Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marie Bruyneel | -- | 3052 | 2022-04-24 14:19:31 | | | |
| 2 | Peter Tang | + 15 word(s) | 3067 | 2022-04-25 04:36:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bruyneel, M.; , . Telemonitoring of Continuous Positive Airway Pressure-Treated Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/22208 (accessed on 07 February 2026).
Bruyneel M, . Telemonitoring of Continuous Positive Airway Pressure-Treated Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/22208. Accessed February 07, 2026.
Bruyneel, Marie, . "Telemonitoring of Continuous Positive Airway Pressure-Treated Patients" Encyclopedia, https://encyclopedia.pub/entry/22208 (accessed February 07, 2026).
Bruyneel, M., & , . (2022, April 24). Telemonitoring of Continuous Positive Airway Pressure-Treated Patients. In Encyclopedia. https://encyclopedia.pub/entry/22208
Bruyneel, Marie and . "Telemonitoring of Continuous Positive Airway Pressure-Treated Patients." Encyclopedia. Web. 24 April, 2022.
Copy Citation
Obstructive sleep apnea–hypopnea (OSA) syndrome is a highly prevalent disease despite still being under-diagnosed. This disorder is responsible for reduced quality of life (QoL), secondary excessive daytime sleepiness (EDS), negative cognitive and psychological impacts, and contributes to risk of cardiovascular disease, stroke, and diabetes. Continuous positive airway pressure (CPAP) telemonitoring (TMg) has become widely implemented in routine clinical care. Objective measures of CPAP compliance, residual respiratory events, and leaks can be easily monitored, but limitations exist.
continuous positive airway pressure
obstructive sleep apnea
telemonitoring
leaks
compliance
1. Introduction
Obstructive sleep apnea–hypopnea (OSA) syndrome is a highly prevalent disease despite still being under-diagnosed. This disorder is responsible for reduced quality of life (QoL), secondary excessive daytime sleepiness (EDS), negative cognitive and psychological impacts, and contributes to risk of cardiovascular disease, stroke, and diabetes [1]. The severity of the disease is currently based on apnea–hypopnea index (AHI), despite the fact that this definition is poorly correlated with the clinical features and other consequences of the disease, and will be probably adapted in the future [2]. The definition of apnea–hypopnea index (AHI) is number of hypopneas + apnea/hour of sleep (or recording time), measured on a polysomnography (comprehensive sleep assessment, able to assess the exact sleep time) or on a polygraphy (simplified sleep recording where only the recording time is measured).
Continuous positive airway pressure (CPAP) remains the first-line treatment in patients with moderate to severe OSA. Recommendations suggest regular patient follow-up in order to ensure treatment effectiveness, tolerance, and adherence as well as symptom resolution. In this context, telemedicine (TM) is a promising way to optimize care.
The use of TM has increased worldwide during the last decade, particularly during the last two years when its use has been motivated by the COVID-19 pandemic [3]. It is anticipated that the use of TM will continue to grow in the future.
TM can be defined as the use of information and communication technologies to improve patient outcomes by increasing access to care and medical information [4].
Since the development of the first CPAP device, assessment of the efficacy of CPAP for the management of respiratory obstructive events has continuously improved. From clinical and self-reported information, built-in CPAP monitoring systems have been developed to record data as well as respiratory parameters [5], moving monitoring from hospital to home.
Today, TM has a preponderant role in the field of sleep medicine and is important to every stage of management of OSA, from diagnosis to CPAP treatment monitoring. Recording system data allows patient follow-up at home and remote adaptation of some parameters, a practice termed telemonitoring (TMg). Recorded data review is also very helpful during consultations, in addition to clinical assessment, in order to objectively document compliance, treatment efficacy, and the cause of side effects (e.g., leaks).
2. Telemonitoring of CPAP-Treated Patients
2.1. Measurement and Accuracy of CPAP-Recorded Data
CPAP tracking systems are built with connectivity to allow remote access by way of Global System for Mobile Communication (GSM) or General Packing Radio System (GPRS), providing information about daily use, pattern of use, respiratory events (residual AHI), type of residual events (central, obstructive), mask leaks and CPAP pressure. Data are transferred on a daily basis and are available via a central secured data center (cloud) for healthcare providers/professionals or, for some devices, via an application, accessed directly by the patient, to stimulate self-management (Figure 1).
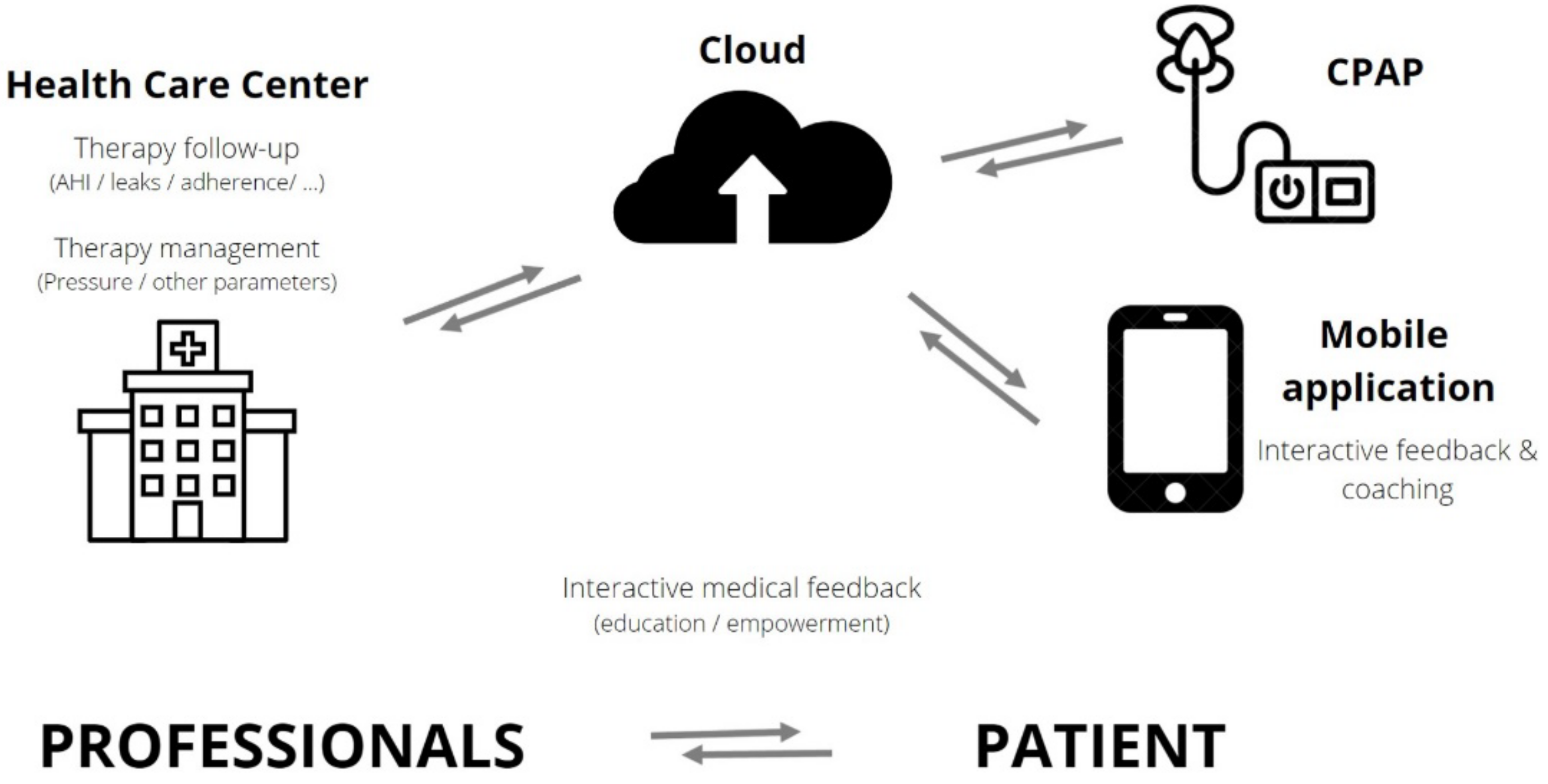
Figure 1. Interactions between patients and healthcare professionals in telemonitored CPAP-treated OSA patients. OSA: obstructive sleep apnea–hypopnea; CPAP: continuous positive airway pressure; AHI: apnea–hypopnea index.
Figure 2A,B show an example of the type of data that can be extracted from TMg as a summary of the data collected from the CPAP device, in patients exhibiting irregular use and residual respiratory events during sleep. The pattern of CPAP use can also be interesting information. For example, in cases of comorbid sleep disorder or when sleep is disturbed due to environmental factors (e.g., shift work, narcolepsy, Ramadan) (Figure 3A,B and Figure 4A,B).
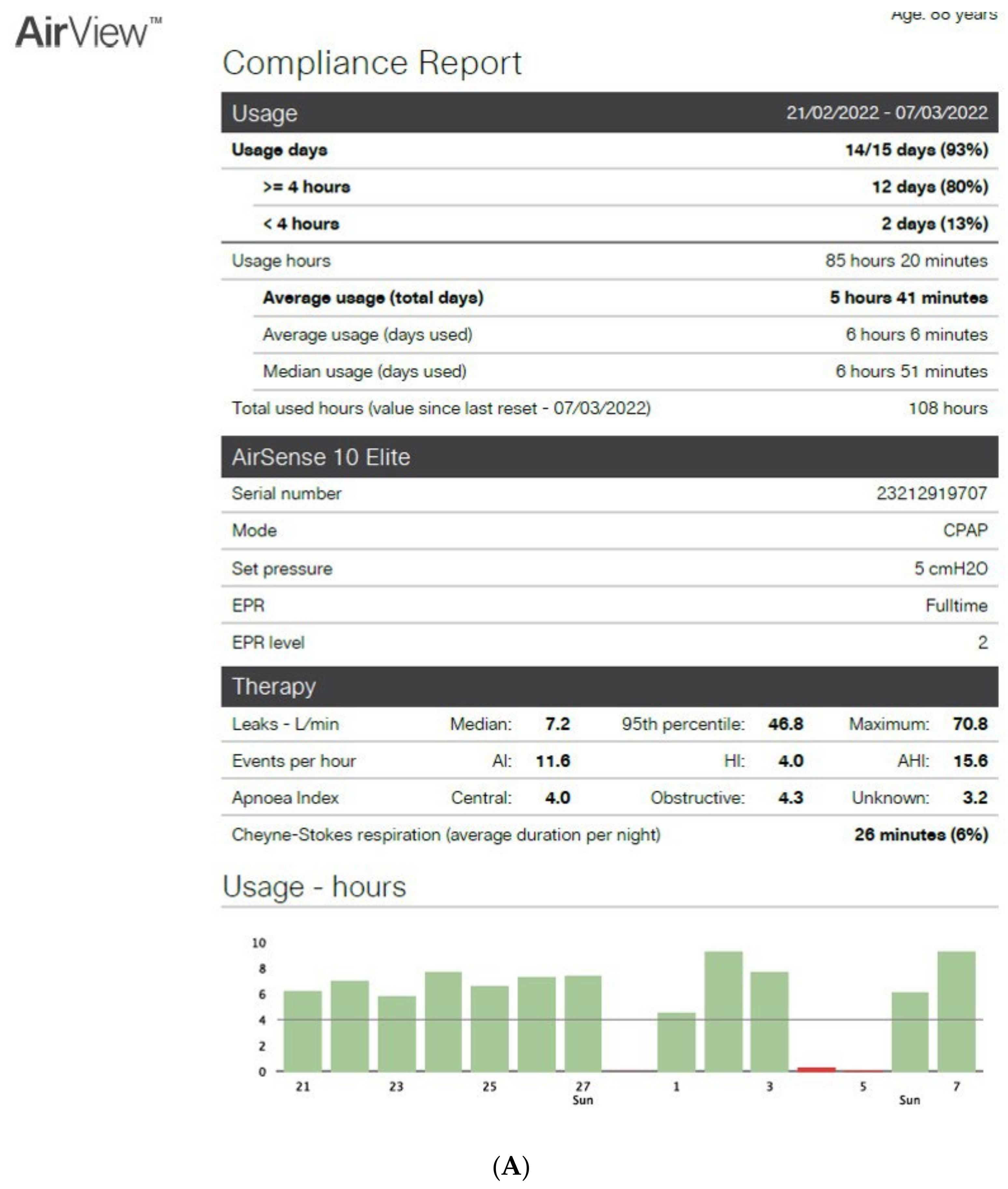
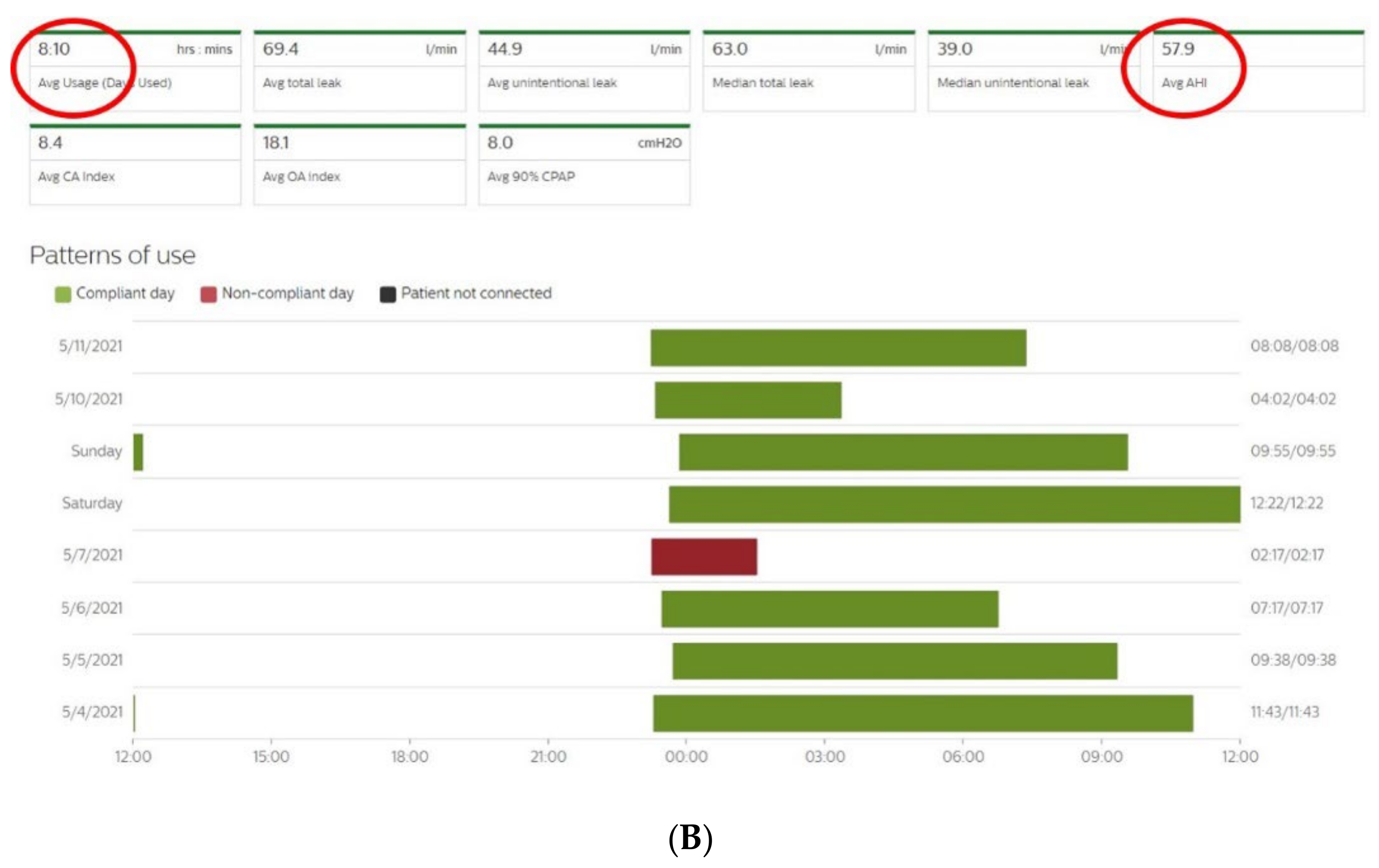
Figure 2. CPAP device data collection ((A): Resmed; (B): Philips) over an 8-to-15-day period in patients exhibiting irregular use and residual respiratory events during sleep. AI: apnea index, HI: hypopnea index, AHI: apnea–hypopnea index, Avg: average, CA: central apnea, OA: obstructive apnea. Red circles highlight average usage and average residual AHI.
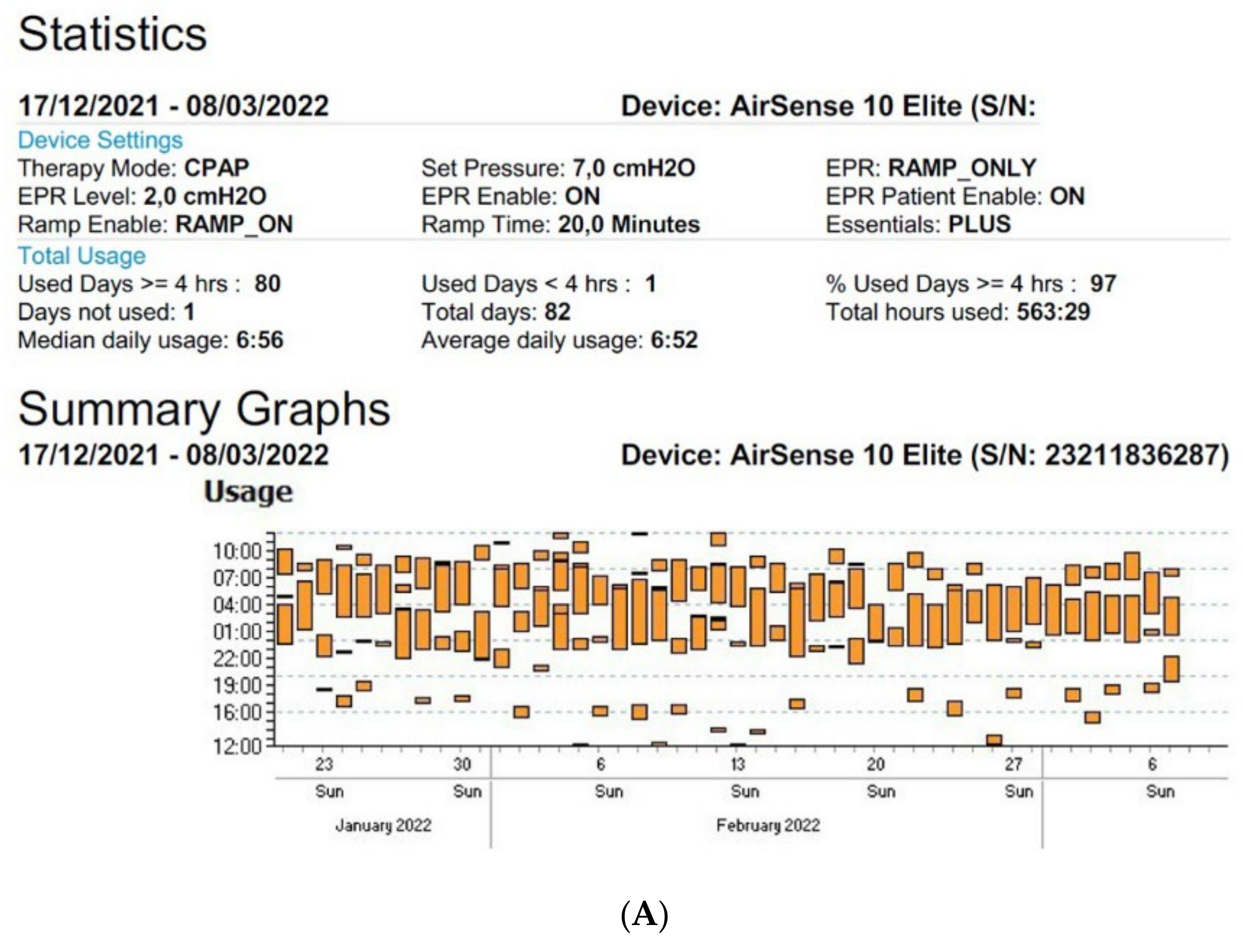
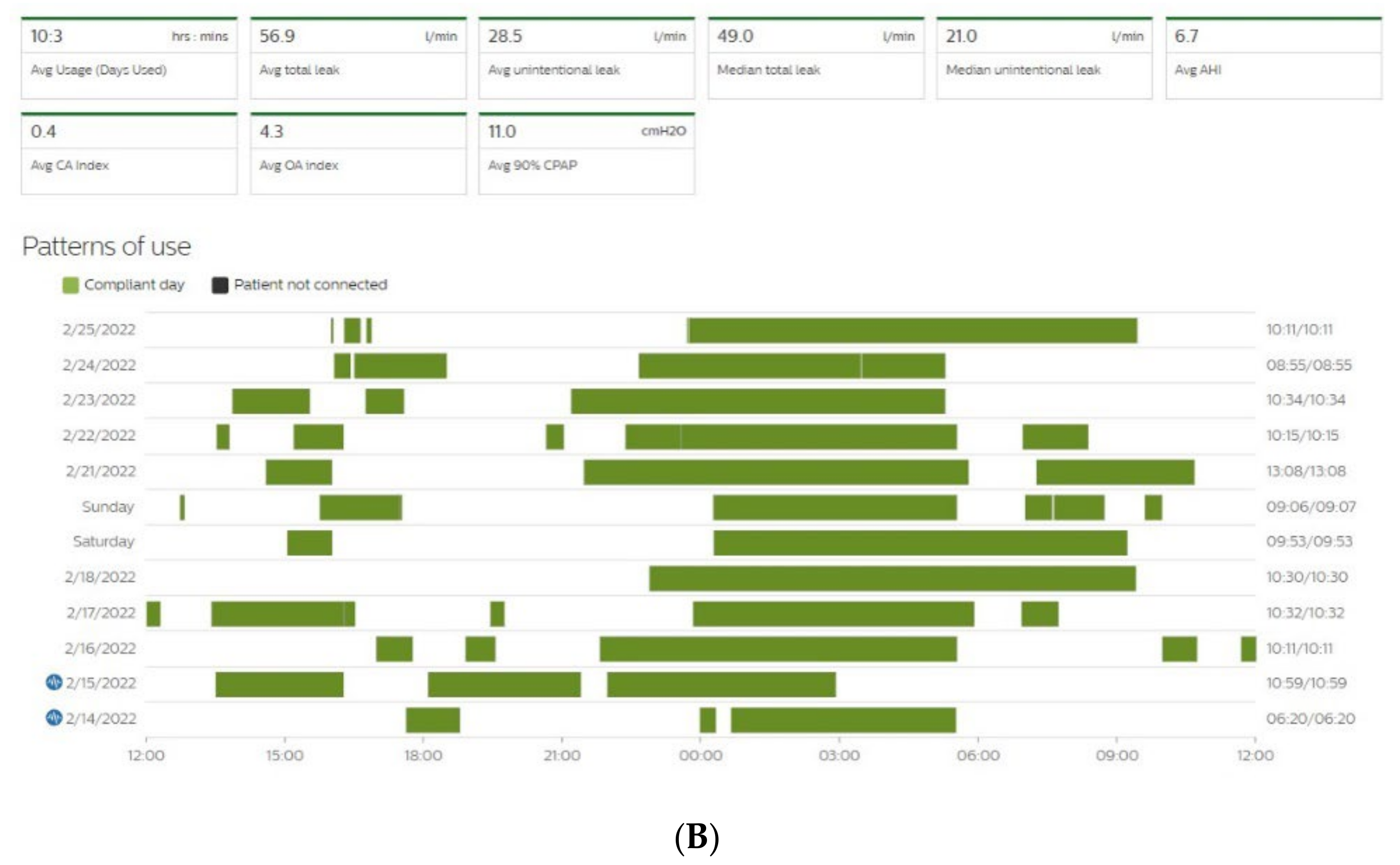
Figure 3. Sleep patterns of CPAP-treated patients suffering from maintenance insomnia. Frequent naps are also observed. CPAP data are collected from Resmed (A) and Philips (B) devices. AHI: apnea–hypopnea index, Avg: average, CA: central apnea, OA: obstructive apnea.
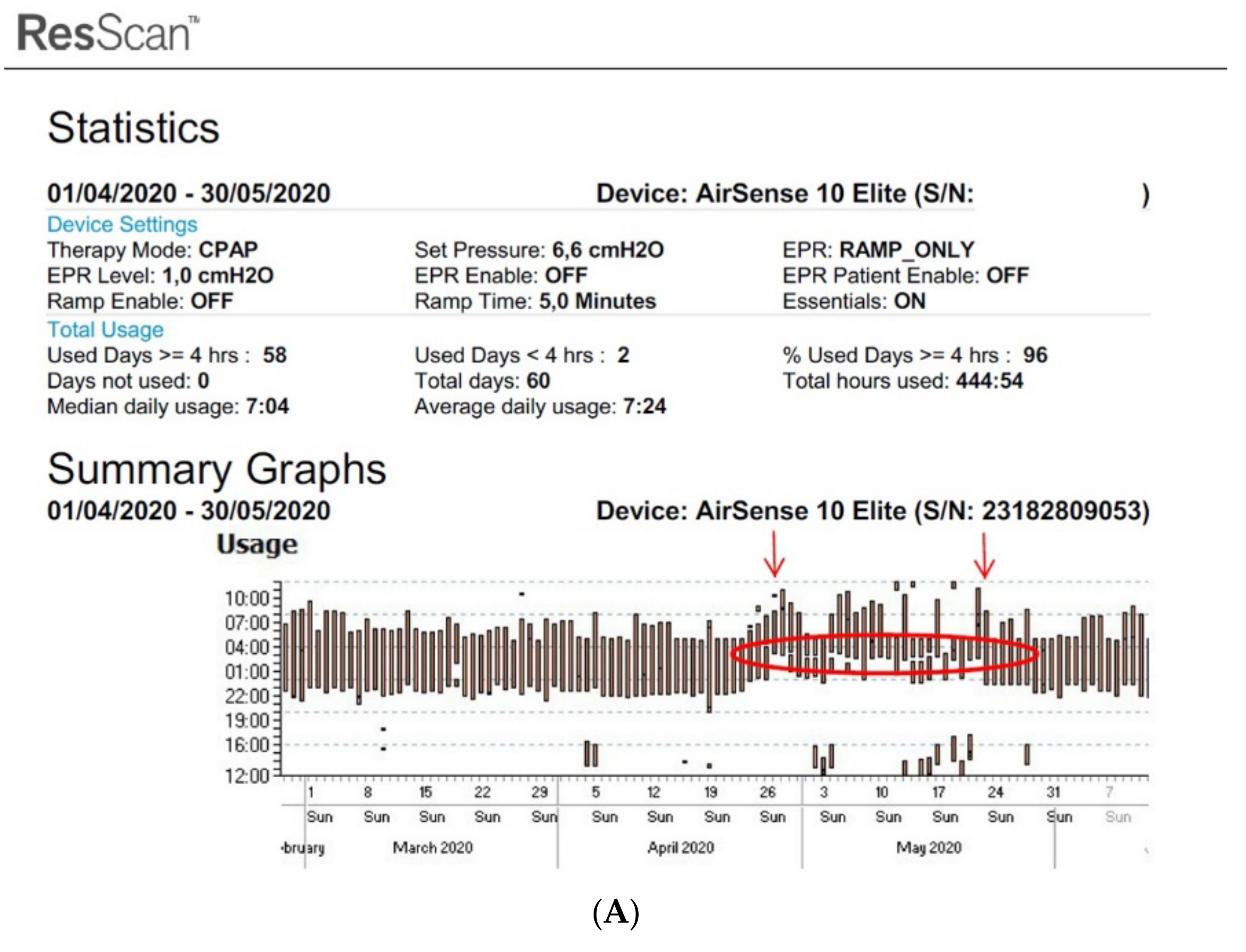
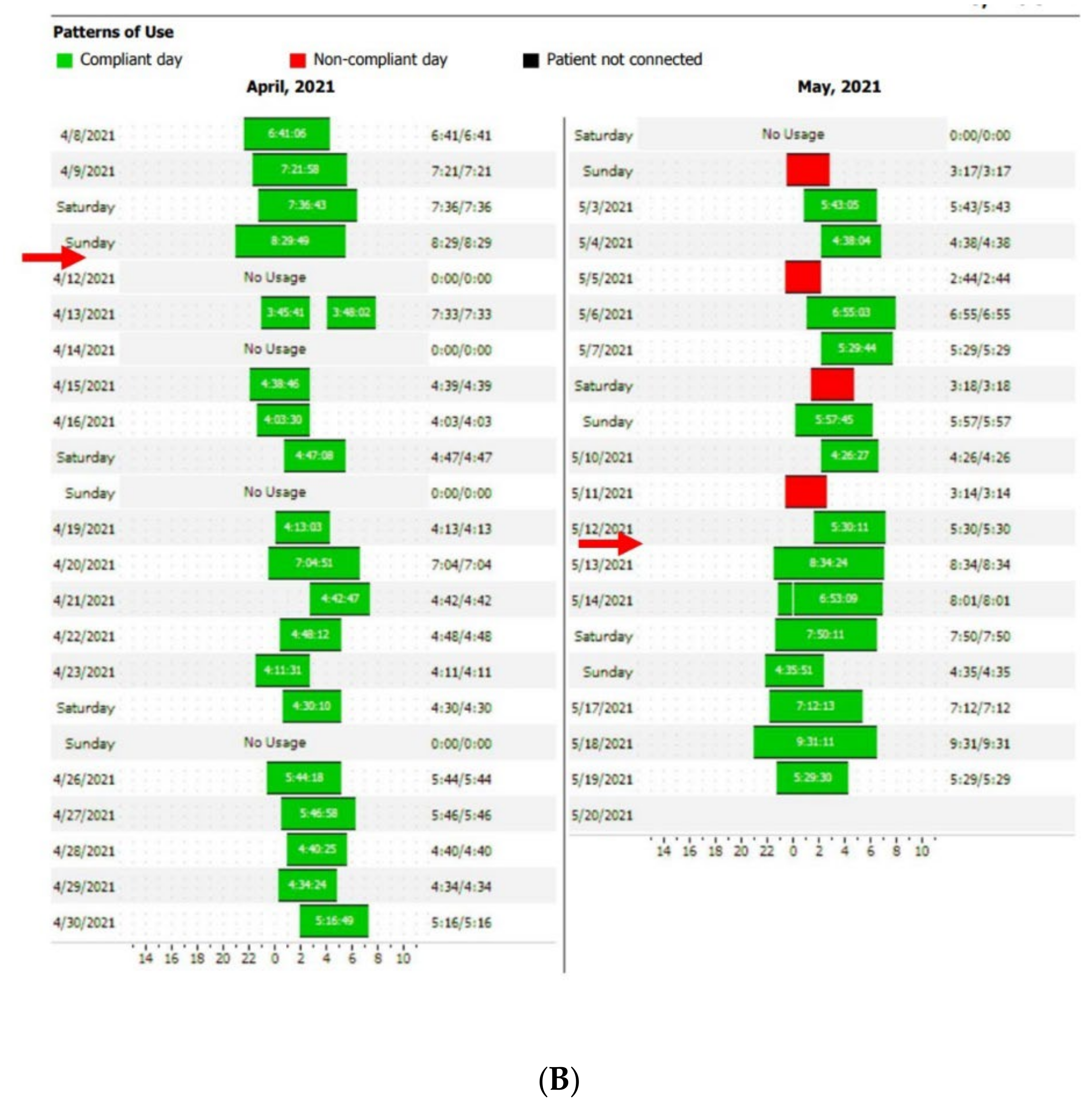 Figure 4. Disturbed sleep pattern of CPAP users during the Ramadan period (arrows). Volitional awakenings, to pray, are observed (A). CPAP data are collected from Resmed (A) and Philips (B) devices. EPR: expiratory pressure relief.
Figure 4. Disturbed sleep pattern of CPAP users during the Ramadan period (arrows). Volitional awakenings, to pray, are observed (A). CPAP data are collected from Resmed (A) and Philips (B) devices. EPR: expiratory pressure relief.At the current time, there is some variability in the definition of the different parameters mentioned above, as each manufacturer of CPAP uses different systems to measure and collect this data. However, it is essential to clearly understand the different definitions to optimize their interpretation and use.
Detection of central apnea–hypopnea (vs. an obstructive event) is based on a forced oscillation technique in the RESMED machine. The RESMED CPAP oscillates the flow when it falls; if oscillations dissipate, it means that the upper airways are open, reflecting a central event. The Philips system is based on a cardiogenic pulse artefact. A pressure pulse is given a few seconds into apnea and, if it is larger than the expected breath at the end of the apnea, it is considered to be obstructive.
The types of residual events provided by the different devices vary by whether or not the device records central hypopneas and inspiratory flow limitation [6].
Understanding the manner by which the presence of residual respiratory events is determined is essential for two reasons:
- Accurate interpretation of the CPAP data report.
- In cases where an auto-adjusting PAP (APAP) is used, the machine will react, by gradually increasing or decreasing the pressure, according to the type of respiratory event detected, and clinicians should be aware of the algorithm used by the device in order to be able to correctly interpret any inappropriate reactions of the APAP device during nighttime recordings.
The denominator used to determine AHI is recording time, in contrast to PSG, providing the real total sleep time. The device calculates the recording time as the length of time the device is turned on, associated with a measurable breathing signal [7].
CPAP devices also provide leak measurement. Leaks are measured in different ways according to different CPAP manufacturers (e.g., RESMED: unintentional leaks + mouth leaks, PHILIPS: intentional leaks subtracted from total flow), leading to different definitions of large leak threshold. Intentional leaks are mandatory for C02 removal and depend on mask type (<24 L/min for nasal mask and <36 L/min for naso-buccal mask for RESMED) and pressure level [5]. Leak compensation is foreseen by CPAP devices to maintain target pressure, and is variable from one device to another. Leaks vary from one breathing cycle to another and unexpected leaks can occur (mouth breathing, lack of seal of the mask). An inappropriate leakage will be compensated for by the device, based on mean expected flow in several breathing cycles, but can be less reliable in the case of large leaks, and lead to difficulties in maintaining target pressure [6][8].
2.2. Clinical Management of CPAP-Recorded Data
When a patient is telemonitored, CPAP data can be reviewed daily on a secured platform. In order to focus on challenging patients, manufacturers have created filters to select patients with low compliance (generally < 4 h/night), high leak levels (different thresholds are proposed), and increased residual AHI. According to these data, a joint statement of the Société Française de recherche et medicine du sommeil (SFRMS) and the Société de pneumologie de langue française (SPLF) has been recently published that proposes an algorithm to manage compliance, leaks, and residual AHI in telemonitored CPAP-treated OSA patients [9]. In cases of low compliance, a consultation is proposed. In cases of high leak levels, mask fitting should be reviewed, and buccal leaks should be identified. Finally, residual AHI > 10/h should be investigated. In cases involving obstructive events, inappropriate mask fitting, pressure, or nasal obstruction should be examined, and, for central events, a medical consultation is required. The recommendations suggest, depending on the variability of CPAP device algorithms, to manage only patients with an AHI flow > 10/h on 7 consecutive days. AHI flow is only considered if leaks are resolved and compliance is optimal.
Based on a compilation of seven studies, Verbraecken has also proposed an algorithm with a similar cut-off [10]. AHI ≥ 10 should be managed. In cases of obstructive events, attention is focused on the level of pressure, nasal obstruction, and mask type, and in cases involving central events, medical support is required [10]. The number of consecutive days over which the problem must persist is not described, but it is between 2–5 days in the majority of the studies on which this algorithm is based.
2.3. Impact of Telemonitoring on CPAP Therapy
2.3.1. Compliance—Adherence
CPAP use is highly dependent on the willingness of the patient to use the device and to apply the mask while sleeping [10]. In order to assess CPAP use, two terms should be distinguished: adherence refers to the real use of CPAP when this treatment has been prescribed (yes/no) while compliance refers to the daily usage expressed in hours (or minutes) per night in persistent users [11].
2.3.2. Patient-Reported Outcomes (PROs)
Assessment of the impact of CPAP telemonitoring on therapy is often focused on CPAP compliance rather than on other outcomes. However, with regard to EDS, TM interventions were associated with an improvement in the Epworth Sleepiness Score (ESS) in 7 out of 16 studies in the meta-analysis Labarca et al. [12]. The mean difference was low, 0.52 (95% CI 0.12–0.93), and did not reach the minimal clinically important difference (MCID) for ESS, which lies between 2 and 3 [13]. This can be explained by the poor performance of the ESS [2], or by the inclusion of non-sleepy patients in seven studies, who generally exhibited an improvement of only 1 point on the ESS with CPAP [14]. Indeed, ESS at baseline was not associated with adherence > 4 h/night. It should also be noted that, when comparing CPAP + TM versus CPAP alone, it is clear that, due to the limited increased in compliance obtained with TM, the subsequent difference in EDS is insignificant.
2.3.3. Cardiac Events: Interpretation of CPAP-Detected Central Sleep Apnea and Cheynes-Stokes Respiration
Particular attention should be given to patients with residual central events, as Prigent et al. have shown that the detection of incident Cheynes-Stokes respiration by CPAP telemonitoring is associated with a high prevalence of heart failure (31%) and arrhythmias (8%) based on a study of 555 patients followed during a one-year period [15]. This highlights another benefit of TMg, an increased ability to consider, detect, and manage cardiovascular comorbidities.
2.3.4. Integrated Care for OSA and Associated Comorbidities, including Telemonitored CPAP
OSA patients, mainly males, exhibit a high prevalence of comorbidities, with half of them suffering from cardiovascular disorders and about 20–30% from diabetes and dyslipidemia [16]. Comorbidities can either be a causal factor of OSA (e.g., diabetes, heart failure) or a consequence (e.g., stroke, hypertension). CPAP is one part of the overall treatment in these patients, that aims to reduce/control cardiometabolic risk. Integrated strategies using telehealth have been studied in OSA. For example, Pépin et al. studied 306 patients suffering from both OSA and high cardiovascular risk [17]. Patients were randomized to CPAP or to multimodal TMg. In the TMg arm, self-measured morning and evening blood pressure (BP) and physical activity were measured by connected devices. The blood pressure monitor and the actigraph (collecting number of steps/day and sleep periods) were connected to a secure website, accessible for physicians and home care providers. CPAP adherence, leaks, and residual events were collected via TMg. Symptoms and quality of life were assessed using a questionnaire-based application. Multimodal TMg provided predefined interventions to home care providers (managing leaks, mask problems, or other side effects) while the medical team managed residual events or CPAP lack of efficacy. After 6 months, no differences in BP reduction or in physical activity were observed, but CPAP compliance (5.28 ± 2.23 vs. 4.75 ± 2.5 h/night), sleepiness, and QoL were significantly improved in the multimodal TMg arm [17]. Further studies need to define the best way to provide a holistic approach to these patients, including TM tools.
2.4. Telemonitored CPAP Therapy: Patient Perspectives
From the patient’s perspective, TMg is often perceived as a mean of reassurance [18]. In several RCTs, satisfaction rates were better in the TM arm using different tools (e.g., feedback by phone, TMg, televisits) compared to the UC group [19][20] or equivalent [21]. However, it is important to fulfill some prerequisites when starting TMg in patients. First of all, patient education about TM methods is important to assure that patients understand the method and are suitable candidates for TM. TM interventions have the theoretical advantage of allowing elderly people or patients living far away to avoid visits to the hospital; however, in real-life settings, the use of TM can be limited by technological barriers/fear, especially if it requires patient intervention to connect to healthcare providers (e.g., for videoconsultation). Costs billed to the patient can also be a source of worry. When restricted to remote TMg for CPAP-treated OSA patients, no special manipulation is required to allow data monitoring, such that there are virtually no limitations.
Considering concerns about privacy protection is also essential. Indeed, despite expressing satisfaction and agreement with the usefulness of online information, some patients have reported concerns about privacy, judging TMg to be intrusive [22]. Bros et al. reported the same concerns, with a majority of patients finding TMg useful but 40% considering TMg to be intrusive [22]. However, in a recent study from Carlier et al. related to interventions performed in telemonitored CPAP patients during the first 6 months of treatment, acceptance (obtained via informed consent) was as high as 87% [23]. Similar data (acceptance of 78%) was also reported in another study [22]. In the latter study, men had fewer concerns about TMg than women, and non-working people were more favorable toward TMg than active people [22].
In addition, acceptance of TMg seems to be an additional predictive factor of adherence. Bros et al. showed that patients refusing TMg were more at risk of CPAP withdrawal [22]. CPAP discontinuation was also more frequent in cases of TMg interruption.
Finally, TM has entered the daily life of many family households through the growing use of connected monitoring tools (e.g., apps, watches, accelerometers) that collect physiological parameters such as blood pressure, sleep measurements, physical activity, and weight. In the future, all of these parameters, including those concerning CPAP treatment, could be integrated into a more comprehensive health monitoring strategy [10]. However, healthcare professionals should be careful to avoid dehumanization of care through TM. Increased use of technology could limit patient engagement if no attention is dedicated to human relationships. The first wave of the COVID pandemic was a good example of this drift, which was experienced as very negative for COVID patients but also for a large part of the general population who experienced limited access to general practitioners, increased use of teleconsultations, and delays in obtaining medical care. Other concerns have also been described in the context of telehealth in COPD patients: it can create high dependency for patients, increase the frequency of nurse–patient interactions, and finally be counterproductive, leading to overtreatment and overmedicalization of patients [24].
2.5. Telemonitored CPAP Therapy: Healthcare Professional Perspectives
Many advantages of the use of TM by medical and paramedical providers are expected or have already been demonstrated. Considering compliance problems and the relationship of these problems to the initial days of use, TMg is a good strategy for identifying patients struggling with their therapy, and setting up intensive early interventions for selected patients. Hoet et al. demonstrated that TMg reduces delay to first technical intervention in CPAP-treated patients, a factor that was associated with improved compliance at 3 months [14]. In this way, TMg also allows practitioners to avoid losing time in unnecessary nurse visits or medical follow-up with patients who do not need it [25][26]. TMg could, therefore, help to forge a compromise between increasing needs in sleep disorder diagnosis and support, and limited health care resources. For example, Anttalainen et al. demonstrated, in 111 patients, a savings of 19 min in nursing time between TMg patients (39 min, range 12–132) and the UC group (58 min, range 40–180) [25]. Reduced staff time was also documented by Munafo et al., who showed that the number of contacts per patient was reduced in the telehealth group (TMg) vs. UC: 2.2 ± 2.6/patient vs. 7.8 ± 4.1/patient [26]. Time spent per patient by the staff was also significantly reduced, even in non-compliant patients. Finally, TMg also allows remote monitoring in patients living in medical desert areas, increasing healthcare accessibility [27].
2.6. Telemonitored CPAP Therapy: Real Costs—Cost-Effectiveness
Few studies have focused on the cost-effectiveness of TMg. Turino et al. have analyzed the treatment and follow-up costs (direct and indirect) in a cohort of 100 TMg-followed CPAP patients [20]. Clinical outcomes were similar in both groups. Three-month costs were lower in the TMg arm vs. UC: 124 Euro versus 171 Euro. In a previous study, Isetta et al. calculated the cost-effectiveness of a TM program versus UC in 139 newly-diagnosed OSA patients [21]. At 6 months, clinical outcomes were similar in both groups and lower costs were billed in the TM arm. When considering medical visits, time to travel to the hospital, and time out of work, the mean cost was 168 Euro in the TM arm and 180 Euro in the control arm, despite the higher number of extra visits to nurses and physicians in the TM arm. The impact of TMg on cost effectiveness seems to be positive but it is not yet clear due to the paucity of the current data [27].
The direct cost of TMg is still very variable from one country to another and from one hospital to another, according to provider policy and to local healthcare reimbursement policies (authorities still lack reimbursement of such tools in many countries). What should really be included in the provider’s billing remains unclear at this time: the access to the cloud? access to the stored data? price per access or all-inclusive price? software maintenance of the cloud or platform? guarantee that the system will be supported for several years?
More than TMg billing, discussion of the generalization of a fee for healthcare professionals managing data and alerts of telemonitored patients should also be started, as this represents additional work for sleep professionals, and is not yet covered by healthcare insurance.
References
- Patel, S.R. Obstructive Sleep Apnea. Ann. Intern. Med. 2019, 171, ITC81–ITC96.
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066.
- Voulgaris, A.; Ferini-Strambi, L.; Steiropoulos, P. Sleep medicine and COVID-19. Has a new era begun? Sleep Med. 2020, 73, 170–176.
- World Health Organization. Telemedicine: Opportunities and Developments in Member States. Report on the Second Global Survey on eHealth. Geneva Switzerland WHO 2010. Available online: https://www.who.int/goe/publications/goe_telemedicine_2010.pdf (accessed on 4 March 2022).
- Schwab, R.J.; Badr, S.M.; Epstein, L.J.; Gay, P.C.; Gozal, D.; Kohler, M.; Lévy, P.; Malhotra, A.; Phillips, B.A.; Rosen, I.M.; et al. An official American Thoracic Society statement: Continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am. J. Respir. Crit. Care Med. 2013, 188, 613–620.
- Johnson, K.G.; Johnson, D.C. Treatment of sleep-disordered breathing with positive airway pressure devices: Technology update. Med. Devices 2015, 8, 425–437.
- Bertelli, F.; Suehs, C.M.; Mallet, J.P.; Rotty, M.C.; Pépin, J.L.; Gagnadoux, F.; Matzner-Lober, E.; Bourdin, A.; Molinari, N.; Jaffuel, D. Apnoea-hypopnoea indices determined via continuous positive airway pressure (AHI-CPAPflow) versus those determined by polysomnography (AHI-PSGgold): A protocol for a systematic review and meta-analysis. BMJ Open 2021, 11, e044499.
- Borel, J.C.; Sabil, A.; Janssens, J.P.; Couteau, M.; Boulon, L.; Lévy, P.; Pépin, J.L. Intentional leaks in industrial masks have a significant impact on efficacy of bilevel noninvasive ventilation: A bench test study. Chest 2009, 135, 669–677.
- Prigent, A.; Gentina, T.; Launois, S.; Meurice, J.C.; Pia d’Ortho, M.; Philippe, C.; Tamisier, R.; Gagnadoux, F.; Jaffuel, D. Groupe de travail de la Société française de recherche et médecine du sommeil et la Société de pneumologie de langue française. . Rev. Mal. Respir. 2020, 37, 550–560. (In French)
- Verbraecken, J. Telemedicine in Sleep-Disordered Breathing: Expanding the Horizons. Sleep Med. Clin. 2021, 16, 417–445.
- Collard, P.; Pieters, T.; Aubert, G.; Delguste, P.; Rodenstein, D.O. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med. Rev. 1997, 1, 33–44.
- Labarca, G.; Schmidt, A.; Dreyse, J.; Jorquera, J.; Barbe, F. Telemedicine interventions for CPAP adherence in obstructive sleep apnea patients: Systematic review and meta-analysis. Sleep Med. Rev. 2021, 60, 101543.
- Patel, S.; Kon, S.S.C.; Nolan, C.M.; Barker, R.E.; Simonds, A.K.; Morrell, M.J.; Man, W.D. The Epworth Sleepiness Scale: Minimum Clinically Important Difference in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 961–963.
- Hoet, F.; Libert, W.; Sanida, C.; Van den Broecke, S.; Bruyneel, A.V.; Bruyneel, M. Telemonitoring in continuous positive airway pressure-treated patients improves delay to first intervention and early compliance: A randomized trial. Sleep Med. 2017, 39, 77–83.
- Prigent, A.; Pellen, C.; Texereau, J.; Bailly, S.; Coquerel, N.; Gervais, R.; Liegaux, J.M.; Luraine, R.; Renaud, J.C.; Serandour, A.L.; et al. CPAP telemonitoring can track Cheyne-Stokes respiration and detect serious cardiac events: The AlertApnée Study. Respirology 2022, 27, 161–169.
- André, S.; Andreozzi, F.; Van Overstraeten, C.; Ben Youssef, S.; Bold, I.; Carlier, S.; Gruwez, A.; Bruyneel, A.V.; Bruyneel, M. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir. Res. 2020, 21, 35.
- Pépin, J.L.; Jullian-Desayes, I.; Sapène, M.; Treptow, E.; Joyeux-Faure, M.; Benmerad, M.; Bailly, S.; Grillet, Y.; Stach, B.; Richard, P.; et al. Multimodal Remote Monitoring of High Cardiovascular Risk Patients With OSA Initiating CPAP: A Randomized Trial. Chest 2019, 155, 730–739.
- Pépin, J.L.; Tamisier, R.; Hwang, D.; Mereddy, S.; Parthasarathy, S. Does remote monitoring change OSA management and CPAP adherence? Respirology 2017, 22, 1508–1517.
- Frasnelli, M.; Baty, F.; Niedermann, J.; Brutsche, M.H.; Schoch, O.D. Effect of telemetric monitoring in the first 30 days of continuous positive airway pressure adaptation for obstructive sleep apnoea syndrome—A controlled pilot study. J. Telemed. Telecare 2016, 22, 209–214.
- Turino, C.; de Batlle, J.; Woehrle, H.; Mayoral, A.; Castro-Grattoni, A.L.; Gómez, S.; Dalmases, M.; Sánchez-de-la-Torre, M.; Barbé, F. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur. Respir. J. 2017, 49, 1601128.
- Isetta, V.; Negrín, M.A.; Monasterio, C.; Masa, J.F.; Feu, N.; Álvarez, A.; Campos-Rodriguez, F.; Ruiz, C.; Abad, J.; Vázquez-Polo, F.J.; et al. A Bayesian cost-effectiveness analysis of a telemedicine-based strategy for the management of sleep apnoea: A multicentre randomised controlled trial. Thorax 2015, 70, 1054–1061.
- Bros, J.S.; Poulet, C.; Arnol, N.; Deschaux, C.; Gandit, M.; Charavel, M. Acceptance of Telemonitoring Among Patients with Obstructive Sleep Apnea Syndrome: How is the Perceived Interest by and for Patients? Telemed. J. E Health 2018, 24, 351–359.
- Carlier, S.; Bruyneel, A.V.; Bruyneel, M. Pressure adjustment is the most useful intervention for improving compliance in telemonitored patients treated with CPAP in the first 6 months of treatment. Sleep Breath 2022, 26, 125–132.
- Brunton, L.; Bower, P.; Sanders, C. The Contradictions of Telehealth User Experience in Chronic Obstructive Pulmonary Disease (COPD): A Qualitative Meta-Synthesis. PLoS ONE 2015, 10, e0139561.
- Anttalainen, U.; Melkko, S.; Hakko, S.; Laitinen, T.; Saaresranta, T. Telemonitoring of CPAP therapy may save nursing time. Sleep Breath 2016, 20, 1209–1215.
- Munafo, D.; Hevener, W.; Crocker, M.; Willes, L.; Sridasome, S.; Mushin, M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacity when compared to standard of care. Sleep Breath 2016, 20, 777–785.
- Bakker, J.P.; Weaver, T.E.; Parthasarathy, S.; Aloia, M.S. Adherence to CPAP: What Should We Be Aiming For, and How Can We Get There? Chest 2019, 155, 1272–1287.
More
Information
Subjects:
Respiratory System
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Remote Sensing Data Fusion
Revisions:
2 times
(View History)
Update Date:
25 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




