
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guangwei Liu | + 1362 word(s) | 1362 | 2020-09-22 11:48:45 | | | |
| 2 | Vicky Zhou | Meta information modification | 1362 | 2020-09-28 05:24:47 | | | | |
| 3 | Vicky Zhou | -6 word(s) | 1356 | 2020-10-14 12:04:15 | | | | |
| 4 | Vicky Zhou | Meta information modification | 1356 | 2020-10-23 11:30:50 | | | | |
| 5 | Vicky Zhou | Meta information modification | 1356 | 2020-10-27 08:56:37 | | |
Video Upload Options
Myeloid-derived suppressor cells (MDSCs) are heterogeneous cells derived from bone marrow. They are precursors of dendritic cells, macrophages and/or granulocytes. They have the ability to significantly inhibit immune cell responses.
1. Introduction
The tumor microenvironment (TME) is a complex immune network that is a vital contributor to the promotion of tumor cell proliferation, metastasis, and immune escape. In the TME, other cells are present in addition to tumor cells, such as fibroblasts, immune and inflammatory cells, adipose cells, and immunosuppressive cells. In the TME, tumor cells incapacitate immune cells, including natural killer (NK) cells and T cells, by themselves and by immunosuppressive cells that are reprogrammed such that the tumor cells are not recognized and killed by the immune system. These “assistants” that assist tumorigenesis consist of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), cancer-associated fibroblasts (CAFs), and myeloid-derived suppressor cells (MDSCs). All members of these suppressive cells secrete large amounts of cytokines, chemokines, and other small molecule metabolites to build a hotbed suitable for the survival of malignant tumors[1][2][3].
MDSCs are a heterogeneous group of cells. Under normal circumstances, MDSCs represent a group of immature myeloid cells (IMCs) derived from bone marrow (BM) of various stages of differentiation and eventually differentiate into macrophages, dendritic cells (DCs), and neutrophils[4]. Therefore, MDSCs have considerable plasticity and diversity. However, under pathological conditions, such as the graft-versus-host disease (GVHD), autoimmune diseases, infections, and cancers, MDSCs are abnormally generated and activated[5]. Especially in the TME, hematopoietic progenitor cells (HPCs) are stimulated by tumor-derived inflammatory factors, e.g., granulocyte-macrophage colony-stimulating factors (GM-CSF), tumor necrosis factor-alpha (TNFα), vascular endothelial growth factor (VEGF), and prostaglandin E2 (PGE2), and differentiate into common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs). GMPs differentiate into monocyte/macrophage and dendritic cell precursors (MDPs) and myeloblasts (MBs) and are ultimately converted into MDSCs [6][7](Figure 1). Activated MDSCs flow through the blood and spleen and are eventually recruited to the tumor site by C–X–C motif chemokine ligand 1 (CXCL1), C–C motif chemokine ligand 2 (CCL2), and other chemokines. MDSCs expressing anti-inflammatory factors such as interleukin (IL)-10 and transforming growth factor-beta (TGFβ) play important immunosuppressive roles in the TME to promote tumor development and expansion[6][8][9]. Given the obvious protumoral capabilities, tumor treatment strategies targeting MDSCs are highly valued.
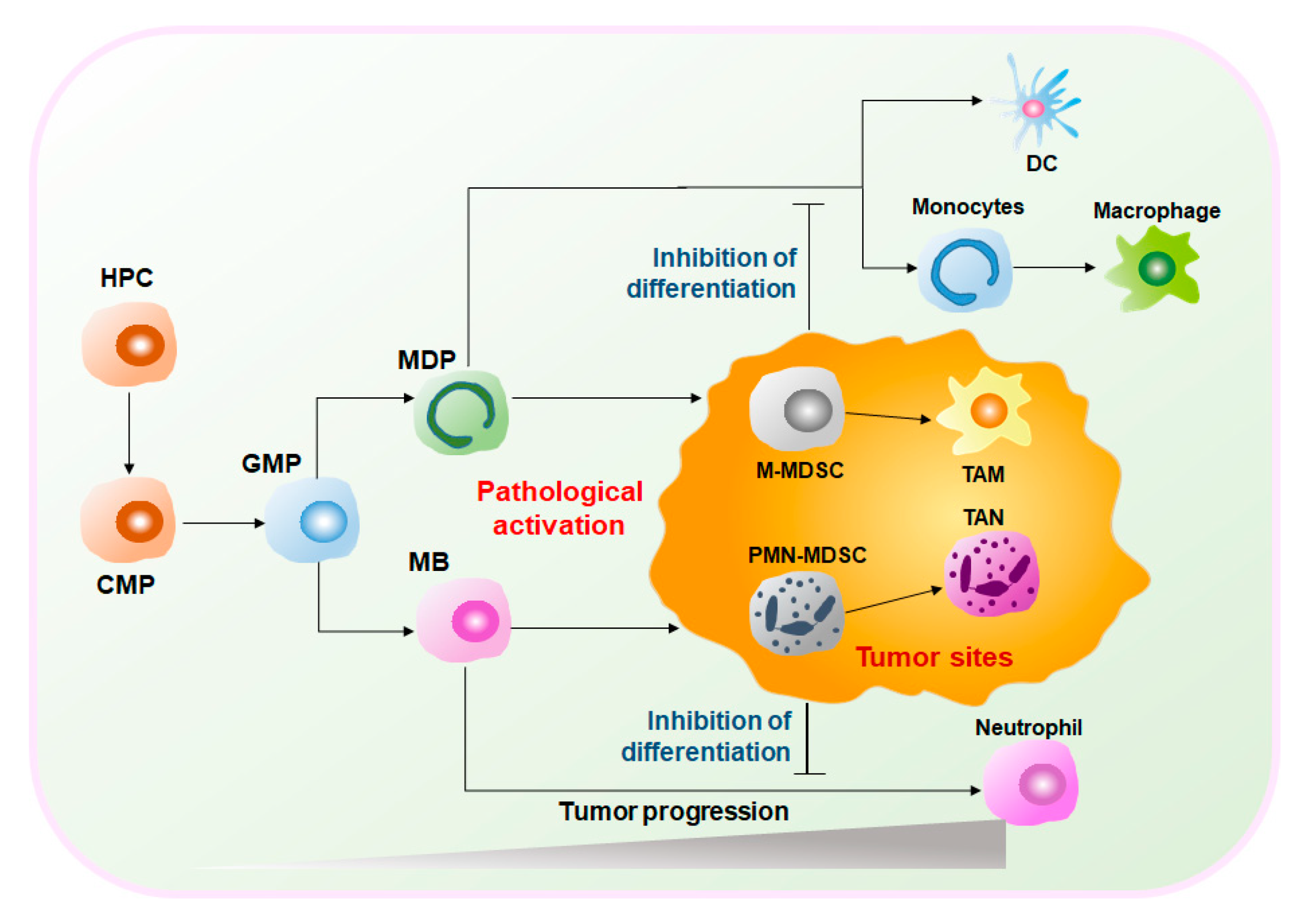
2. The Therapeutic Effects of Targeting MDSCs
Immunotherapy is currently the mainstream cancer therapy and can effectively save the lives of cancer patients through an immune checkpoint blockade (ICB)[10]. However, immunotherapy is not effective for every patient. Only a few patients can be cured, and it is limited to specific types of cancer. The immunosuppressive function of MDSCs is considered to make a major contribution to tumor development given their extensive inhibition of antitumor responses and promotion of tumorigenesis. Studies have shown that MDSCs are the main contributors to the poor clinical outcome of immunotherapy[11][12]. Therefore, in recent years, a variety of cancer treatment strategies have been developed to reduce the number of MDSCs and impede the immunosuppressive function of MDSCs. In addition, some traditional treatment approaches, such as radiotherapy or other methods, can also effectively damage the inhibitory activity of MDSCs[13][14]. Furthermore, a large number of studies have combined treatment methods targeting MDSCs with immunotherapy, which has exhibited potential antitumor effects (Figure 2 and Figure 3).
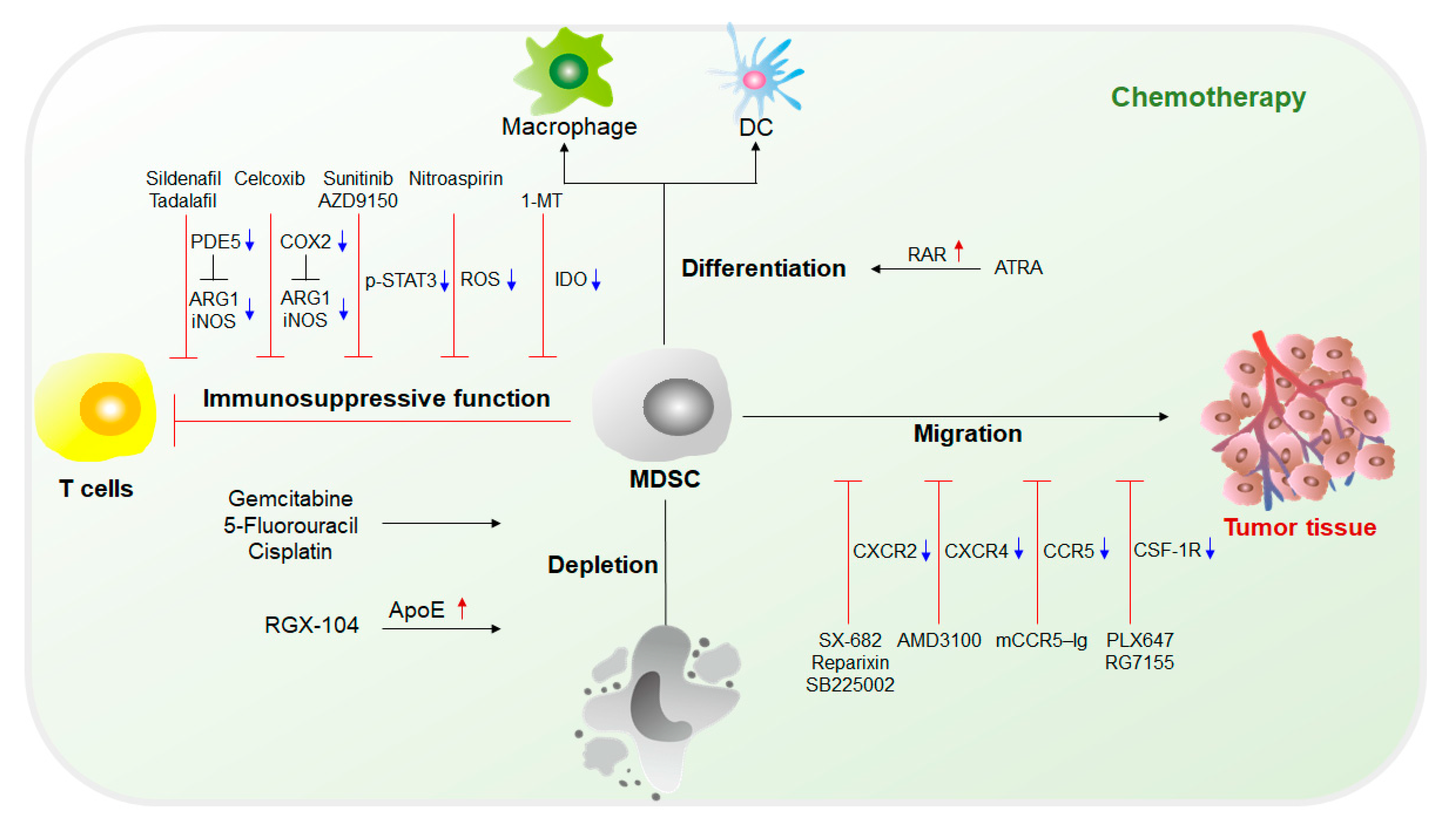
2.1. Chemotherapy Targeting MDSCs
The fundamental purpose of therapy targeting MDSCs is to eliminate MDSCs. Without the immunosuppression mediated by MDSCs, the limitation of the antitumor response can be lifted, and tumor development can be suppressed. Current chemotherapy approaches targeting MDSCs mainly include (1) inhibition of immunosuppressive functions of MDSCs; (2) elimination of MDSCs in both tumor sites and the circulatory system; (3) blockade of MDSC recruitment to the TME; and (4) induction of the differentiation of MDSCs into mature myeloid cells that lack suppressive activity [4][15][16](Figure 2).
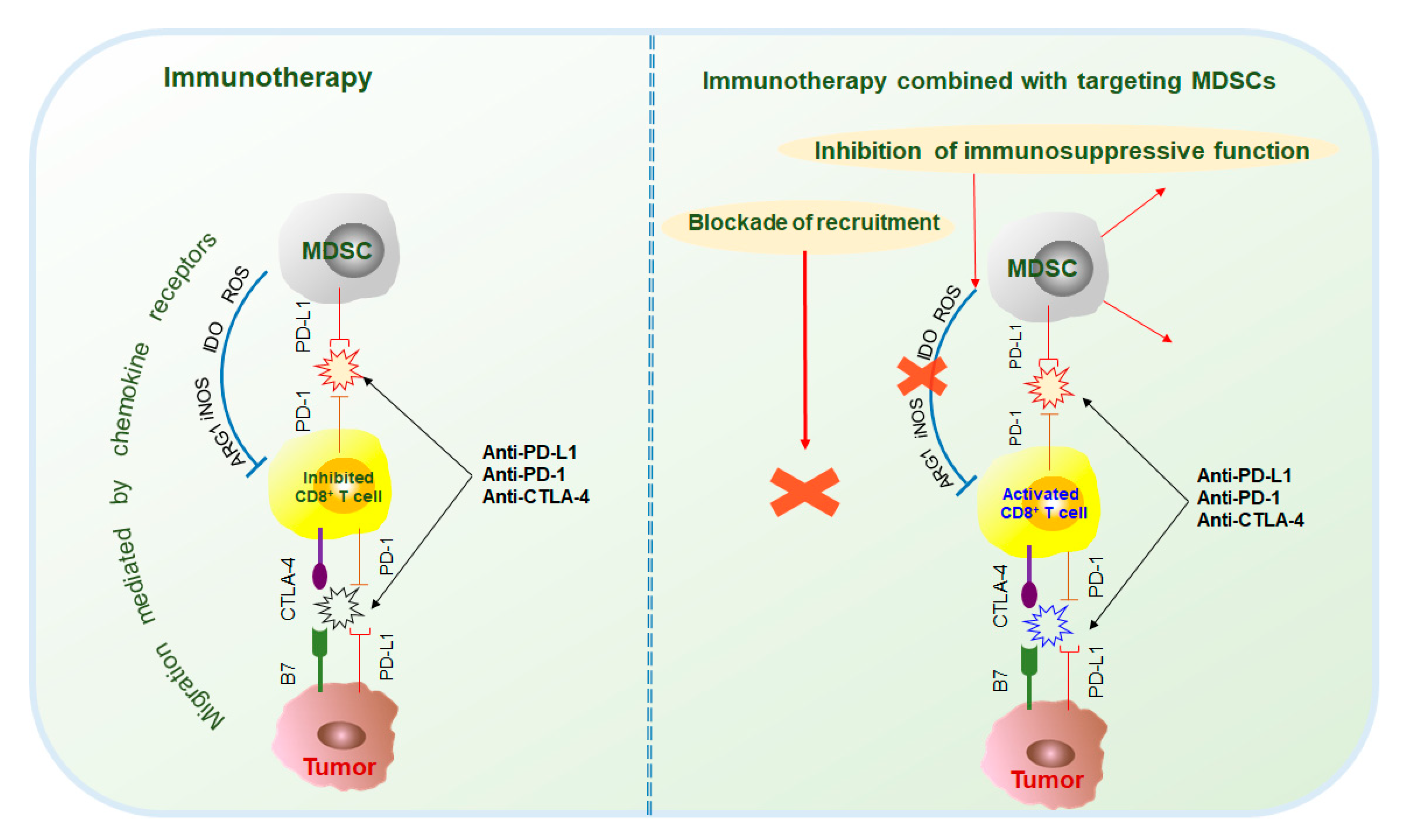
2.2. Immunotherapy in Combination with MDSC Targeted Therapy
Tumor and immunosuppressive cells, such as MDSCs, also inhibit antitumor responses through the interaction of immune checkpoint molecules, such as PD-1/PD-L1, CTLA-4/B7, and Gal-9/TIM-3[17]. Current studies mainly focus on the immunotherapy of PD-1, PD-L1, and CTLA-4. PD-1 antibodies include pembrolizumab and nivolumab; PD-L1 antibodies include atezolizumab, durvalumab, and avelumab; and CTLA-4 antibodies include ipilimumab and tremelimumab[17][18]. However, because MDSCs are the main contributors to immunosuppression, the effects of immunotherapy are often hindered. Therefore, the combination of immunotherapy and targeted MDSCs has been thoroughly researched and has made great progress (Figure 3).
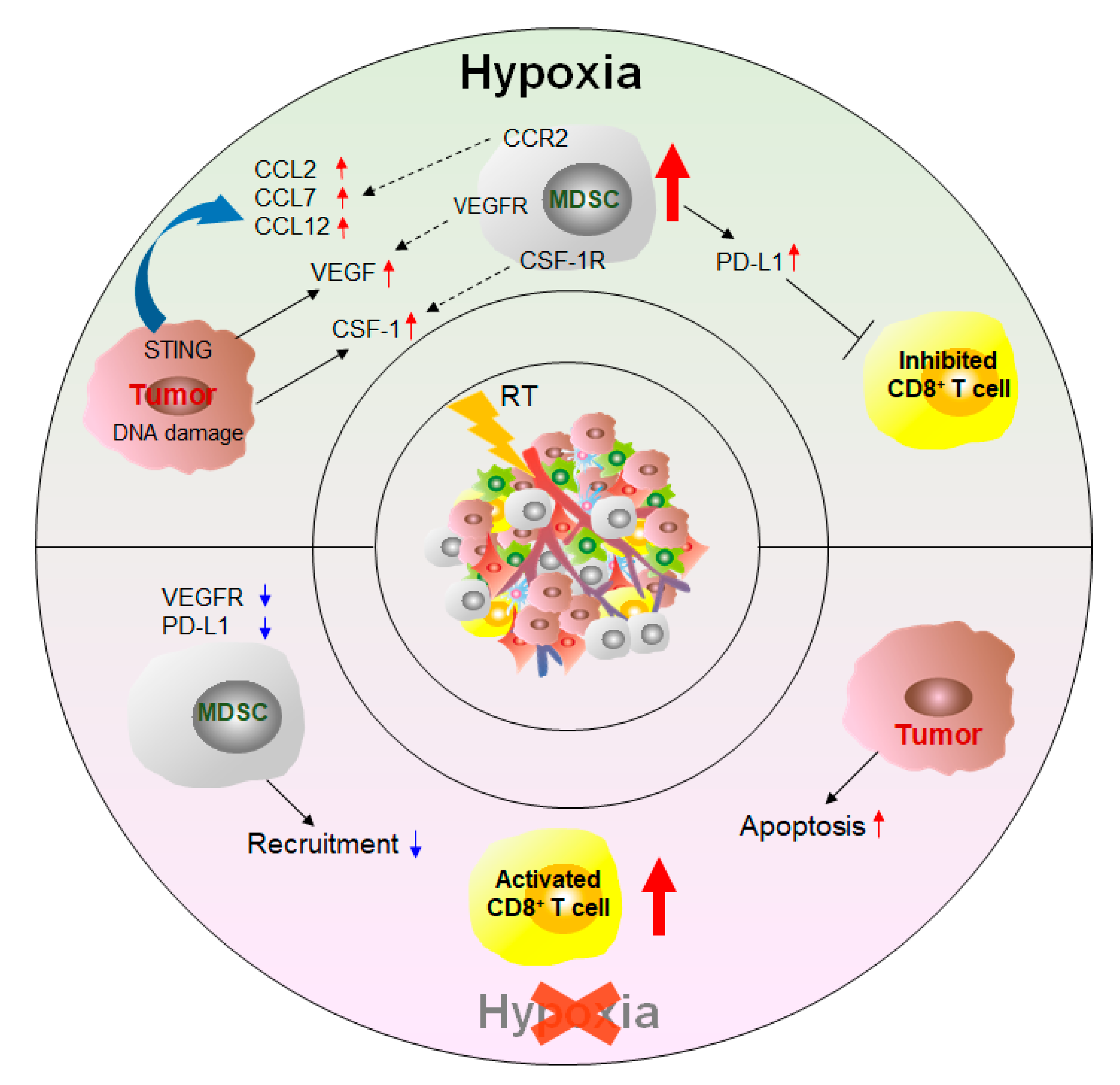
2.3. Other Therapy Strategies
In addition to the targeted MDSC approaches, other treatments can affect the number and function of MDSCs and thus achieve the purpose of inhibiting tumor growth and improving survival (Figure 4).
3. Conclusions
Overall, MDSCs are one of the main promoters of cancer. MDSCs abolish the antitumor response by exerting immunosuppressive functions, promote the formation of the tumor microenvironment, and provide comfortable conditions for tumor growth. At present, research on MDSCs remains insufficient, and how to distinguish MDSCs from other myeloid cells remains controversial. Emerging high-throughput technologies may help to better identify the phenotype of MDSCs. Therapeutic methods targeting MDSCs have been shown to effectively limit the accumulation of MDSCs in tumor tissue and peripheral organs. In the future, the combination of targeted MDSCs and immunotherapy may become the main cancer treatment strategy.
References
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220.
- De Sanctis, F.; Solito, S.; Ugel, S.; Molon, B.; Bronte, V.; Marigo, I. MDSCs in cancer: Conceiving new prognostic and therapeutic targets. Biochim. Biophys. Acta 2016, 1865, 35–48.
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8.
- Thyagarajan, A.; Alshehri, M.S.A.; Miller, K.L.R.; Sherwin, C.M.; Travers, J.B.; Sahu, R.P. Myeloid-derived suppressor cells and pancreatic cancer: Implications in novel therapeutic approaches. Cancers (Basel) 2019, 11, 1627.
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: Not only in tumor immunity. Front. Immunol. 2019, 10, 1099.
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119.
- Dong, L.; Bi, Y.; Jia, A.; Yu, Q.; Wang, Y.; Wang, Y.; Yang, Q.; Cao, Y.; He, Y.; Liu, R.; et al. Crucial role of histone deacetylase SIRT1 in myeloid-derived suppressor cell-mediated reprogramming of CD4 + T-cell differentiation. Cell. Mol. Immunol. 2020, 17, 785–787.
- Wang, Y.; Jia, A.; Bi, Y.; Wang, Y.; Liu, G. Metabolic regulation of myeloid-derived suppressor cell function in cancer. Cells 2020, 9, 1011.
- Sica, A.; Guarneri, V.; Gennari, A. Myelopoiesis, metabolism and therapy: A crucial crossroads in cancer progression. Cell Stress 2019, 3, 284–294.
- Popovic, A.; Jaffee, E.M.; Zaidi, N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Invest. 2018, 128, 3209–3218.
- Zhang, Y.; Guoqiang, L.; Sun, M.; Lu, X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol. Med. 2020, 17, 32–43.
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 2020, 9, 561.
- Yin, Z.; Li, C.; Wang, J.; Xue, L. Myeloid-derived suppressor cells: Roles in the tumor microenvironment and tumor radiotherapy. Int. J. Cancer 2019, 144, 933–946.
- Darragh, L.B.; Oweida, A.J.; Karam, S.D. Overcoming resistance to combination radiation-immunotherapy: A focus on contributing pathways within the tumor microenvironment. Front. Immunol. 2019, 9.
- Tesi, R.J. MDSC; the most important cell you have never heard of. Trends Pharmacol. Sci. 2019, 40, 4–7.
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting myeloid-derived suppressor cell, a promising strategy to overcome resistance to immune checkpoint inhibitors. Front. Immunol. 2020, 11, 783.
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086.
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39.




