Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sung Un Huh | -- | 2002 | 2022-04-27 10:53:19 | | | |
| 2 | Dean Liu | Meta information modification | 2002 | 2022-04-28 03:34:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Huh, S.U. Metacaspases. Encyclopedia. Available online: https://encyclopedia.pub/entry/22355 (accessed on 08 February 2026).
Huh SU. Metacaspases. Encyclopedia. Available at: https://encyclopedia.pub/entry/22355. Accessed February 08, 2026.
Huh, Sung Un. "Metacaspases" Encyclopedia, https://encyclopedia.pub/entry/22355 (accessed February 08, 2026).
Huh, S.U. (2022, April 27). Metacaspases. In Encyclopedia. https://encyclopedia.pub/entry/22355
Huh, Sung Un. "Metacaspases." Encyclopedia. Web. 27 April, 2022.
Copy Citation
Metacaspases, a family of cysteine proteases, play critical roles in programmed cell death during plant development and defense responses. Plant metacaspases are further subdivided into types I, II, and III. In the type I Arabidopsis MCs, AtMC1 and AtMC2 have similar structures, but antagonistically regulate hypersensitive response cell death upon immune receptor activation.
caspase
metacaspase
programmed cell death
1. Basic Features of Caspase and Metacaspase
Apoptosis is one of the programmed cell deaths that is crucial for tissue development and homeostasis [1]. Programmed cell death is promoted by caspases, a highly conserved set of intracellular proteases. Caspase is a term used to describe the two functional roles of this group of enzymes. In this case, “c” stands for cysteine protease, and “aspase” stands for its ability to cleave aspartic acid residues [2]. Based on caspase functions, mammalian caspases are divided into the apoptotic and inflammatory caspases groups. Inflammatory caspases trigger a form of inflammation known as pyroptosis. These caspases play important roles in the activation of the inflammasome to initiate inflammation and initiate programmed cell death. Inflammatory caspases contain caspase-1, caspase-4, caspase-5, caspase-11, and caspase-12 (Figure 1A). On the other hand, apoptotic caspases initiate and execute an immunologically silent form of programmed cell death known as apoptosis. Apoptotic caspases are subgrouped into initiator and effector caspases according to their functional order in the execution of apoptosis [3]. Initiator caspases, including caspase-2, caspase-8, caspase-9, and caspase-10, function as proteolytic signal amplifiers to activate effector caspases. Effector caspases, including caspase-3, caspase-6, and caspase-7 enhance apoptosis through the proteolysis of several cellular proteins at their target sites (Figure 1A).
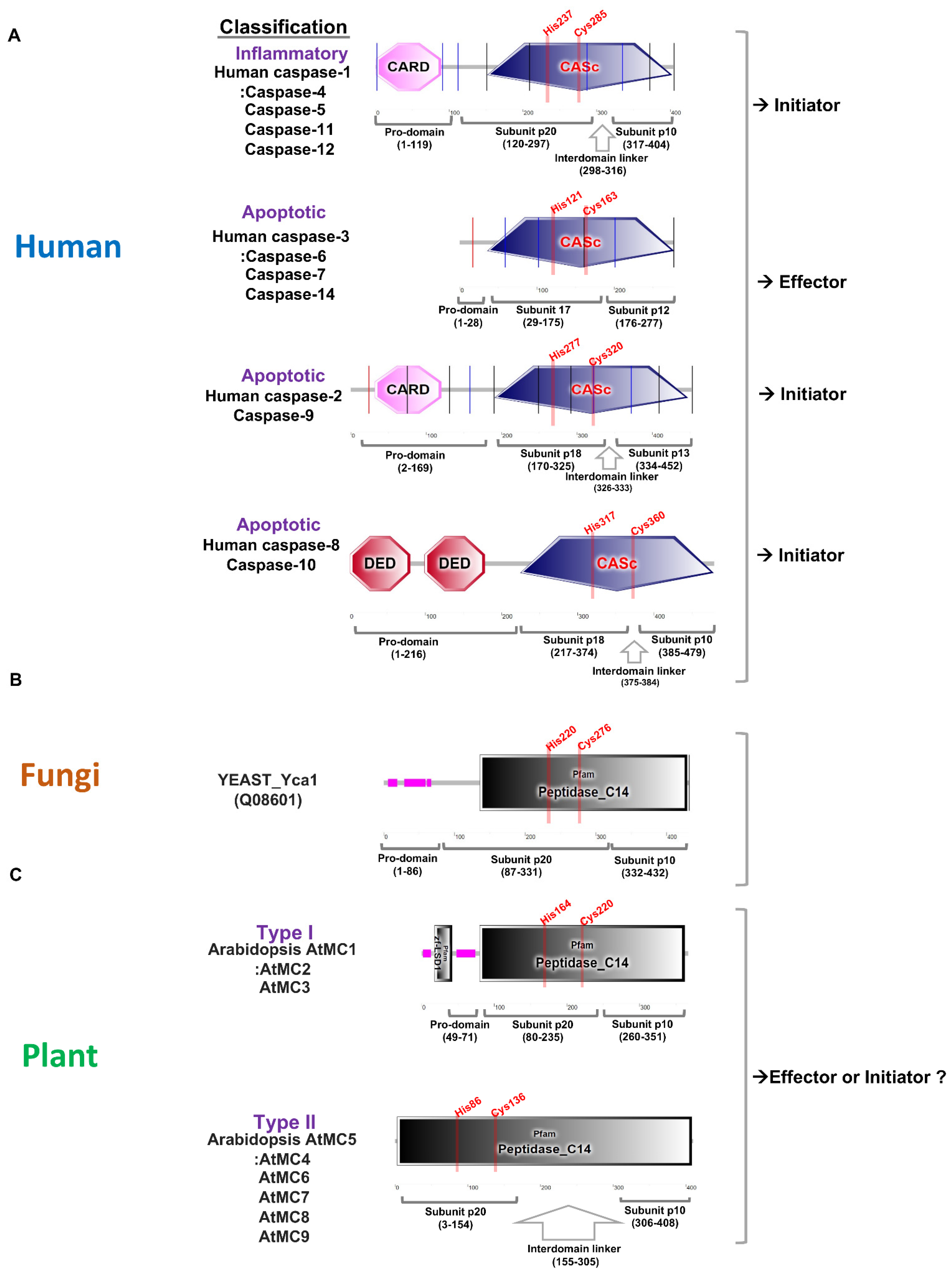
Figure 1. Comparative analysis of protein domains for caspase and metacaspase in human, yeast, and plant. (A). Protein domains for human caspase-1, caspase-2, and caspase-3 are marked using a simple modular architecture research tool (SMART) [4]. Caspases are classified into inflammatory and apoptotic according to their functions, and apoptotic caspases are further divided into initiators and effectors. Initiator caspases have a pro-domain containing a CARD, or death effector domain (DED), at the N-terminus, and effector caspases have a very short pro-domain. Human caspases have conserved active sites (His and Cys) in the large subunit p20. (B). Yeast contains one metacaspase in its genome and has a pro-domain in the N-terminus. There is no special linker between subunits p20 and p10, and active sites (His and Cys) are conserved in subunit p20. (C). Arabidopsis thaliana contains three type I and six type II metacaspases, and type I has an N-terminal pro-domain with or without a zinc-finger at the N-terminus. Type II lacks a pro-domain, but it is characterized by a long linker between subunits p20 and p10. The active sites (His and Cys) are conserved in subunit p20.
Caspases are comprised of a pro-domain and a mature chain, which is folded into a caspase/hemoglobinase. After the removal of the pro-domain, caspases undergo proteolytic cleavage that releases two peptide fragments with the large subunit p20 (about 20 kDa) and small subunit p10 (about 10 kDa), respectively. Caspase-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11, and caspase-12 also have amino-terminal pro-domains including caspase recruitment domains, or caspase activation and recruitment domains (CARDs), which are interaction motifs. Effector caspase-3, caspase-6, and caspase-7 contain short pro-domains and these caspases are activated by the initiator caspases (Figure 1A). Caspase-1 can, in basic terms, induce pyroptotic cell death in response to pathogen- associated signals, and is, therefore, critical for innate immunity [5]. The full-length pro-caspase-1 is activated by proximity-induced autologous proteolysis. Activated caspase-1 cleaves the inflammatory cytokines pro-IL-1β and pro-IL-18 for activating cytokines [6]. Caspase-1, including caspase-4, -5, and -11, can specifically cleave pore-forming protein gasdermin D (GSDMD), and cleavage of GSDMD is required for pyroptosis [6][7]. Interestingly, interdomain linker cleavage of caspase-1 is also required for pyroptosis [8]. Although caspase cleavage occurs in various domains, it can be expected that the activation step is highly conserved in the caspases of other species. Many metacaspases were not found in fungi compared to plants. The yeast genome contains only one metacaspase, called Yca1, which has a long pro-domain and undergoes autocatalytic processing in a Ca2+-dependent manner [9]. Yca1 has a protein fold similar to the canonical caspases (Figure 1B). Yca1 is known to play an important role as a positive regulator of apoptosis [10][11][12]. The protease activity of Yca1 is believed to degrade protein aggregates rather than limit aggregate formation [13]. The rice blast fungus Magnaporthe oryzae has two metacaspase genes, MoMca1 and MoMca2 [14]. It has been reported that these metacaspase proteins exhibit functional redundancy and can complement the yeast Yca1 mutant [14]. Double mutant Momca1mca2 strain contains increased insoluble aggregates during vegetative growth. MoMca1 and MoMca2 promote the clearance of the insoluble aggregates in M. oryzae [14]. Metacaspase function in fungi may help maintain the fitness of fungal cells by eliminating insoluble aggregates under stress conditions.
The caspase family is the only family of cysteine proteases with members in all kingdoms according to the MEROPS database [15][16]. Plant genomes also contain evolutionarily conserved caspase-like genes. However, plant proteases are not cysteine-dependent aspartate-directed proteases, which are characterized as mammalian caspases, but structural homologues called metacaspases [17]. In a previous report, phylogenetic analysis found that eukaryotic caspases, metacaspases, and paracaspases were equally distant from each other. It is classified within the clade CD of cysteine protease [17]. Metacaspases are only found in eukaryotes such as plants, fungi, and protists. As in caspases, metacaspases contain a caspase-specific catalytic dyad of histidine and cysteine in the large subunit p20. Although plant metacaspases lack substrate specificity for aspartate residues, metacaspases share structural homology with mammalian caspases. Based on the presence or absence of an N-terminal pro-domain, three types of metacaspases, containing subunit p20 and subunit p10 caspase domains, are identified [15][18]. Type I metacaspases contain an N-terminal pro-domain containing a proline-rich repeat motif and a zinc finger motif in plant members (Figure 1C). There are nine metacaspases in Arabidopsis, of which AtMC1, AtMC2, and AtMC3 have the characteristics of type I metacaspases. Type II metacaspases lack a pro-domain at the N-terminus but present a linker region between the putative large subunit p20 and small subunit p10. AtMC4-AtMC9 have the characteristics of type II (Figure 1C). The AtMC4 crystal structure is determined and AtMC4 can modulate Ca2+-dependent as a damage-induced plant immune defense [19]. Large linker domain of AtMC4 acts as inhibitory conformation and suppresses metacaspase activation [19]. Type II metacaspases appear to have evolved functions to regulate protein activity through a long linker domain between p20 and p10 instead of at the N-terminal extension. Recently, type III metacaspases were discovered in the genome of the Guillardia theta algae. [20]. G. theta metacaspases exhibit 1 type I (GtMC1), 1 type III (GtMC2), and 11 metacaspase-like types (GtMC3-GtMC14) [20]. Type III differs from type I in that the protein position of p20–p10 is switched only in the order of p10–p20. More research is needed to determine what kind of functional effect this arrangement has on protease. Although studies on metacaspase-like types are still lacking, it can be determined that algae have evolved in a considerable number and in various forms.
2. Subcellular Localization of Caspase and Metacaspase
The intracellular localization of caspase and metacaspase shows various distributions [21][22]. In particular, their intracellular localization in the activated state may be important as caspase and metacaspase undergo autoproteolysis and interact with target proteins. Pro-caspase-1 is present in the cytoplasm in an inactive form and requires inflammasomes for proteolytic activation. Interestingly, pro-caspase-1, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), and ASCs (apoptosis-associated speckle-like proteins with carboxy-terminal CARDs) translocate between the nucleus and the cytoplasm as a reaction to an inflammatory response [23][24][25].
Caspase-1 can target and cleave the GATA4 transcription factor, which regulates cardiac cell fate [26]. Mature caspase-1 can be expected to have various functions in the nucleus when activated. In addition, GATA4 was found to be evolutionarily conserved in a similar way to transcriptional regulators in plants and fungi [27][28][29][30][31][32]. GATA factors can bind to the 5′-WGATAR-3′ motif via a C-terminal zinc finger domain, and an N-terminal zinc finger lends supports in order to stabilize the interaction [33]. Although there is no known metacaspase-GATA protein interaction yet, it is likely that plant and fungal metacaspases bind to nuclear GATA transcription factors and participate in transcriptional regulation. Furthermore, caspase-2 contains a classical nuclear localization signal peptide, and also contains a putative mitochondrial targeting sequence [34]. Caspase-2 has been observed in the mitochondria, and it has been determined that it is essential for mitochondrial oxidative stress-induced apoptosis. Casp2−/− primary skin fibroblasts are protected upon oxidant treatment [34]. A general fact is that caspase-2 activation occurs mainly in the cytoplasm, but its function is exerted at various subcellular locations [35]. Additionally, caspase-1 or caspase-3 have been detected in the plasma membrane to promote pyroptosis and apoptosis-induced proliferation [21][36]. It can be expected that caspases with these diverse intracellular localizations can be translocated depending on the targets.
The yeast genome only has a single type I metacaspase, Yca1, which localizes in insoluble protein aggregates via its N-terminal pro-domain and promotes aggregate clearance [37]. The loss of Yca1 results in increased retention of aggregated material within the insoluble aggregates. It has been found that Yca1 associates with components of the ubiquitin protease system (UPS), such as E3 ligase Rsp5 and ubiquitinated Yca1 is located in the juxtanuclear quality control compartment (JUNQ) which is tethered to the nucleus [38]. As in Yca1, the Arabidopsis full-length AtMC1 is found in the microsomal, in the insoluble fraction, but processed AtMC1 is located in the soluble fraction [39]. Interestingly, the catalytic dead mutant AtMC1 (C99A-C220A) protein remained mostly insoluble. Independent of the catalytic activity, it can be detected that the intra-cellular localization of AtMC1 is very similar to that of Yca1. During programmed cell semi-death of sieve elements in Tritium aestivum, type II metacaspase TaeMCAII has been detected in dynamic localizations [40]. The authors collected spikelet samples from 0 to 7 days post-flowering (DAF) to detect TaeMCAII localization using immunoelectron microscopy. In the first step (1 and 2 DAF), TaeMCAII was mainly located to the nucleus. In the middle stage (3, 4, and 5 DAF), it was generally distributed around the cytoplasm and nuclear fragments. In the last stage (6 and 7 DAF), which started at the last stage of sieve element developments, translocation of TaeMCAII from the cytoplasm to the cell wall was found [40]. These results implied that the intracellular localization of metacaspases was also shifted, almost similar to the movement of Ca2+. Sieve elements are different from typical programmed cell death, but it can be expected that the function of metacaspases during development is determined by changes in their subcellular localization that are dependent on Ca2+.
Two grapevine (Vitis rupestris L.) metacaspases, VrMC2 and VrMC5 have been identified as type I and type II metacaspases, respectively [41]. VrMC2-GFP, which contains a putative retention-like motif (KPFI) in the C-terminal region, is localized around the nucleus area as aggregated dots, and is merged with the ER marker protein. On the other hand, VrMC5-GFP, which lacks any canonical organelle-targeting signal, is detected mainly in the cytoplasm and nucleus [41]. Thus, it is expected that type I and type II metacaspases may function differently at different subcellular locations in the cell. However, both VrMC2 and VrMC5 participate in cell death-related immunity and act as executors of hypersensitive cell death. The VrMC2 homologue from Oryza sativa OsMC1, which is localized exclusively in the nucleus, has putative nuclear localization signals (NLS) in the N-terminal region [22]. The type II OsMC5, OsMC5, and OsMC8 were normally found in the cytoplasm [22]. Similarly, Arabidopsis AtMC4 was predominantly localized in the cytosol, but was also detected in the nucleus [42][43]. Interestingly, type II AtMC4, AtMC5, AtMC6, and AtMC7 participate in processing of tonoplast-localized plant elicitor peptide 1 (PROPEP1), to active Pep-mediated plant immunity and these type II metacaspases might translocate in diverse cellular positions. Maize type I metacaspases, ZmMC1 and ZmMC2, exhibited partial localization with the autophagic marker ATG8a in maize protoplasts [44]. If coexpressed with intracellular nucleotide-binding, leucine-rich repeat (NLR or NB-LRR) protein, Rp1-D21 or coiled-coil (CC) domain of Rp1-D21, ZmMC1, and ZmMC2 proteins localize to the nucleocytoplasm and dot-like structure [44]. As in animal caspases, plant metacaspases can move to various subcellular locations in the cell. The dynamic intracellular localization of metacaspases can be influenced by protein interaction partners or targets within the cell.
References
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120.
- Alnemri, E.S.; Livingston, D.J.; Nicholson, D.W.; Salvesen, G.; Thornberry, N.A.; Wong, W.W.; Yuan, J. Human ICE/CED-3 protease nomenclature. Cell 1996, 87, 171.
- Man, S.M.; Kanneganti, T.D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016, 16, 7–21.
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496.
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420.
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665.
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671.
- Ball, D.P.; Taabazuing, C.Y.; Griswold, A.R.; Orth, E.L.; Rao, S.D.; Kotliar, I.B.; Vostal, L.E.; Johnson, D.C.; Bachovchin, D.A. Caspase-1 interdomain linker cleavage is required for pyroptosis. Life Sci. Alliance 2020, 3.
- Wong, A.H.; Yan, C.; Shi, Y. Crystal structure of the yeast metacaspase Yca1. J. Biol. Chem. 2012, 287, 29251–29259.
- Longo, V.; Zdralevic, M.; Guaragnella, N.; Giannattasio, S.; Zolla, L.; Timperio, A.M. Proteome and metabolome profiling of wild-type and YCA1-knock-out yeast cells during acetic acid-induced programmed cell death. J. Proteom. 2015, 128, 173–188.
- Zdralevic, M.; Longo, V.; Guaragnella, N.; Giannattasio, S.; Timperio, A.M.; Zolla, L. Differential proteome-metabolome profiling of YCA1-knock-out and wild type cells reveals novel metabolic pathways and cellular processes dependent on the yeast metacaspase. Mol. Biosyst. 2015, 11, 1573–1583.
- Chaves, S.R.; Rego, A.; Martins, V.M.; Santos-Pereira, C.; Sousa, M.J.; Corte-Real, M. Regulation of Cell Death Induced by Acetic Acid in Yeasts. Front. Cell Dev. Biol. 2021, 9, 642375.
- Shrestha, A.; Puente, L.G.; Brunette, S.; Megeney, L.A. The role of Yca1 in proteostasis. Yca1 regulates the composition of the insoluble proteome. J. Proteom. 2013, 81, 24–30.
- Fernandez, J.; Lopez, V.; Kinch, L.; Pfeifer, M.A.; Gray, H.; Garcia, N.; Grishin, N.V.; Khang, C.H.; Orth, K. Role of Two Metacaspases in Development and Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae. mBio 2021, 12, e03471-20.
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632.
- McLuskey, K.; Mottram, J.C. Comparative structural analysis of the caspase family with other clan CD cysteine peptidases. Biochem. J. 2015, 466, 219–232.
- Uren, A.G.; O’Rourke, K.; Aravind, L.A.; Pisabarro, M.T.; Seshagiri, S.; Koonin, E.V.; Dixit, V.M. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 2000, 6, 961–967.
- Minina, E.A.; Coll, N.S.; Tuominen, H.; Bozhkov, P.V. Metacaspases versus caspases in development and cell fate regulation. Cell Death Differ. 2017, 24, 1314–1325.
- Zhu, P.; Yu, X.H.; Wang, C.; Zhang, Q.; Liu, W.; McSweeney, S.; Shanklin, J.; Lam, E.; Liu, Q. Structural basis for Ca(2+)-dependent activation of a plant metacaspase. Nat. Commun. 2020, 11, 2249.
- Klemencic, M.; Funk, C. Type III metacaspases: Calcium-dependent activity proposes new function for the p10 domain. New Phytol. 2018, 218, 1179–1191.
- Amcheslavsky, A.; Wang, S.; Fogarty, C.E.; Lindblad, J.L.; Fan, Y.; Bergmann, A. Plasma Membrane Localization of Apoptotic Caspases for Non-apoptotic Functions. Dev. Cell 2018, 45, 450–464.e3.
- Huang, L.; Zhang, H.; Hong, Y.; Liu, S.; Li, D.; Song, F. Stress-Responsive Expression, Subcellular Localization and Protein-Protein Interactions of the Rice Metacaspase Family. Int. J. Mol. Sci. 2015, 16, 16216–16241.
- Bryan, N.B.; Dorfleutner, A.; Rojanasakul, Y.; Stehlik, C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 2009, 182, 3173–3182.
- Wang, S.L.; Zhao, G.; Zhu, W.; Dong, X.M.; Liu, T.; Li, Y.Y.; Song, W.G.; Wang, Y.Q. Herpes simplex virus-1 infection or Simian virus 40-mediated immortalization of corneal cells causes permanent translocation of NLRP3 to the nuclei. Int. J. Ophthalmol. 2015, 8, 46–51.
- Mao, P.L.; Jiang, Y.; Wee, B.Y.; Porter, A.G. Activation of caspase-1 in the nucleus requires nuclear translocation of pro-caspase-1 mediated by its prodomain. J. Biol. Chem. 1998, 273, 23621–23624.
- Aries, A.; Whitcomb, J.; Shao, W.; Komati, H.; Saleh, M.; Nemer, M. Caspase-1 cleavage of transcription factor GATA4 and regulation of cardiac cell fate. Cell Death Dis. 2014, 5, e1566.
- He, C.; Cheng, H.; Zhou, R. GATA family of transcription factors of vertebrates: Phylogenetics and chromosomal synteny. J. Biosci. 2007, 32, 1273–1280.
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004, 134, 1718–1732.
- Kim, M.; Xi, H.; Park, J. Genome-wide comparative analyses of GATA transcription factors among 19 Arabidopsis ecotype genomes: Intraspecific characteristics of GATA transcription factors. PLoS ONE 2021, 16, e0252181.
- Kim, M.; Xi, H.; Park, S.; Yun, Y.; Park, J. Genome-wide comparative analyses of GATA transcription factors among seven Populus genomes. Sci. Rep. 2021, 11, 16578.
- Manzoor, M.A.; Sabir, I.A.; Shah, I.H.; Wang, H.; Yu, Z.; Rasool, F.; Mazhar, M.Z.; Younas, S.; Abdullah, M.; Cai, Y. Comprehensive Comparative Analysis of the GATA Transcription Factors in Four Rosaceae Species and Phytohormonal Response in Chinese Pear (Pyrus bretschneideri) Fruit. Int. J. Mol. Sci. 2021, 22, 12492.
- Yu, M.; Yu, J.; Cao, H.; Yong, M.; Liu, Y. Genome-wide identification and analysis of the GATA transcription factor gene family in Ustilaginoidea virens. Genome 2019, 62, 807–816.
- Tanaka, H.; Takizawa, Y.; Takaku, M.; Kato, D.; Kumagawa, Y.; Grimm, S.A.; Wade, P.A.; Kurumizaka, H. Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat. Commun. 2020, 11, 4136.
- Lopez-Cruzan, M.; Sharma, R.; Tiwari, M.; Karbach, S.; Holstein, D.; Martin, C.R.; Lechleiter, J.D.; Herman, B. Caspase-2 resides in the mitochondria and mediates apoptosis directly from the mitochondrial compartment. Cell Death Dis. 2016, 2, 16005.
- Brown-Suedel, A.N.; Bouchier-Hayes, L. Caspase-2 Substrates: To Apoptosis, Cell Cycle Control, and Beyond. Front. Cell Dev. Biol. 2020, 8, 610022.
- McKenzie, B.A.; Fernandes, J.P.; Doan, M.A.L.; Schmitt, L.M.; Branton, W.G.; Power, C. Activation of the executioner caspases-3 and -7 promotes microglial pyroptosis in models of multiple sclerosis. J. Neuroinflam. 2020, 17, 253.
- Lee, R.E.; Brunette, S.; Puente, L.G.; Megeney, L.A. Metacaspase Yca1 is required for clearance of insoluble protein aggregates. Proc. Natl. Acad. Sci. USA 2010, 107, 13348–13353.
- Shrestha, A.; Brunette, S.; Stanford, W.L.; Megeney, L.A. The metacaspase Yca1 maintains proteostasis through multiple interactions with the ubiquitin system. Cell Discov. 2019, 5, 6.
- Coll, N.S.; Smidler, A.; Puigvert, M.; Popa, C.; Valls, M.; Dangl, J.L. The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: Functional linkage with autophagy. Cell Death Differ. 2014, 21, 1399–1408.
- Zhang, Z.; Lv, Y.; Zhou, Z.; Mei, F.; Wang, L. Type II metacaspase protein localization and gene transcription during programmed cell semi-death of sieve elements in developing caryopsis of Tritium aestivum. Biologia 2017, 72, 398–406.
- Gong, P.; Riemann, M.; Dong, D.; Stoeffler, N.; Gross, B.; Markel, A.; Nick, P. Two grapevine metacaspase genes mediate ETI-like cell death in grapevine defence against infection of Plasmopara viticola. Protoplasma 2019, 256, 951–969.
- Watanabe, N.; Lam, E. Calcium-dependent activation and autolysis of Arabidopsis metacaspase 2d. J. Biol. Chem. 2011, 286, 10027–10040.
- Watanabe, N.; Lam, E. Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biotic and abiotic stresses. Plant J. 2011, 66, 969–982.
- Luan, Q.L.; Zhu, Y.X.; Ma, S.; Sun, Y.; Liu, X.Y.; Liu, M.; Balint-Kurti, P.J.; Wang, G.F. Maize metacaspases modulate the defense response mediated by the NLR protein Rp1-D21 likely by affecting its subcellular localization. Plant J. 2021, 105, 151–166.
More
Information
Subjects:
Evolutionary Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
970
Revisions:
2 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




