
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arif Ashraf | -- | 2593 | 2022-04-22 16:32:12 | | | |
| 2 | Peter Tang | -1 word(s) | 2592 | 2022-04-24 03:45:51 | | |
Video Upload Options
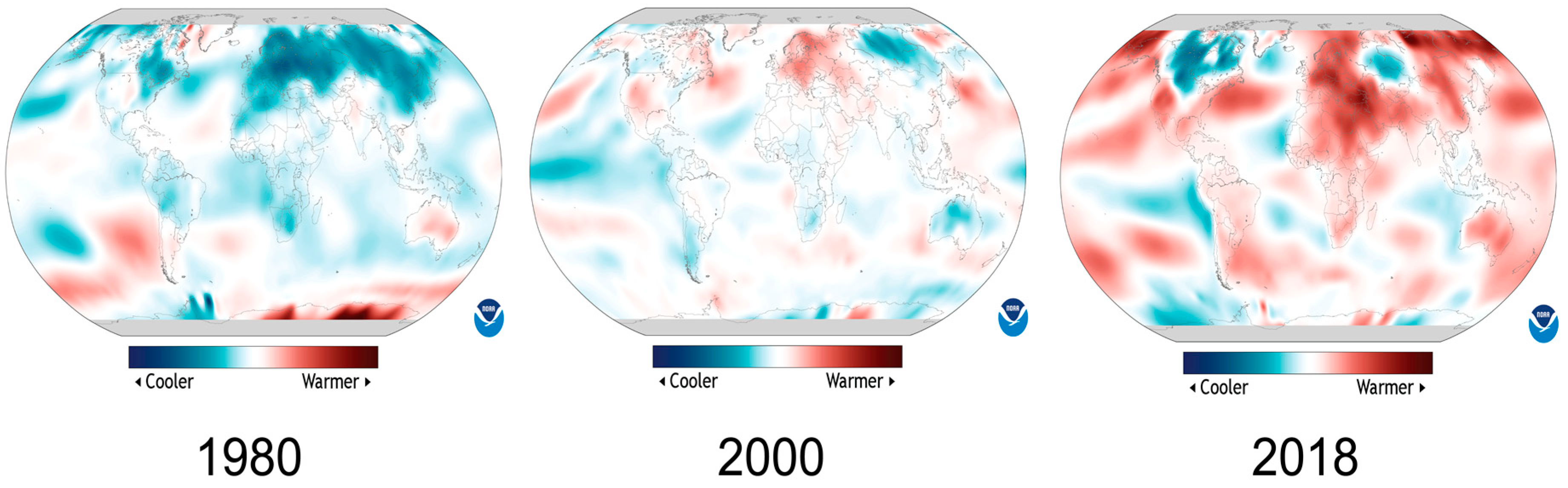
Over the years, although substantial progress has been made in understanding low-temperature response mechanisms in plants, the research is more focused on aerial parts of the plants rather than on the root or whole plant, and more efforts have been made in identifying and testing the major regulators of this pathway preferably in the model organism rather than in crop plants. For the low-temperature stress response mechanism, ICE-CBF regulatory pathway turned out to be the solely established pathway, and historically most of the low-temperature research is focused on this single pathway instead of exploring other alternative regulators.
1. Climate Change and Plant Growth
2. Major Low Temperature-Responsive Pathway
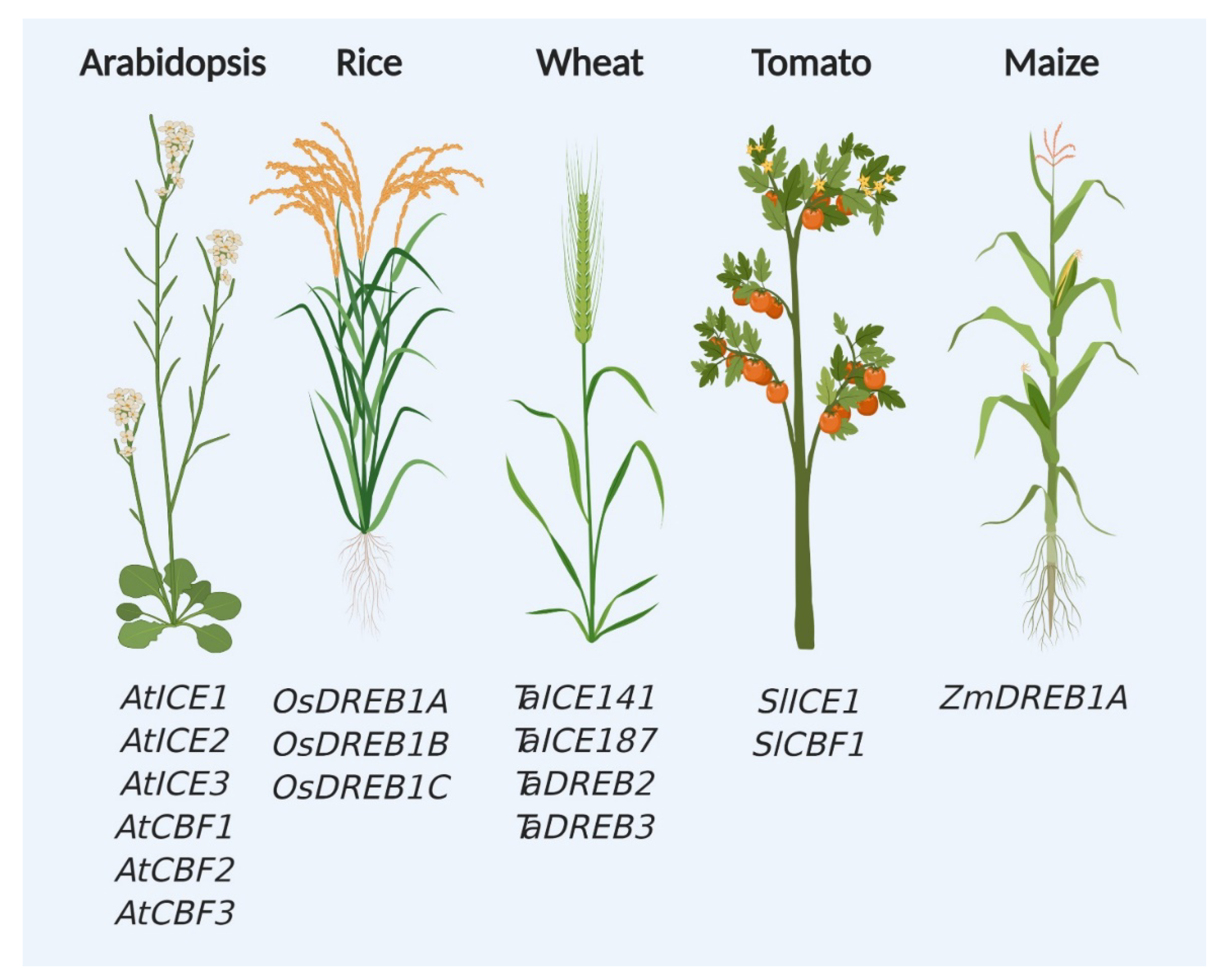
3. CBF-Dependent Pathway in Crop Plant Engineering
|
Gene |
Source |
Host |
Reference |
|---|---|---|---|
|
AtICE1 |
Arabidopsis thaliana |
Arabidopsis thaliana |
[32] |
|
AtICE2 |
Arabidopsis thaliana |
Arabidopsis thaliana |
[34] |
|
AtICE3 |
Arabidopsis thaliana |
Cucumis sativus |
[35] |
|
AtDREB1B/ AtCBF1 |
Arabidopsis thaliana |
Arabidopsis thaliana, Brassica napus, Fragaria ananassa, Populus tremula x alba |
|
|
AtDREB1C/ AtCBF2 |
Arabidopsis thaliana |
Arabidopsis thaliana, Brassica napus |
|
|
AtDREB1A/ AtCBF3 |
Arabidopsis thaliana |
Arabidopsis thaliana, Brassica napus, Solanum tuberosum, Triticum aestivum, Nicotiana tabacum, Manihot esculenta |
|
|
SlICE1 |
Solanum lycopersicum |
Solanum lycopersicum |
|
|
SlCBF1 |
Solanum lycopersicum |
Arabidopsis thaliana |
|
|
OsDREB1A |
Oryza sativa |
Oryza sativa |
[50] |
|
OsDREB1A |
Oryza sativa |
Arabidopsis thaliana |
[51] |
|
OsDREB1B |
Oryza sativa |
Oryza sativa |
[50] |
|
OsDREB1B |
Oryza sativa |
Nicotiana plumbaginifolia |
[52] |
|
OsDREB1C |
Oryza sativa |
Oryza sativa |
[50] |
|
TaICE141 |
Triticum aestivum |
Arabidopsis thaliana |
[53] |
|
TaICE187 |
Triticum aestivum |
Arabidopsis thaliana |
[53] |
|
TaDREB2 |
Triticum aestivum |
Triticum aestivum |
[54] |
|
TaDREB3 |
Triticum aestivum |
Triticum aestivum |
[54] |
|
HvCBF3 |
Hordeum vulgare |
Arabidopsis thaliana |
[55] |
|
HvCBF4 |
Hordeum vulgare |
Oryza sativa |
[56] |
|
ZmDREB1A |
Zea mays |
Arabidopsis thaliana |
[57] |
|
GmDREB3 |
Glycine max |
Arabidopsis thaliana |
[58] |
|
VrCBF1 |
Vitis riparia |
Arabidopsis thaliana |
[59] |
|
VrCBF4 |
Vitis riparia |
Arabidopsis thaliana |
[59] |
|
LpCBF3 |
Lolium perenne |
Arabidopsis thaliana |
|
|
MbDREB1 |
Malus baccata |
Arabidopsis thaliana |
[62] |
4. CBF-Independent Cold-Responsive Pathway
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448.
- Lancaster, L.T.; Humphreys, A.M. Global variation in the thermal tolerances of plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13580–13587.
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19.
- Prasad, P.V.V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441.
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183.
- Raju, S.K.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86.
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180.
- Ashraf, M.A.; Biswas, S.; Razzaque, S.; Haque, T.; Seraj, Z.I. Cloning and characterization of alcohol dehydrogenase (Adh) promoter region for expression under submergence and salinity stress. Plant Tissue Cult. Biotechnol. 2014, 24, 111–120.
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756.
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451.
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599.
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637.
- Zhao, C.; Lang, Z.; Zhu, J.-K. Cold responsive gene transcription becomes more complex. Trends Plant Sci. 2015, 20, 466–468.
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022.
- Lee, H.-J.; Seo, P.J. Ca2+ talyzing initial responses to environmental stresses. Trends Plant Sci. 2021, 26, 849–870.
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.; Wang, J.; Xie, H.; Wu, X.; Haq, S.I.U.; Qiu, Q.-S. Small signaling molecules in plant response to cold stress. J. Plant Physiol. 2021, 266, 153534.
- Verma, N.; Giri, S.K.; Singh, G.; Gill, R.; Kumar, A. Epigenetic regulation of heat and cold stress responses in crop plants. Plant Gene 2022, 29, 100351.
- Thomashow, M.F.; Torii, K.U. SCREAMing Twist on the Role of ICE1 in Freezing Tolerance. Plant Cell 2020, 32, 816–819.
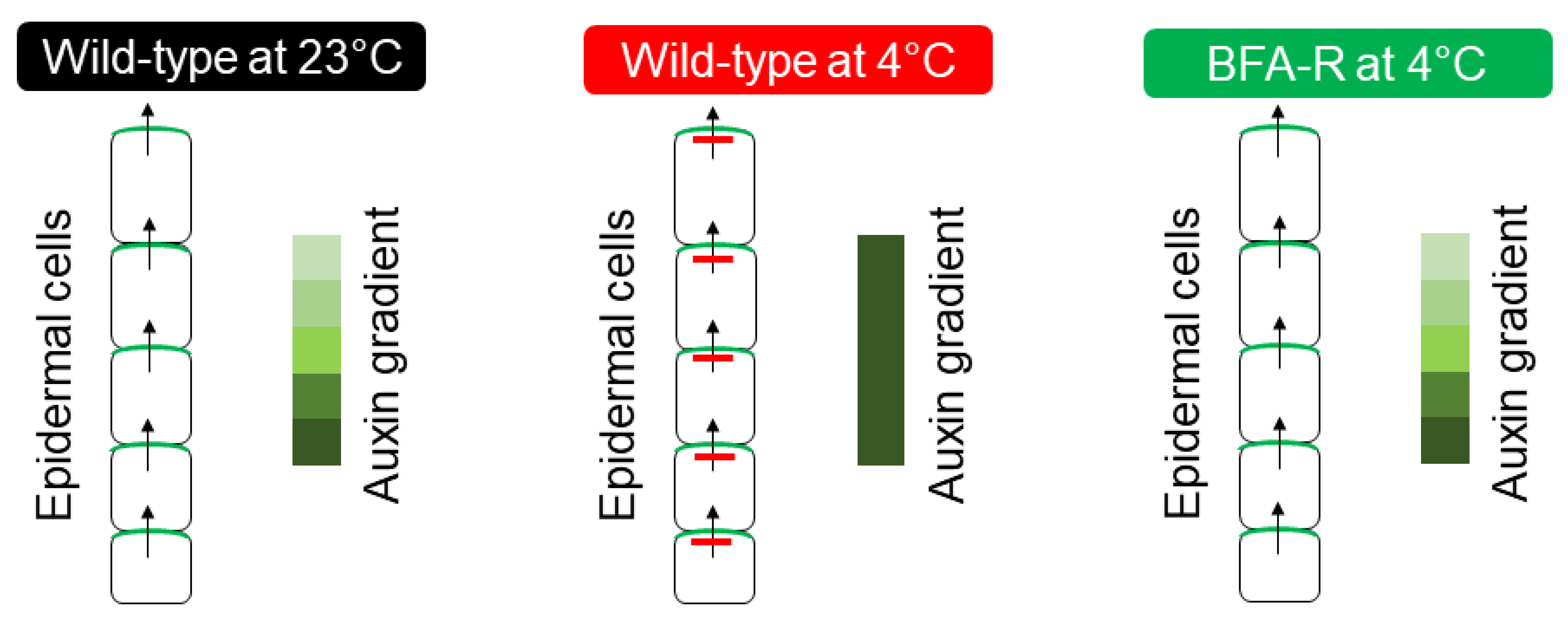
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin Response in Arabidopsis under Cold Stress: Underlying Molecular Mechanisms. Plant Cell 2009, 21, 3823–3838.
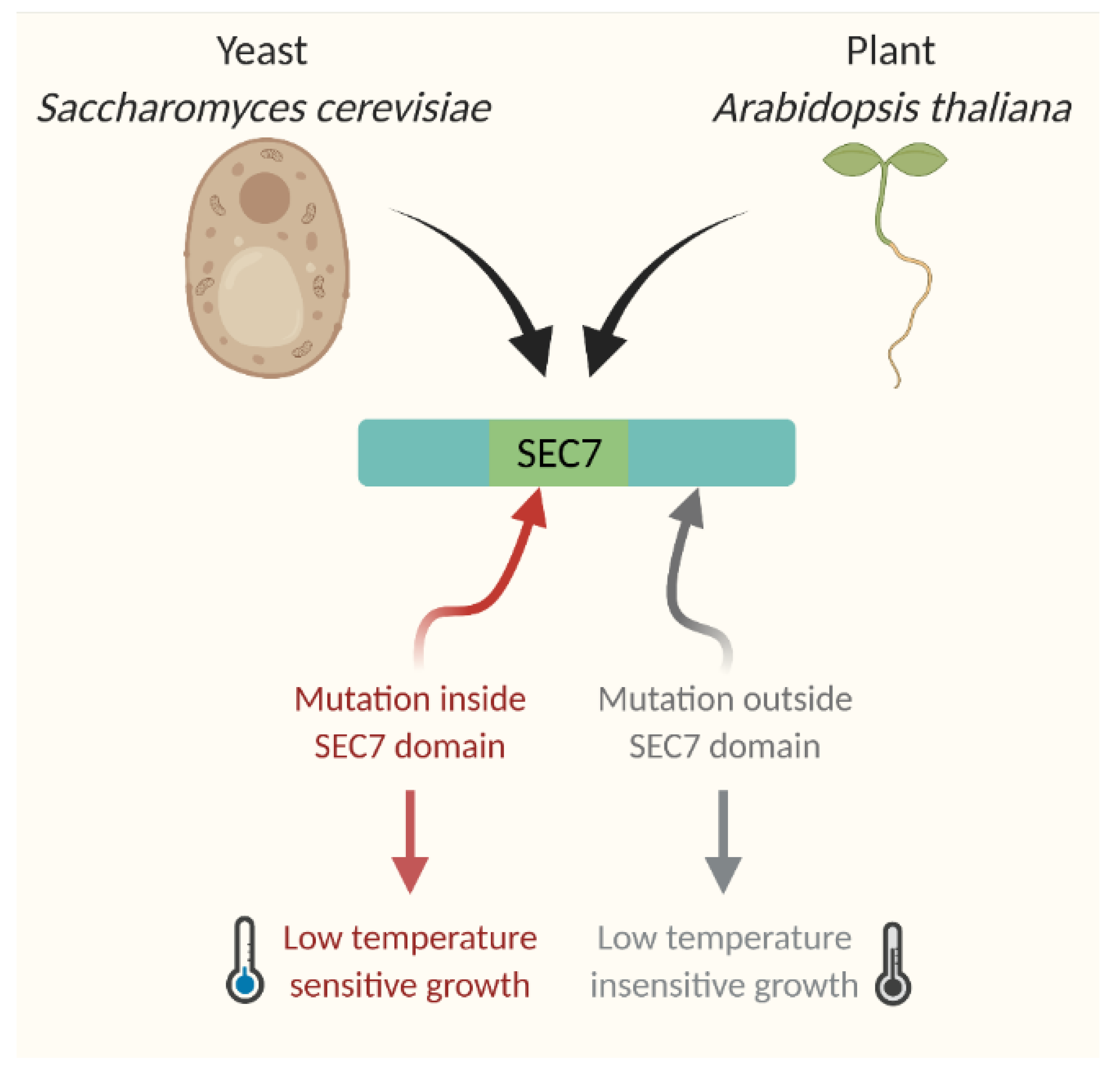
- Ashraf, M.A.; Rahman, A. Cold stress response in Arabidopsis thaliana is mediated by GNOM ARF-GEF. Plant J. 2019, 97, 500–516.
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221.
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Ramakanth, K.K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell 2017, 170, 102–113.
- Wang, X.; Li, W.; Li, M.; Welti, R. Profiling lipid changes in plant response to low temperatures. Physiol. Plant. 2006, 126, 90–96.
- Hong-Bo, S.; Li-Ye, C.; Ming-An, S.; Shi-Qing, L.; Ji-Cheng, Y. Bioengineering plant resistance to abiotic stresses by the global calcium signal system. Biotechnol. Adv. 2008, 26, 503–510.
- Jeon, J.; Kim, N.Y.; Kim, S.; Kang, N.Y.; Novák, O.; Ku, S.-J.; Cho, C.; Lee, D.J.; Lee, E.-J.; Strnad, M. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 2010, 285, 23371–23386.
- Jeon, J.; Kim, J. Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiol. 2013, 161, 408–424.
- Baker, S.S.; Wilhelm, K.S.; Thomashow, M.F. The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought-and ABA-regulated gene expression. Plant Mol. Biol. 1994, 24, 701–713.
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264.
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353.
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759.
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984.
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.; Hong, X.; Agarwal, M.; Zhu, J.-K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054.
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.-K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785.
- Fursova, O.V.; Pogorelko, G.V.; Tarasov, V.A. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene 2009, 429, 98–103.
- Liu, L.; Duan, L.; Zhang, J.; Zhang, Z.; Mi, G.; Ren, H. Cucumber (Cucumis sativus L.) over-expressing cold-induced transcriptome regulator ICE1 exhibits changed morphological characters and enhances chilling tolerance. Sci. Hortic. 2010, 124, 29–33.
- Gilmour, S.J.; Sebolt, A.M.; Salazar, M.P.; Everard, J.D.; Thomashow, M.F. Overexpression of the Arabidopsis CBF3transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000, 124, 1854–1865.
- Jaglo, K.R.; Kleff, S.; Amundsen, K.L.; Zhang, X.; Haake, V.; Zhang, J.Z.; Deits, T.; Thomashow, M.F. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved inbrassica napus and other plant species. Plant Physiol. 2001, 127, 910–917.
- Owens, C.L.; Thomashow, M.F.; Hancock, J.F.; Iezzoni, A.F. CBF1 orthologs in sour cherry and strawberry and the heterologous expression of CBF1 in strawberry. J. Am. Soc. Hortic. Sci. 2002, 127, 489–494.
- Benedict, C.; Skinner, J.S.; Meng, R.; Chang, Y.; Bhalerao, R.; Huner, N.P.A.; Finn, C.E.; Chen, T.H.H.; Hurry, V. The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ. 2006, 29, 1259–1272.
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106.
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406.
- Behnam, B.; Kikuchi, A.; Celebi-Toprak, F.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Watanabe, K.N. Arabidopsis rd29A: DREB1A enhances freezing tolerance in transgenic potato. Plant Cell Rep. 2007, 26, 1275–1282.
- Pellegrineschi, A.; Reynolds, M.; Pacheco, M.; Brito, R.M.; Almeraya, R.; Yamaguchi-Shinozaki, K.; Hoisington, D. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 2004, 47, 493–500.
- Kasuga, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought-and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol. 2004, 45, 346–350.
- An, D.; Ma, Q.; Yan, W.; Zhou, W.; Liu, G.; Zhang, P. Divergent regulation of CBF regulon on cold tolerance and plant phenotype in cassava overexpressing Arabidopsis CBF3 gene. Front. Plant Sci. 2016, 7, 1866.
- Miura, K.; Shiba, H.; Ohta, M.; Kang, S.W.; Sato, A.; Yuasa, T.; Iwaya-Inoue, M.; Kamada, H.; Ezura, H. SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum. Plant Biotechnol. 2012, 29, 253–260.
- Miura, K.; Sato, A.; Shiba, H.; Kang, S.W.; Kamada, H.; Ezura, H. Accumulation of antioxidants and antioxidant activity in tomato, Solanum lycopersicum, are enhanced by the transcription factor SlICE1. Plant Biotechnol. 2012, 29, 261–269.
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919.
- Hsieh, T.-H.; Lee, J.-T.; Yang, P.-T.; Chiu, L.-H.; Charng, Y.; Wang, Y.-C.; Chan, M.-T. Heterology expression of the ArabidopsisC-repeat/dehydration response element binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002, 129, 1086–1094.
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153.
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 2003, 33, 751–763.
- Gutha, L.R.; Reddy, A.R. Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol. Biol. 2008, 68, 533.
- Badawi, M.; Reddy, Y.V.; Agharbaoui, Z.; Tominaga, Y.; Danyluk, J.; Sarhan, F.; Houde, M. Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol. 2008, 49, 1237–1249.
- Morran, S.; Eini, O.; Pyvovarenko, T.; Parent, B.; Singh, R.; Ismagul, A.; Eliby, S.; Shirley, N.; Langridge, P.; Lopato, S. Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol. J. 2011, 9, 230–249.
- Skinner, J.S.; von Zitzewitz, J.; Szűcs, P.; Marquez-Cedillo, L.; Filichkin, T.; Amundsen, K.; Stockinger, E.J.; Thomashow, M.F.; Chen, T.H.H.; Hayes, P.M. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Mol. Biol. 2005, 59, 533–551.
- Oh, S.; Kwon, C.; Choi, D.; Song, S.I.; Kim, J. Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol. J. 2007, 5, 646–656.
- Qin, F.; Sakuma, Y.; Li, J.; Liu, Q.; Li, Y.-Q.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004, 45, 1042–1052.
- Chen, M.; Xu, Z.; Xia, L.; Li, L.; Cheng, X.; Dong, J.; Wang, Q.; Ma, Y. Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.). J. Exp. Bot. 2009, 60, 121–135.
- Siddiqua, M.; Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011, 34, 1345–1359.
- Xiong, Y.; Fei, S.-Z. Functional and phylogenetic analysis of a DREB/CBF-like gene in perennial ryegrass (Lolium perenne L.). Planta 2006, 224, 878–888.
- Zhao, H.; Bughrara, S.S. Isolation and characterization of cold-regulated transcriptional activator LpCBF3 gene from perennial ryegrass (Lolium perenne L.). Mol. Genet. Genom. 2008, 279, 585–594.
- Yang, W.; Liu, X.-D.; Chi, X.-J.; Wu, C.-A.; Li, Y.-Z.; Song, L.-L.; Liu, X.-M.; Wang, Y.-F.; Wang, F.-W.; Zhang, C. Dwarf apple MbDREB1 enhances plant tolerance to low temperature, drought, and salt stress via both ABA-dependent and ABA-independent pathways. Planta 2011, 233, 219–229.
- Fukaki, H.; Fujisawa, H.; Tasaka, M. Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol. 1996, 110, 933–943.
- Wyatt, S.E.; Rashotte, A.M.; Shipp, M.J.; Robertson, D.; Muday, G.K. Mutations in the gravity persistence signal loci in Arabidopsis disrupt the perception and/or signal transduction of gravitropic stimuli. Plant Physiol. 2002, 130, 1426–1435.
- Nadella, V.; Shipp, M.J.; Muday, G.K.; Wyatt, S.E. Evidence for altered polar and lateral auxin transport in the gravity persistent signal (gps) mutants of Arabidopsis. Plant Cell Environ. 2006, 29, 682–690.
- Geldner, N.; Friml, J.; Stierhof, Y.; Jürgens, G.; Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413, 425–428.
- Geldner, N.; Anders, N.; Wolters, H.; Keicher, J.; Kornberger, W.; Muller, P.; Delbarre, A.; Ueda, T.; Nakano, A.; Jürgens, G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003, 112, 219–230.
- Moriwaki, T.; Miyazawa, Y.; Fujii, N.; Takahashi, H. GNOM regulates root hydrotropism and phototropism independently of PIN-mediated auxin transport. Plant Sci. 2014, 215, 141–149.
- Steinmann, T.; Geldner, N.; Grebe, M.; Mangold, S.; Jackson, C.L.; Paris, S.; Gälweiler, L.; Palme, K.; Jürgens, G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 1999, 286, 316–318.
- Geldner, N.; Richter, S.; Vieten, A.; Marquardt, S.; Torres-Ruiz, R.A.; Mayer, U.; Jürgens, G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 2004, 131, 389–400.
- Chantalat, S.; Park, S.-K.; Hua, Z.; Liu, K.; Gobin, R.; Peyroche, A.; Rambourg, A.; Graham, T.R.; Jackson, C.L. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J. Cell Sci. 2004, 117, 711–722.
- Jones, S.; Jedd, G.; Kahn, R.A.; Franzusoff, A.; Bartolini, F.; Segev, N. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics 1999, 152, 1543–1556.




