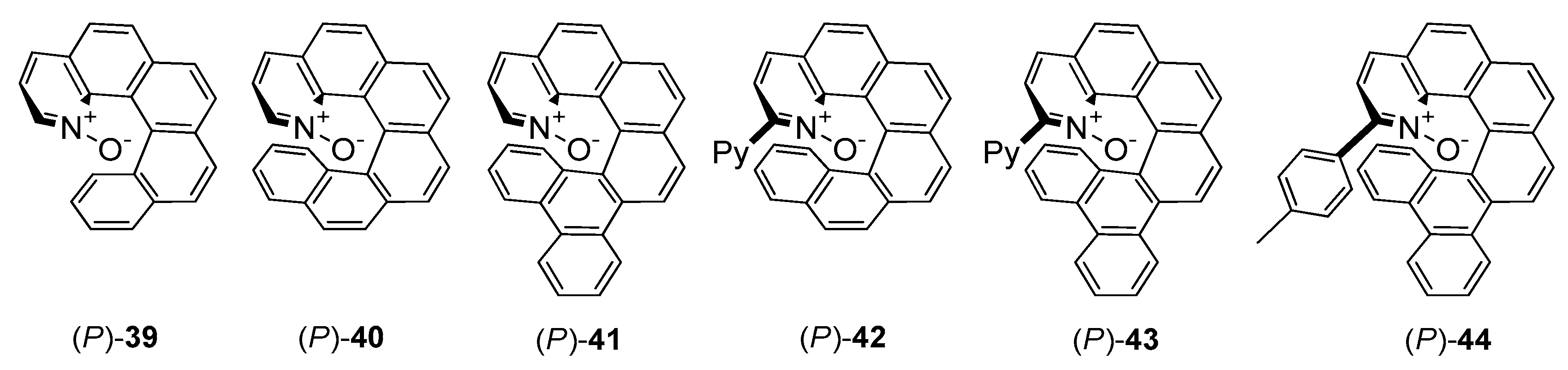
Figure 4. Helically chiral N-oxides.
1.5. N-Oxides Possessing Planar Chirality
Compounds bearing [2.2] paracyclophane moiety 45–48 (see Figure 5), which represents planar chiral N-oxides should also be briefly mentioned. Rowlands et al. presented a facile synthesis of the mentioned compounds in two steps from [2.2] paracyclophane, based on Fagnou’s direct arylation [31]. Then, obtained compounds were applied in the allylation of benzaldehyde with allyltrichlorosilane. For catalysts 45b and 46b, the presence of methoxy group in their structure caused decrease of the yield and enantioselectivity and also inversion of configuration from R to S, in comparison to corresponding unsubstituted structures 45a and 46a–from 65%, 38% ee to 52%, 36% ee and from 72%, 38% ee to 58%, 28% ee, respectively. Unsubstituted, mixed pyridine/pyridine N-oxide catalyst 47 was less effective (55% yield and 30% ee) compare to 46a and inversion of product configuration was also observed, which might indicate that presence of N-oxide group plays a crucial role. It is also worth to compare the results obtained by Rowlands and coworkers with those obtained by Andrus and coworkers [32]. The latter group synthesized aza-paracyclophane N-oxide catalysts 48. Among them (S)-48a turned out to be a brilliant catalyst for allylation of various aromatic and aliphatic aldehydes with allyltrichlorosilane, giving 87–95% yield and 87–96% ee [32]. It is puzzling whether a structure containing aza-paracyclophane N-oxide would be effective catalysts or the presence of an oxazoline group is essential.
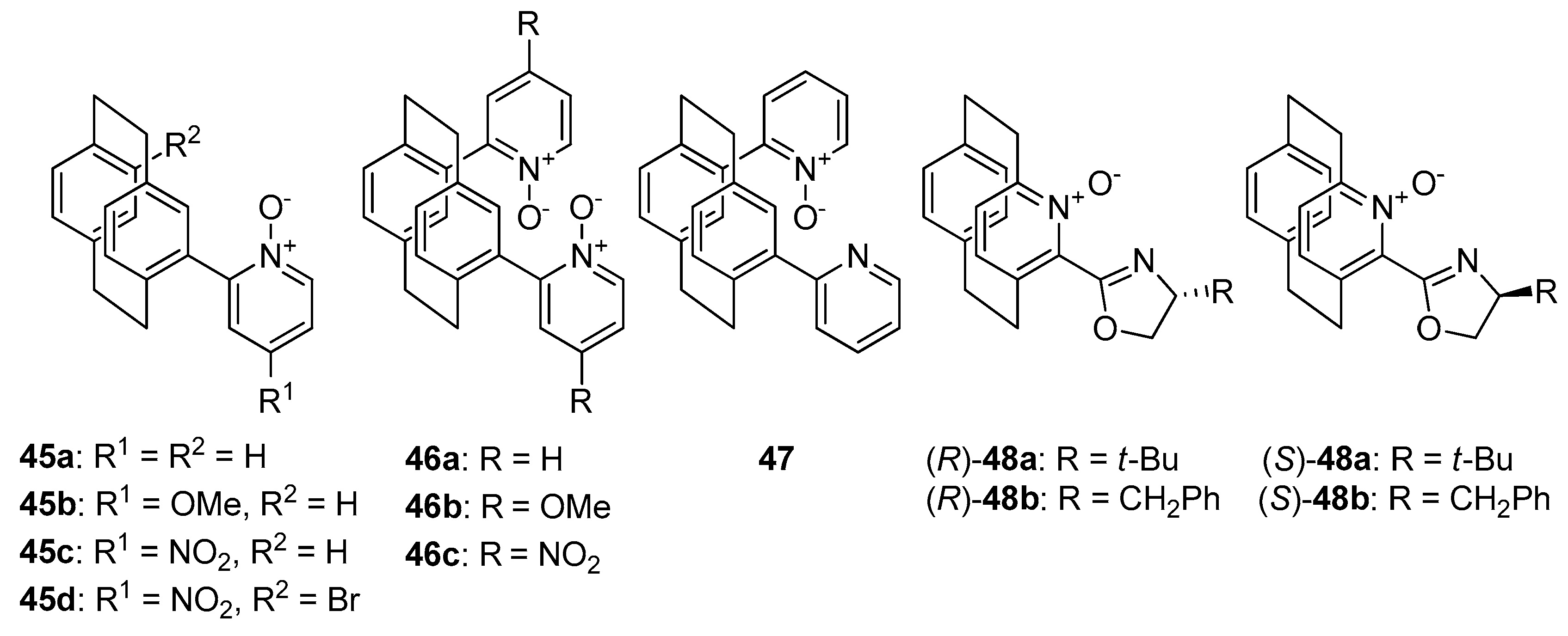
Figure 5. Planar chiral N-oxides.
2. Chiral Heteroaromatic N-Oxides as Ligands for Metal Catalysis
Over the past decade, relatively little attention has been paid to the use of chiral heteroaromatic N-oxides as ligands for metal complexes catalyzed reactions. Several mentions come from 2002–2003 and include (S)-1-CdI2 complex as a catalyst for conjugate addition of thiol to enone or enal, providing 70–78% ee [33], and (R)-1-Sc(OTf)3 complex as a catalyst for Michael addition of β-keto ester to methyl vinyl ketone or acrolein, giving almost quantitative yield with moderate enantioselectivity of 38–84% ee [34]. Chiral copper (II)-terpyridine mono-N-oxide and di-N-oxide complexes were used in asymmetric cyclopropanation of styrene [35]. Enantiomeric excess up to 83% and yield up to 97% were achieved. Currently, rather the applications of chiral alkyl amine N-oxides (mostly proline N-oxide derivatives) as ligands for metal complexes, in various types of asymmetric reactions, can be found in the literature [36][37][38]. The different direction was presented by Wolińska, who used chiral pyridine N-oxide derivatives possessing oxazoline moiety 49–51 (see Figure 6) for the asymmetric nitroaldol reaction, catalyzed by a copper complex. Catalysts 49, used in Henry reaction of 3-nitrobenzaldehyde [39], gave high efficiency (80–88%) but unfortunately, the enantioselectivity was low (11–14% ee). Chiral 3-oxazoline pyridine N-oxides substituted by with 1,2,4-triazine ring (50, 51) [40] were also examined in nitroaldol reaction of m-nitrobenzaldehyde but all attempts resulted in racemic nitroalcohol. An external base addition was tried and some improvement has been observed [41]. However, enantioselectivity grows (from racemate to 41% ee) only for bases with low pKa value. The reason for the low catalytic effectiveness may be the relatively large distance between the complexation site (N-O) and the stereogenic center.
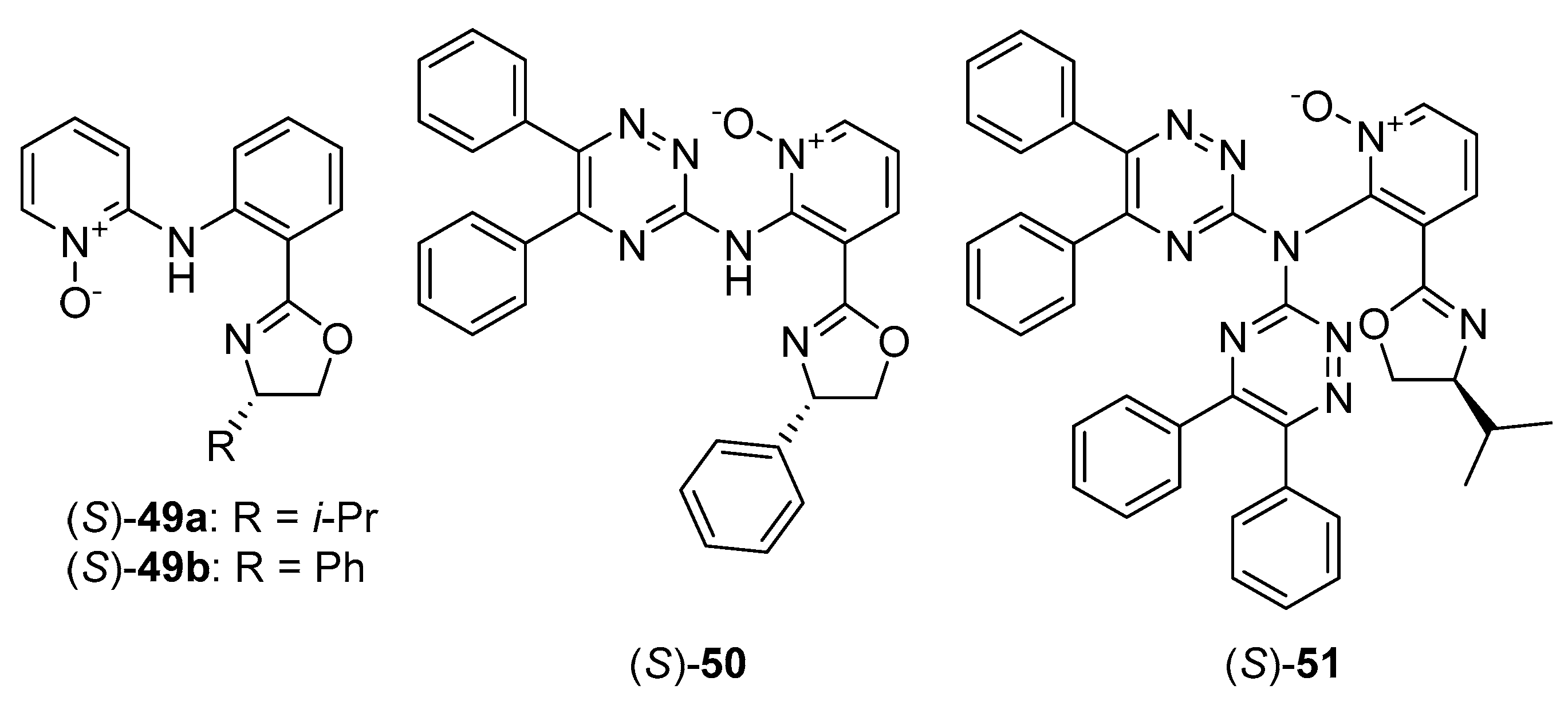
Figure 6. N-oxide ligands used in Cu-catalyzed nitroaldol reaction.
3. Other Applications of Pyridine N-Oxides
Without any doubt, N-oxides are used in asymmetric organocatalysis, but also their applications in different branches of science are of great importance. They have significant synthetic value as intermediates in multi-step syntheses. They are widely used to various functionalization of N-heteroaromatic compounds-this mainly concerns the C-H bond in position 2. The examples of the application of N-oxides as synthetic intermediates in the industrial synthesis of some pharmaceuticals are also described, e.g., pranoprofen or omeprazole [42]. Compounds containing in their structure the 2-mercaptopyridine-N-oxide moiety have anti-cancer, bactericidal, and fungicidal activity [43][44]. N-oxides are also a crucial component in personal care products such as soaps, toothpaste, washing agents, shampoos and cosmetics [45]. Interesting properties of the N-O bond caused that N-oxides are used also in materials engineering. They consist of a wide group of polymer additives, e.g., crosslinkers, vulcanization accelerators, epoxy resin hardeners, UV absorbers or additives for stereospecific polymerization of polypropylene [46]. The most attention is focused on polymers with N-oxide groups e.g., hyperbranched polyimide N-oxide, which is used as photocatalyst [47]. Their greatest advantages, in comparison to photocatalysts based on inorganic compounds, are easy and cost-efficient synthesis and, particularly, the possibility of visible light absorption without the necessity of structural modifications. Another example of the photocatalyst is light crosslinked polymers, based on triazine N-oxide fragment. It has been shown that they are effective photocatalysts, causing degradation of methyl orange, an azo dye employed as a pH indicator [48]. Most dyes have a very stable structure, which makes their degradation especially difficult and uncontrolled entry of these compounds into water affects flora and fauna. In the case of water reservoirs where there is no flow of water, it might cause eutrophication. N-oxides are also used in coordination polymers, among which semiconductor luminescent materials with tunable luminescence are sought. This type of material can be applied in lighting and displays, as well as in-memory devices and sensors. As an example can be mentioned coordination polymers with symmetric and unsymmetrical ligands-4,4′- and 2,2′-bipyridine N,N’-dioxides and N-oxides [49]. Recent reports concern also pH-responsive polystyrene-b-poly(4-vinylpyridine-N-oxide) membranes [50] and the possibility of applying the coatings from a solution of cellulose-N-methylmorpholine-N-oxide to paper [51]. In the first case, at low pH the pores open (the solution flow increases), and at high pH, the pores close (the solution flow is reduced). The membrane is synthesized by oxidation of polystyrene-b-poly(4-vinylpyridine), which shows an inverse pH response and the presence of both forms in membrane opens up an attractive way for pH-based separations [50]. In other cases, depending on the composition of the coating and whether is it continuous or porous, it is possible to improve the tear strength, print quality as well as the adhesive or antibacterial properties of the paper. It also affects fire resistance, thermal and electrical conductivity, and the friction coefficient.
References
- Váňa, J.; Roithová, J.; Kotora, M.; Beran, P.; Rulíšek, L.; Kočovský, P. Proton Affinities of Organocatalysts Derived from Pyridine N-oxide. Croat. Chem. Acta 2014, 87, 349–356.
- Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Pyridine N-Oxides and Derivatives Thereof in Organocatalysis. In Heterocyclic N-Oxides. Topics in Heterocyclic Chemistry; Larionov, O., Ed.; Springer: Cham, Switzerland, 2017; Volume 53, pp. 29–58.
- Chelucci, G.; Murineddu, G.; Pinna, G.A. Chiral pyridine N-oxides: Useful ligands for asymmetric catalysis. Tetrahedron: Asymmetry 2004, 15, 1373–1389.
- Ikai, T.; Moro, M.; Maeda, K.; Kanoh, S. Synthesis of polysaccharide derivatives bearing pyridine N-oxide groups and their use as asymmetric organocatalysts. React. Funct. Polym. 2011, 71, 1055–1058.
- Malkov, A.V.; Stončius, S.; Bell, M.; Castelluzzo, F.; Ramírez-López, P.; Biedermannová, L.; Langer, V.; Rulišek, L.; Kočovský, P. Mechanistic Dichotomy in the Asymmetric Allylation of Aldehydes with Allyltrichlorosilanes Catalyzed by Chiral Pyridine N-Oxides. Chem. Eur. J. 2013, 19, 9167–9185.
- Motloch, P.; Valterová, I.; Kotora, M. Enantioselective Allylation of Thiophene-2-carbaldehyde: Formal Total Synthesis of Duloxetine. Adv. Synth. Catal. 2014, 356, 199–204.
- Hessler, F.; Korotvička, A.; Nečas, D.; Valterová, I.; Kotora, M. Syntheses of a Flobufen Metabolite and Dapoxetine Based on Enantioselective Allylation of Aromatic Aldehydes. Eur. J. Org. Chem. 2014, 2543–2548.
- O’Hora, P.S.; Incerti-Pradillos, C.A.; Kabeshov, M.A.; Shipilovskikh, S.A.; Rubstov, A.E.; Elsegood, M.R.J.; Malkov, A.V. Catalytic Asymmetric Crotylation of Aldehydes: Application in Total Synthesis of (−)-Elisabethadione. Chem. Eur. J. 2015, 21, 4551–4555.
- Koukal, P.; Kotora, M. Enantioselective Allylation of (2E,4E)-2,4-Dimethylhexadienal: Synthesis of (5R,6S)-(+)-Pteroenone. Chem. Eur. J. 2015, 21, 7408–7412.
- Kadlčίková, A.; Valterová, I.; Ducháčková, L.; Roithová, J.; Kotora, M. Lewis Base Catalyzed Enantioselective Allylation of α,β-Unsaturated Aldehydes. Chem. Eur. J. 2010, 16, 9442–9944.
- Vlašaná, K.; Betík, R.; Valterová, I.; Nečas, D.; Kotora, M. Enantioselective Allylations of Selected α,β,γ,δ-Unsaturated Aldehydes by Axially Chiral, N,N′-dioxides. Synthesis of the Left-hand Part of Papulacandin D. Curr. Organocatal. 2016, 3, 301–305.
- Neniškis, A.; Stončius, S. Enantioselective Ring Opening of meso-Epoxides with Silicon Tetrachloride Catalyzed by Pyridine N-Oxides Fused with the Bicyclononane Framework. Eur. J. Org. Chem. 2015, 6359–6369.
- Kadlčíková, A.; Vlašaná, K.; Kotora, M. Enantioselective epoxide ring opening catalyzed by bis(tetrahydroisoquinoline) N,N′-dioxides. Collect. Czech. Chem. Commun. 2011, 76, 415–422.
- Gnanamani, E.; Someshwar, N.; Sanjeevi, J.; Ramanathan, C.R. Conformationally Rigid Chiral Bicyclic Skeleton-Tethered Bipyridine N,N’-Dioxide as Organocatalyst: Asymmetric Ring Opening of meso-Epoxides. Adv. Synth. Catal. 2014, 356, 2219–2223.
- Takenaka, N.; Saranghtem, R.S.; Captain, B. Helical chiral pyridine N-oxides: A new family of asymmetric catalysts. Angew. Chem. Int. Ed. 2008, 47, 9708–9710.
- Chen, J.; Captain, B.; Takenaka, N. Helical Chiral 2,2′-Bipyridine N-Monoxides as Catalysts in the Enantioselective Propargylation of Aldehydes with Allenyltrichlorosilane. Org. Lett. 2011, 13, 1654–1657.
- Peng, Z.; Takenaka, N. Applications of helical-chiral pyridines as organocatalysts in asymmetric synthesis. Chem. Rec. 2013, 13, 28–42.
- Bednářová, E.; Nečas, D.; Cisařová, I.; Dušek, M.; Lamaty, F.; Kotora, M. Synthesis of new bipyridine N,N’-dioxides and their application in asymmetric allylation of benzaldehyde and aldol addition to acetophenone. Monatsh. Chem. 2019, 150, 29–48.
- Sugiura, M.; Sato, N.; Sonoda, Y.; Kotani, S.; Nakajima, M. Diastereo- and Enantioselective Reductive Aldol Reaction with Trichlorosilane Using Chiral Lewis Bases as Organocatalysts. Chem. Asian J. 2010, 5, 478–481.
- Pei, Y.-N.; Deng, Y.; Li, J.-L.; Liu, L.; Zhu, H.-J. New chiral biscarboline N,N′-dioxide derivatives as catalyst in enantioselective reduction of ketoimines with trichlorosilane. Tetrahedron Lett. 2014, 55, 2948–2952.
- Pan, W.; Deng, Y.; He, J.B.; Bai, B.; Zhu, H.J. Highly efficient asymmetric-axle-supported N-O amides in enantioselective hydrosilylation of ketimines with trichlorosilane. Tetrahedron 2013, 69, 7253–7257.
- Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S. (S)-3,3′-Dimethyl-2,2′-biquinoline N,N′-Dioxide as an Efficient Catalyst for Enantioselective Addition of Allyltrichlorosilanes to Aldehydes. J. Am. Chem. Soc. 1998, 120, 6419–6420.
- Jiao, Z.; Feng, X.; Liu, B.; Chen, F.; Zhang, G.; Jiang, Y. Enantioselective Strecker Reactions between Aldimines and Trimethylsilyl Cyanide Promoted by Chiral N,N′-Dioxides. Eur. J. Org. Chem. 2003, 3818–3826.
- Hrdina, R.; Valterová, I.; Hodačová, J.; Cisařová, I.; Kotora, M. A Simple Approach to Unsymmetric Atropoisomeric Bipyridine N,N’-Dioxides and Their Application in Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2007, 349, 822–826.
- Hrdina, R.; Boyd, T.; Valterová, I.; Hodačová, J.; Kotora, M. Catalytic Asymmetric Allylation of Aliphatic Aldehydes by Chiral Bipyridine N,N′-Dioxides. Synlett 2008, 20, 3141–3144.
- Kadlčíková, A.; Hrdina, R.; Valterová, I.; Kotora, M. Simple and Fast Synthesis of New Axially Chiral Bipyridine N,N’-Dioxides for Highly Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2009, 351, 1279–1283.
- Kwak, J.; Ohk, J.; Jung, Y.; Chang, S. Rollover Cyclometalation Pathway in Rhodium Catalysis: Dramatic NHC Effects in the C–H Bond Functionalization. J. Am. Chem. Soc. 2012, 134, 17778–17788.
- Kwiatkowski, P.; Mucha, P.; Mlostoń, G.; Jurczak, J. Novel Chiral C2-Symmetric Bisimidazole-N-oxide s as Promising Organocatalysts for Enantioselective Allylation of Aromatic Aldehydes. Synlett. 2009, 11, 1757–1760.
- Boyd, D.R.; Sharma, N.D.; Sbircea, L.; Murphy, D.; Malone, J.F.; James, S.L.; Allen, C.C.R.; Hamilton, J.T.G. Chemoenzymatic synthesis of chiral 2,2′-bipyridine ligands and their N-oxide derivatives: Applications in the asymmetric aminolysis of epoxides and asymmetric allylation of aldehydes. Org. Biomol. Chem. 2010, 8, 1081–1090.
- Hrdina, R.; Dračínský, M.; Valterová, I.; Hodačová, J.; Císařová, I.; Kotora, M. New Pathway to C2-Symmetric Atropoisomeric Bipyridine N,N’-Dioxides and Solvent Effect in Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2008, 350, 1449–1456.
- Fulton, J.R.; Glover, J.E.; Kamara, L.; Rowlands, G.J. Facile synthesis of planar chiral N-oxides and their use in Lewis base catalysis. Chem. Commun. 2011, 47, 433–435.
- Chai, Q.; Song, C.; Sun, Z.; Ma, Y.; Ma, C.; Dai, Y.; Andrus, M.B. Asymmetric allylation of aldehydes with allyltrichlorosilane using aza-paracyclophane-oxazoline-N-oxide catalysts. Tetrahedron Lett. 2006, 47, 8611–8615.
- Nakajima, M. Chiral N-Oxides as Catalysts or Ligands in Enantioselective Reactions. J. Synth. Org. Chem. Jpn. 2003, 61, 1081–1087.
- Malkov, A.V.; Kysilka, O.; Edgar, M.; Kadlčiková, A.; Kotora, M.; Kočovský, P. A Novel Bifunctional Allyldisilane as a Triple Allylation Reagent in theStereoselective Synthesis of Trisubstituted Tetrahydrofurans. Chem. Eur. J. 2011, 17, 7162–7166.
- Wong, W.-L.; Lee, W.-S.; Kwong, H.-L. Synthesis of new chiral terpyridine mono-N-oxide and di- N-oxide ligands and their applications in copper-catalyzed asymmetric cyclopropanation. Tetrahedron: Asymmetry 2002, 13, 1485–1492.
- Liu, X.; Lin, L.; Feng, X. Chiral N,N′-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions. ACC Chem. Res. 2011, 44, 574–587.
- Liu, X.; Lin, L.; Feng, X. Chiral N,N′-dioxide ligands: Synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014, 1, 298–302.
- Wang, J.; Zuo, Y.; Hu, C.; Su, Z. heoretical and experimental studies on the structure–property relationship of chiral N,N′-dioxide–metal catalysts probed by the carbonyl–ene reaction of isatin. Catal. Sci. Technol. 2017, 7, 2183–2193.
- Wolińska, E. A study of chiral oxazoline ligands with a 1,2,4-triazine and other six-membered aza-heteroaromatic rings and their application in Cu-catalysed asymmetric nitroaldol reactions. Tetrahedron Asymmetry 2014, 25, 1478–1487.
- Wolińska, E. Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings - synthesis and application in the Cu-catalyzed nitroaldol reaction. Heterocycl. Commun. 2016, 22, 85–94.
- Wolińska, E.; Karczmarczyk, Z.; Wysocki, W. Structural characterization of copper complexes with chiral 1,2,4-triazine-oxazoline ligands. Heterocycl. Commun. 2016, 22, 265–274.
- Larionov, O.V.; Stephens, D.; Mfuh, A.M.; Arman, H.D.; Naumova, A.S.; Chavez, G.; Skenderi, B. Insights into the mechanistic and synthetic aspects of the Mo/P-catalyzed oxidation of N-heterocycles. Org. Biomol. Chem. 2014, 12, 3026–3036.
- Mfuh, A.M.; Larionov, O.V. Heterocyclic N-Oxides—An Emerging Class of Therapeutic Agents. Curr. Med. Chem. 2015, 22, 2819–2857.
- Herrmann, A.; Hedman, H.; Rosén, J.; Jansson, D.; Haraldsson, B.; Hellenäs, K.-E. Analysis of the Mushroom Nephrotoxin Orellanine and Its Glucosides. J. Nat. Prod. 2012, 75, 1690–1696.
- Limnios, D.; Kokotos, C.G. 2,2,2-Trifluoroacetophenone as an Organocatalyst for the Oxidation of Tertiary Amines and Azines to N-Oxides. Chem. Eur. J. 2014, 20, 559–563.
- Albini, A.; Pietra, S. Heterocyclic N-Oxides, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 285–287.
- Yang, J.; Chu, S.; Guo, Y.; Luo, L.; Kong, F.; Wang, Y.; Zou, Z. Hyperbranched polymeric N-oxide: A novel kind of metal-free photocatalyst. Chem. Commun. 2012, 48, 3533–3535.
- Li, Y.; Zhang, W.; Wang, J.; Lu, H.; Liu, Y.; Liu, Z.; Xie, Z. Light-induced synthesis of triazine N-oxide-based cross-linked polymers for effective photocatalytic degradation of methyl orange. RSC Adv. 2017, 7, 9309–9315.
- Toma, O.; Mercier, N.; Allain, M.; Meinardi, F.; Botta, C. Lead(II) 4,4’-Bipyridine N-Oxide Coordination Polymers—Highly Phosphorescent Materials with Mechanochromic Luminescence Properties. Eur. J. Inorg. Chem. 2017, 844–850.
- Shevate, R.; Karunakaran, M.; Kumar, M.; Peinemann, K.-V. Polyanionic pH-responsive polystyrene-b-poly(4-vinyl pyridine-N-oxide) isoporous membranes. J. Membrane Sci. 2016, 501, 161–168.
- Krysztof, M.; Olejnik, K.; Kulpinski, P.; Stanislawska, A.; Khadzhynova, S. Regenerated cellulose from N-methylmorpholine N-oxide solutions as a coating agent for paper materials. Cellulose 2018, 25, 3595–3607.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
28 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





