
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisabetta Giorgini | -- | 2837 | 2022-04-21 17:31:01 | | | |
| 2 | Elisabetta Giorgini | Meta information modification | 2837 | 2022-04-21 17:33:12 | | | | |
| 3 | Elisabetta Giorgini | Meta information modification | 2837 | 2022-04-21 17:34:20 | | | | |
| 4 | Amina Yu | -26 word(s) | 2811 | 2022-04-22 06:11:35 | | | | |
| 5 | Amina Yu | Meta information modification | 2811 | 2022-04-22 06:12:30 | | | | |
| 6 | Amina Yu | Meta information modification | 2811 | 2022-04-22 06:14:52 | | | | |
| 7 | Amina Yu | Meta information modification | 2811 | 2022-04-25 09:45:59 | | |
Video Upload Options
Raman Microspectroscopy (RMS) represents an innovative tool to in vitro situation hard dental tissues. In fact, this vibrational technique has the advantage of providing, at the same time and on the same sample, a morpho-chemical correlation between the microscopic information from the visual analysis of the sample and its chemical and macromolecular composition. Moreover, thanks to the light scattering, it is possible to simultaneously perform the imaging analysis of both the inorganic and organic components of teeth, at a high spatial resolution level and in a confocal mode. The identification of specific Raman markers representative of sound and pathological hard dental tissues is crucial to improve the diagnosis of several dental pathologies and to detect dental lesions at an early stage when they are not visually detectable .
1. RMS and ATR-FTIR Characterization of Hard Dental Tissues
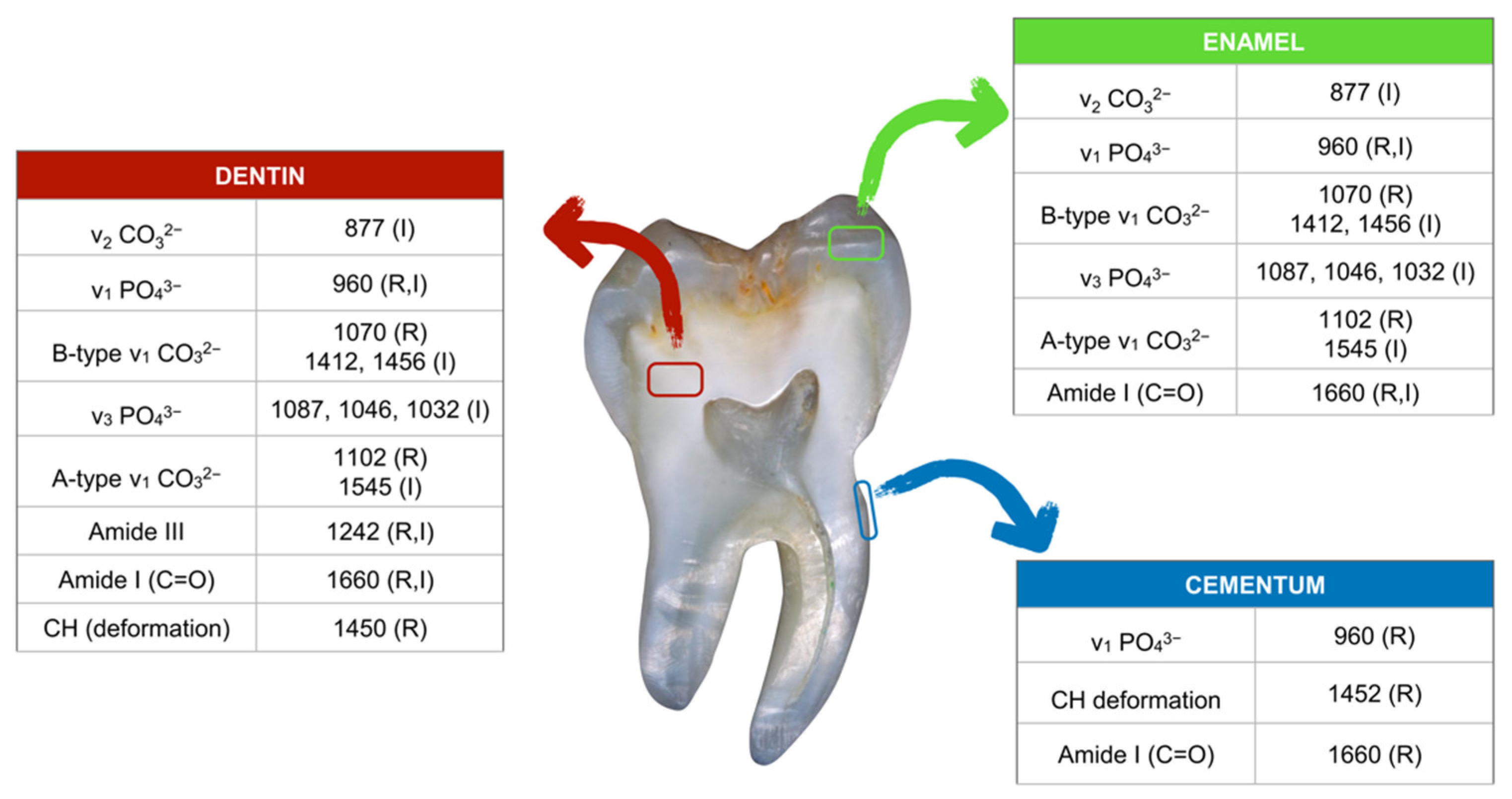
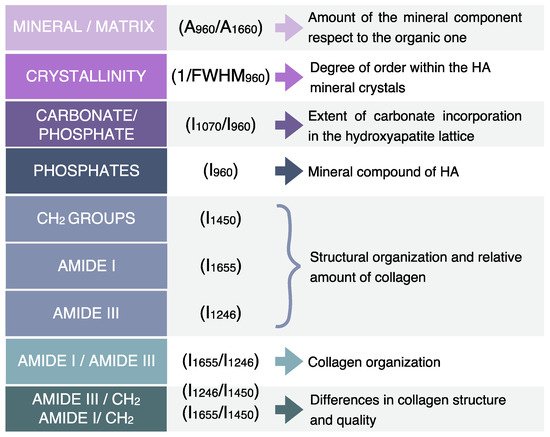
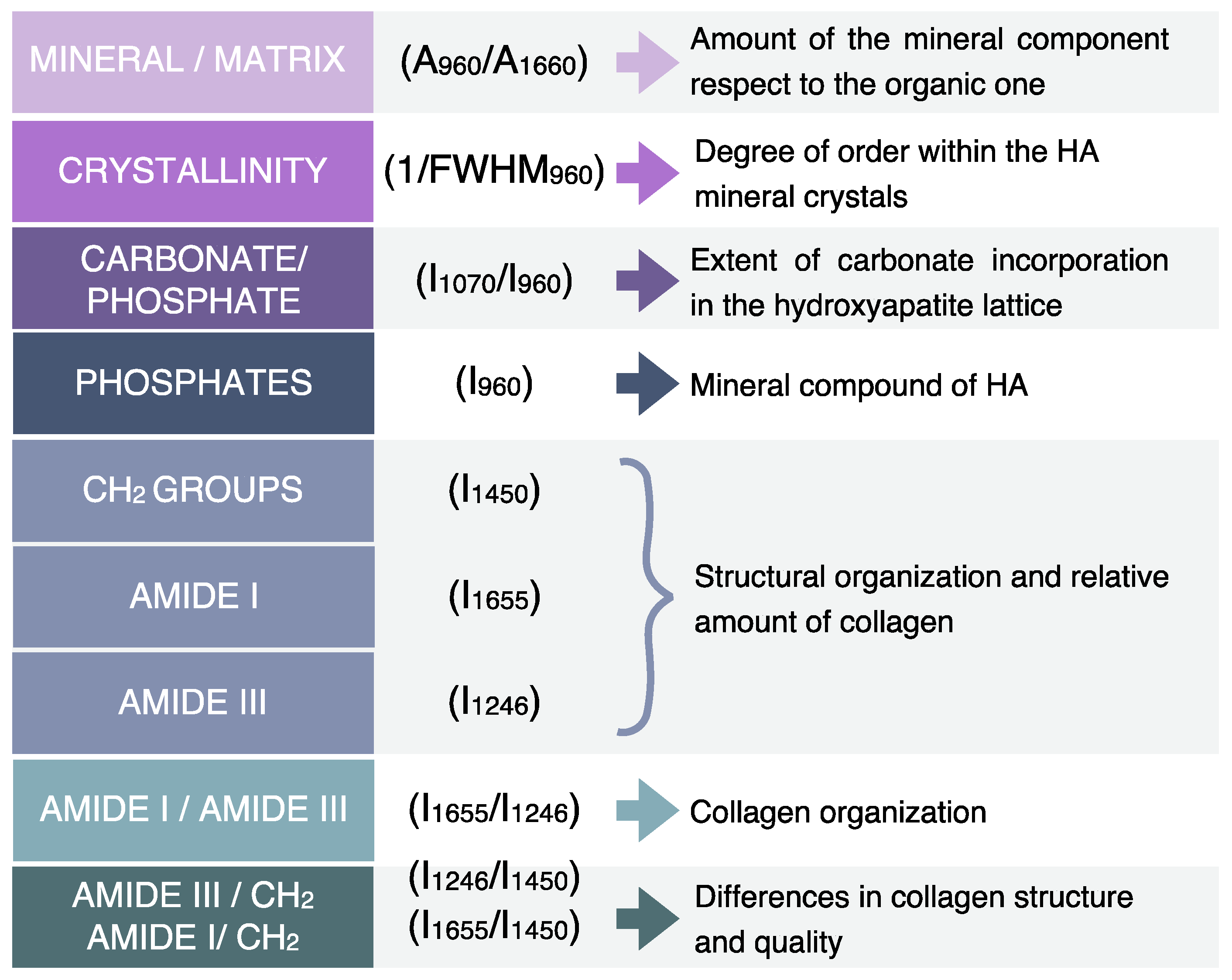
2. RMS Characterization of Dental Lesions
2.1. RMS of Amelogenesis and Dentinogenesis Imperfectae
2.2. RMS Characterization of Early Enamel Lesions
2.3. RMS Characterization of Dental Caries
References
- Rodrigo S. Lacruz; Stefan Habelitz; J. Timothy Wright; Michael L. Paine; Dental Enamel Formation and Implications for Oral Health and Disease. Physiological Reviews 2017, 97, 939-993, 10.1152/physrev.00030.2016.
- Kamna Srivastava; Tripti Tikku; Rohit Khanna; Kiran Sachan; Risk factors and management of white spot lesions in orthodontics. journal of orthodontic science 2013, 2, 43-49, 10.4103/2278-0203.115081.
- M. Anwar Alebrahim; C. Krafft; W. Sekhaneh; B. Sigusch; J. Popp; ATR-FTIR and Raman spectroscopy of primary and permanent teeth. Biomedical Spectroscopy and Imaging 2014, 3, 15-27, 10.3233/BSI-130059.
- Tsuneyuki Yamamoto; Tomoka Hasegawa; Tomomaya Yamamoto; Hiromi Hongo; Norio Amizuka; Histology of human cementum: Its structure, function, and development. Japanese Dental Science Review 2016, 52, 63-74, 10.1016/j.jdsr.2016.04.002.
- R.P. Shellis; Relationship between human enamel structure and the formation of caries-like lesions in vitro. Archives of Oral Biology 1984, 29, 975-981, 10.1016/0003-9969(84)90144-4.
- Agr Targino; Aronita Rosenblatt; Andressa Oliveira; Amb Chaves; Ve Santos; The relationship of enamel defects and caries: a cohort study. Oral Diseases 2010, 17, 420-426, 10.1111/j.1601-0825.2010.01770.x.
- Neslihan Efeoglu; David Wood; Candan Efeoglu; Microcomputerised tomography evaluation of 10% carbamide peroxide applied to enamel. Journal of Dentistry 2005, 33, 561-567, 10.1016/j.jdent.2004.12.001.
- Neslihan Efeoglu; David J. Wood; Candan Efeoglu; Thirty-five percent carbamide peroxide application causes in vitro demineralization of enamel. Dental Materials 2007, 23, 900-904, 10.1016/j.dental.2006.06.032.
- G. Penel; G. Leroy; C. Rey; E. Bres; MicroRaman Spectral Study of the PO 4 and CO 3 Vibrational Modes in Synthetic and Biological Apatites. Calcified Tissue Research 1998, 63, 475-481, 10.1007/s002239900561.
- R. Al-Obaidi; H. Salehi; A. Desoutter; L. Bonnet; P. Etienne; E. Terrer; B. Jacquot; B. Levallois; H. Tassery; F. J. G. Cuisinier; et al. Chemical & Nano-mechanical Study of Artificial Human Enamel Subsurface Lesions. Scientific Reports 2018, 8, 1-8, 10.1038/s41598-018-22459-7.
- Ozlem Marti Akgun; Sevgi Haman Bayari; Semra Ide; Gunseli Guven Polat; Ceren Yildirim; Ilgar Orujalipoor; Evaluation of the protective effect on enamel demineralization of CPP‐ACP paste and ROCS by vibrational spectroscopy and SAXS : An in vitro study. Microscopy Research and Technique 2021, 84, 2977–2987, 10.1002/jemt.23857.
- Mark T. Fulmer; Ira C. Ison; Christine R. Hankermayer; Brent R. Constantz; John Ross; Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002, 23, 751-755, 10.1016/s0142-9612(01)00180-6.
- Ravikumar Ramakrishnaiah; Ghufran Ur Rehman; Santhosh Basavarajappa; Abdulaziz Abdullah Al Khuraif; B. H. Durgesh; Abdul Samad Khan; Ihtesham Ur Rehman; Applications of Raman Spectroscopy in Dentistry: Analysis of Tooth Structure. Applied Spectroscopy Reviews 2014, 50, 332-350, 10.1080/05704928.2014.986734.
- Ana Flávia Sanches Borges; Renata Andrade Bitar; Kamila Rosamilia Kantovitz; Américo Bortollazo Correr; Airton Abrahão Martin; Regina Maria Puppin-Rontani; New perspectives about molecular arrangement of primary and permanent dentin. Applied Surface Science 2007, 254, 1498-1505, 10.1016/j.apsusc.2007.07.018.
- Changqi Xu; Rosi Jan Reed; Jeffrey P. Gorski; Yiguo Wang; Mary P. Walker; The distribution of carbonate in enamel and its correlation with structure and mechanical properties. Journal of Materials Science 2012, 47, 8035-8043, 10.1007/s10853-012-6693-7.
- Giulia Orilisi; Riccardo Monterubbianesi; Valentina Notarstefano; Vincenzo Tosco; Flavia Vitiello; Giampaolo Giuliani; Angelo Putignano; Giovanna Orsini; New insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the microstructure and chemical composition of vestibular and lingual surfaces in permanent and deciduous human teeth. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 260, 119966, 10.1016/j.saa.2021.119966.
- Mohamed Khalid; Tanujjal Bora; Ahmed Al Ghaithi; Sharanjit Thukral; Joydeep Dutta; Raman Spectroscopy detects changes in Bone Mineral Quality and Collagen Cross-linkage in Staphylococcus Infected Human Bone. Scientific Reports 2018, 8, 1-9, 10.1038/s41598-018-27752-z.
- Y.W. Liu; Xin Yao; Yunxia Wang; A Fourier Transform Infrared Spectroscopy Analysis of Carious Dentin from Transparent Zone to Normal Zone. Caries Research 2014, 48, 320-329, 10.1159/000356868.
- Amel Slimani; Fares Nouioua; Alban Desoutter; Bernard Levallois; Frédéric J. G. Cuisinier; Hervé Tassery; Elodie Terrer; Hamideh Salehi; Confocal Raman mapping of collagen cross-link and crystallinity of human dentin–enamel junction. Journal of Biomedical Optics 2017, 22, 086003, 10.1117/1.jbo.22.8.086003.
- Tattiana Enrich-Essvein; Cristina Benavides-Reyes; Pedro Álvarez-Lloret; María Victoria Bolaños-Carmona; Alejandro B. Rodríguez-Navarro; Santiago González-López; Influence of de-remineralization process on chemical, microstructural, and mechanical properties of human and bovine dentin. Clinical Oral Investigations 2020, 25, 841-849, 10.1007/s00784-020-03371-9.
- Antonio José Ortiz-Ruiz; Juan De Dios Teruel-Fernández; Luis Alberto Alcolea-Rubio; Ana Hernández-Fernández; Yolanda Martínez-Beneyto; Francesc Gispert Guirado; Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Annals of Anatomy - Anatomischer Anzeiger 2018, 218, 7-17, 10.1016/j.aanat.2017.12.012.
- Hamideh Salehi; Elodie Terrer; Ivan Panayotov; Bernard Levallois; Bruno Jacquot; Hervé Tassery; Frédéric Cuisinier; Functional mapping of human sound and carious enamel and dentin with Raman spectroscopy. Journal of Biophotonics 2012, 6, 765-774, 10.1002/jbio.201200095.
- Manuel Toledano; Fátima S. Aguilera; Estrella Osorio; Inmaculada Cabello; Manuel Toledano-Osorio; Raquel Osorio; Functional and molecular structural analysis of dentine interfaces promoted by a Zn-doped self-etching adhesive and an in vitro load cycling model. Journal of the Mechanical Behavior of Biomedical Materials 2015, 50, 131-149, 10.1016/j.jmbbm.2015.05.026.
- Claire E. L. Smith; James Poulter; Agne Antanaviciute; Jennifer Kirkham; Steven Brookes; Chris F. Inglehearn; Alan J. Mighell; Amelogenesis Imperfecta; Genes, Proteins, and Pathways. Frontiers in Physiology 2017, 8, 435-435, 10.3389/fphys.2017.00435.
- K. Gadhia; S. McDonald; N. Arkutu; K. Malik; Amelogenesis imperfecta: an introduction. British Dental Journal 2012, 212, 377-379, 10.1038/sj.bdj.2012.314.
- Anna Akkus; Asya Akkus; Renato Roperto; Ozan Akkus; Thiago Porto; Sorin Teich; Lisa Lang; Evaluation of mineral content in healthy permanent human enamel by Raman spectroscopy. Journal of Clinical and Experimental Dentistry 2016, 8, e546-e549, 10.4317/jced.53057.
- G. Orsini; A. Majorana; A. Mazzoni; A. Putignano; M. Falconi; A. Polimeni; L. Breschi; Immunocytochemical detection of dentin matrix proteins in primary teeth from patients with dentinogenesis imperfecta associated with osteogenesis imperfecta. European Journal of Histochemistry 2014, 58, 2405, 10.4081/ejh.2014.2405.
- Kārlis Bērziņš; Joshua J. Sutton; Carolina Loch; Deanna Beckett; Benjamin J. Wheeler; Bernadette K. Drummond; Sara J. Fraser‐Miller; Keith C. Gordon; Application of low‐wavenumber Raman spectroscopy to the analysis of human teeth. Journal of Raman Spectroscopy 2019, 50, 1375-1387, 10.1002/jrs.5648.
- Felicity A. Crombie; David Manton; Joseph E.A. Palamara; Ilya Zalizniak; Nathan J. Cochrane; Eric C. Reynolds; Characterisation of developmentally hypomineralised human enamel. Journal of Dentistry 2013, 41, 611-618, 10.1016/j.jdent.2013.05.002.
- Jiajie Mao; Lin Wang; Yun Jiang; Haoran Cheng; Ning Li; Shi Shi; Fan Fan; Jianfeng Ma; Shengbin Huang; Nanoscopic wear behavior of dentinogenesis imperfecta type II tooth dentin. Journal of the Mechanical Behavior of Biomedical Materials 2021, 120, 104585, 10.1016/j.jmbbm.2021.104585.
- Fredrik Bergstrand; Svante Twetman; A Review on Prevention and Treatment of Post-Orthodontic White Spot Lesions - Evidence-Based Methods and Emerging Technologies. The Open Dentistry Journal 2011, 5, 158-162, 10.2174/1874210601105010158.
- Alex C.-T. Ko; Lin-P'ing Choo-Smith; Mark Hewko; Michael Sowa; Cecilia C. S. Dong; Blaine Cleghorn; Detection of early dental caries using polarized Raman spectroscopy. Optics Express 2006, 14, 203-215, 10.1364/opex.14.000203.
- H. Kinoshita; N. Miyoshi; Y. Fukunaga; T. Ogawa; T. Ogasawara; K. Sano; Functional mapping of carious enamel in human teeth with Raman microspectroscopy. Journal of Raman Spectroscopy 2008, 39, 655-660, 10.1002/jrs.1908.
- Alban Desoutter; Amel Slimani; Rand Al‐Obaidi; Stephane Barthélemi; Frederic Cuisinier; Hervé Tassery; Hamideh Salehi; Cross striation in human permanent and deciduous enamel measured with confocal Raman microscopy. Journal of Raman Spectroscopy 2019, 50, 548-556, 10.1002/jrs.5555.
- Giuseppe Pezzotti; Tetsuya Adachi; Isabella Gasparutti; Giulio Vincini; Wenliang Zhu; Marco Boffelli; Alfredo Rondinella; Elia Marin; Hiroaki Ichioka; Toshiro Yamamoto; et al.Yoshinori MarunakaNarisato Kanamura Vibrational monitor of early demineralization in tooth enamel after in vitro exposure to phosphoridic liquid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2017, 173, 19-33, 10.1016/j.saa.2016.08.036.
- Pavel Seredin; Dmitry Goloshchapov; Tatiana Prutskij; Yury Ippolitov; Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008-e0124008, 10.1371/journal.pone.0124008.
- Giovanna Orsini; Vincenzo Tosco; Riccardo Monterubbianesi; Scilla Sparabombe; Giulia Orilisi; Angelo Putignano; In Vitro Investigation of the Effect of Different Remineralizing Agents on Human Enamel. Modern Approaches in Dentistry and Oral Health Care 2020, 4, 369-375, 10.32474/madohc.2020.04.000189.
- Masatoshi Ando; Chien-Sheng Liao; George J. Eckert; Ji-Xin Cheng; Imaging of demineralized enamel in intact tooth by epidetected stimulated Raman scattering microscopy. Journal of Biomedical Optics 2018, 23, 105005, 10.1117/1.jbo.23.10.105005.
- M. Alturki; G. Koller; F. Warburton; U. Almhöjd; A. Banerjee; Biochemical characterisation of carious dentine zones using Raman spectroscopy. Journal of Dentistry 2020, 105, 103558, 10.1016/j.jdent.2020.103558.
- Tomasz Buchwald; Zuzanna Buchwald; Assessment of the Raman spectroscopy effectiveness in determining the early changes in human enamel caused by artificial caries. The Analyst 2018, 144, 1409-1419, 10.1039/c8an01494a.
- Shuvashis Das Gupta; Markus Killenberger; Tarja Tanner; Lassi Rieppo; Simo Saarakkala; Jarkko Heikkilä; Vuokko Anttonen; Mikko A. J. Finnilä; Mineralization of dental tissues and caries lesions detailed with Raman microspectroscopic imaging. The Analyst 2020, 146, 1705-1713, 10.1039/d0an01938k.
- Elia Marin; Noriko Hiraishi; Taigi Honma; Francesco Boschetto; Matteo Zanocco; Wenliang Zhu; Tetsuya Adachi; Narisato Kanamura; Toshiro Yamamoto; Giuseppe Pezzotti; et al. Raman spectroscopy for early detection and monitoring of dentin demineralization. Dental Materials 2020, 36, 1635-1644, 10.1016/j.dental.2020.10.005.




