
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hanieh Gholizadeh | -- | 3518 | 2022-04-21 06:31:48 | | | |
| 2 | Vivi Li | -15 word(s) | 3503 | 2022-04-21 08:22:20 | | |
Video Upload Options
Developing novel drug formulations and progressing them to the clinical environment relies on preclinical in vitro studies and animal tests to evaluate efficacy and toxicity. However, these current techniques have failed to accurately predict the clinical success of new therapies with a high degree of certainty. The main reason for this failure is that conventional in vitro tissue models lack numerous physiological characteristics of human organs, such as biomechanical forces and biofluid flow. Moreover, animal models often fail to recapitulate the physiology, anatomy, and mechanisms of disease development in human. These shortfalls often lead to failure in drug development, with substantial time and money spent. To tackle this issue, organ-on-chip technology offers realistic in vitro human organ models that mimic the physiology of tissues, including biomechanical forces, stress, strain, cellular heterogeneity, and the interaction between multiple tissues and their simultaneous responses to a therapy. For the latter, complex networks of multiple-organ models are constructed together, known as multiple-organs-on-chip. Numerous studies have demonstrated successful application of organ-on-chips for drug testing, with results comparable to clinical outcomes.
1. Introduction
| Refs. | Focus of the Review |
|---|---|
| [3][4][5] |
|
| [3][9][10][11] |
|
| [6][7] |
|
| [8] |
|
| This entry |
|
2. Drug Testing Capabilities Using Liver-, Kidney-, and Lung-on-Chip Models
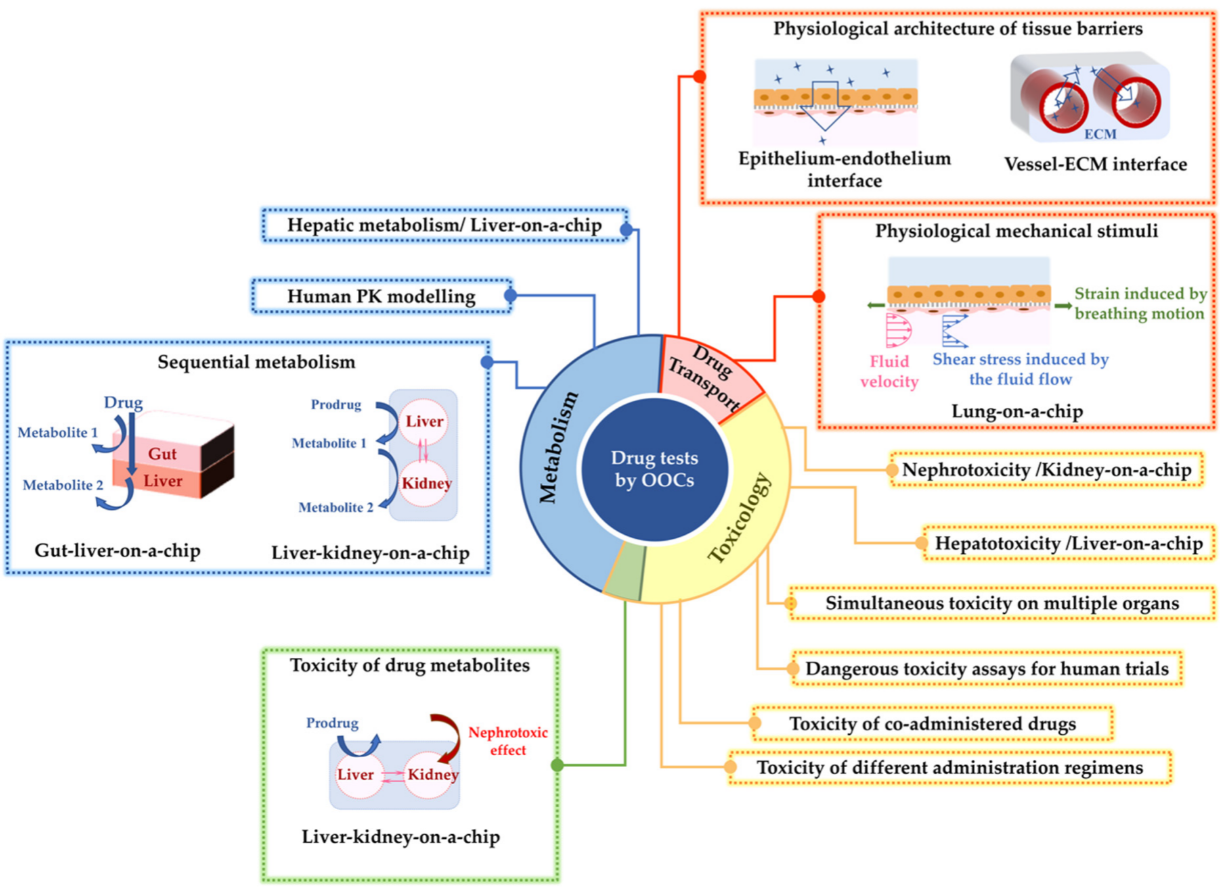
2.1. Drug Metabolism Studies
| Drug | Toxicology | Metabolism | Tissue(s) | Reference |
|---|---|---|---|---|
| diclofenac acetaminophen |
✓ | liver | [18] | |
| troglitazone | ✓ | liver | [19] | |
| acetaminophen | ✓ | liver | [32][33][34][35][36] | |
| acetaminophen | ✓ | ✓ | liver | [37][38] |
| acetaminophen isoniazid rifampicin |
✓ | ✓ | liver | [39] |
| rifampin ketoconzazole acetaminophen |
✓ | ✓ | liver | [40] |
| bupropion tolbutamide omeprazole testosterone |
✓ | liver | [24] | |
| 7-ethoxy-4-trifluoromethyl coumarin | ✓ | liver | [25] | |
| acetaminophen chlorpromazine tacrine |
✓ | liver | [41] | |
| ccetaminophen fialuridine |
✓ | ✓ | liver | [42] |
| diclofenac | ✓ | ✓ | liver | [43] |
| cadmium aspirin caffein troglitazone rosiglitazone pioglitazone acetaminophen |
✓ | liver | [2] | |
| cisplatin | ✓ | kidney | [14] | |
| adriamycin | ✓ | kidney | [44] | |
| gentamicin | ✓ | kidney | [45] | |
| polymyxin B | ✓ | kidney | [46] | |
| carboxylated polystyrene nanoparticles | ✓ | GI tract–liver | [47] | |
| troglitazone | ✓ | ✓ | liver–intestine liver–skin |
[48] |
| apigenin | ✓ | gut–liver | [28] | |
| epirubicine irinotecan cyclophosphamide |
✓ | small intestine–liver–lung | [29] | |
| ifosfamide verapamil |
✓ | ✓ | liver–kidney | [49] |
| paracetamol | ✓ | liver–gut | [31] | |
| mannitol propranolol caffeine |
✓ | GI–liver | [50] | |
| combination of genistein and dacarbazine | ✓ | intestine–liver | [51] | |
| 5-fluorouracil | ✓ | liver–tumor–marrow | [52] | |
| paracetamol | ✓ | ✓ | liver–kidney | [53] |
| diclofenac ketoconazole hydrocortisone acetaminophen |
✓ | liver–heart–skin | [54] | |
| luteolin | ✓ | liver–tumor | [30] | |
| capecitabine tegafur |
✓ | liver–cancer intestine–liver–cancer–connective tissue | [27] | |
| digoxin | ✓ | intestine–kidney | [55] | |
| ifosfamide | ✓ | ✓ | liver–kidney | [56] |
| vitamin D | ✓ | liver–kidney | [26] |
2.2. Toxicology
2.2.1. Toxicology Studies by the Kidney- or Liver-on-Chip Models
2.2.2. Toxicology Studies by the Kidney- and Liver-on-Chip Models Interconnected with Other Organs
2.3. Drug Delivery/Transport
2.3.1. Simulation of In Vivo-Level Barrier Functions On-Chip
2.3.2. Simulation of Multiple Drug-Delivery Routes On-Chip
2.3.3. Drug Delivery Tests under In Vivo-Inspired Dynamic Conditions On-Chip
2.3.4. Further Improvements of Drug Delivery Studies by Organ-on-Chips
References
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171.
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126.
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discoov. 2015, 14, 248–260.
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 1–19.
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacok. 2018, 33, 43–48.
- Deng, J.; Qu, Y.; Liu, T.; Jing, B.; Zhang, X.; Chen, Z.; Luo, Y.; Zhao, W.; Lu, Y.; Lin, B. Recent organ-on-a-chip advances toward drug toxicity testing. Development 2018, 19, 20.
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug toxicity evaluation based on organ-on-a-chip technology: A review. Micromachines 2020, 11, 381.
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93.
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A new paradigm for drug development. Trends Pharmacol. Sci. 2020, 42, 119–133.
- Vulto, P.; Joore, J. Adoption of organ-on-chip platforms by the pharmaceutical industry. Nat. Rev. Drug Discov. 2021, 20, 961–962.
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361.
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668.
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310.
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129.
- Abaci, H.E.; Shuler, M.L. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 2015, 7, 383–391.
- Sung, J.H.; Wang, Y.I.; Narasimhan Sriram, N.; Jackson, M.; Long, C.; Hickman, J.J.; Shuler, M.L. Recent advances in body-on-a-chip systems. Anal. Chem. 2018, 91, 330–351.
- Van Den Berg, A.; Mummery, C.L.; Passier, R.; Van der Meer, A.D. Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip 2019, 19, 198–205.
- Yu, F.; Deng, R.; Tong, W.H.; Huan, L.; Chan Way, N.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528.
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240.
- Hornberg, J.J.; Laursen, M.; Brenden, N.; Persson, M.; Thougaard, A.V.; Toft, D.B.; Mow, T. Exploratory toxicology as an integrated part of drug discovery. Part I: Why and how. Drug Discov. Today 2014, 19, 1131–1136.
- Kumar, G.N.; Surapaneni, S. Role of drug metabolism in drug discovery and development. Med. Res. Rev. 2001, 21, 397–411.
- Tscheik, C.; Blasig, I.E.; Winkler, L. Trends in drug delivery through tissue barriers containing tight junctions. Tissue Barriers 2013, 1, e24565.
- Weng, Y.S.; Chang, S.F.; Shih, M.C.; Tseng, S.H.; Lai, C.H. Scaffold-free liver-on-a-chip with multiscale organotypic cultures. Adv. Mater. 2017, 29, 1701545.
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126.
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004.
- Liu, X.; Wang, H.; Liang, X.; Roberts, M.S. Hepatic Metabolism in Liver Health and Disease. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 391–400.
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2018, 18, 115–125.
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101.
- Lee, H.; Kim, D.S.; Ha, S.K.; Choi, I.; Lee, J.M.; Sung, J.H. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic–pharmacodynamic (PK–PD) model. Biotechnol. Bioeng. 2017, 114, 432–443.
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018, 18, 3606–3616.
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89.
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 1–14.
- Snyder, J.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H.; Sun, W. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 2011, 3, 034112.
- Shuler, M.L. Organ-, body-and disease-on-a-chip systems. Lab Chip 2017, 17, 2345–2346.
- Hongmao, S. (Ed.) Chapter 7—In Silico ADMET Profiling: Predictive Models for CYP450, P-gp, PAMPA, and hERG. In A Practical Guide to Rational Drug Design; Woodhead Publishing: Cambridge, UK, 2016; pp. 225–268.
- Larrey, D.; Ursic-Bedoya, J.; Meunier, L. Drug-induced Hepatotoxicity. Schiff Dis. Liver 2017, 32, 740–773.
- Theobald, J.; el Maaty, M.A.A.; Kusterer, N.; Wetterauer, B.; Wink, M.; Cheng, X.; Wölfl, S. In vitro metabolic activation of vitamin D3 by using a multi-compartment microfluidic liver-kidney organ on chip platform. Sci. Rep. 2019, 9, 4616.
- Jie, M.; Lin, H.; He, Z.; Liu, H.; Li, H.; Lin, J.M. An on-chip intestine-liver model for multiple drugs absorption and metabolism behavior simulation. Sci. China Chem. 2018, 61, 236–242.
- Kim, S.; LesherPerez, S.C.; Yamanishi, C.; Labuz, J.M.; Leung, B.; Takayama, S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication 2016, 8, 015021.
- Mi, S.; Yi, X.; Du, Z.; Xu, Y.; Sun, W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication 2018, 10, 025010.
- Jang, M.; Neuzil, P.; Volk, T.; Manz, A.; Kleber, A. On-chip three-dimensional cell culture in phaseguides improves hepatocyte functions in vitro. Biomicrofluidics 2015, 9, 034113.
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.W. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019, 11, 025001.
- Ozkan, A.; Ghousifam, N.; Hoopes, P.J.; Yankeelov, T.E.; Rylander, M.N. In vitro vascularized liver and tumor tissue microenvironments on a chip for dynamic determination of nanoparticle transport and toxicity. Biotechnol. Bioeng. 2019, 116, 1201–1219.
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M.; et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. 2017, 1, 69.
- Chen, H.J.; Miller, P.; Shuler, M.L. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip 2018, 18, 2036–2046.
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; MacDonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.M.; et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 2018, 3, 123673.
- Delalat, B.; Cozzi, C.; Rasi Ghaemi, S.; Polito, G.; Kriel, F.H.; Michl, T.D.; Harding, F.J.; Priest, C.; Barillaro, G.; Voelcker, N.H. Microengineered bioartificial liver chip for drug toxicity screening. Adv. Funct. Mater. 2018, 28, 1801825.
- Ma, C.; Zhao, L.; Zhou, E.M.; Xu, J.; Shen, S.; Wang, J. On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal. Chem. 2016, 88, 1719–1727.
- Choucha-Snouber, L.; Aninat, C.; Grsicom, L.; Madalinski, G.; Brochot, C.; Poleni, P.E.; Razan, F.; Guillouzo, C.G.; Legallais, C.; Corlu, A.; et al. Investigation of ifosfamide nephrotoxicity induced in a liver–kidney co-culture biochip. Biotechnol. Bioeng. 2013, 110, 597–608.
- Choe, A.; Ha, S.K.; Choi, I.; Choi, N.; Sung, J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices 2017, 19, 4.
- Lee, D.W.; Ha, S.K.; Choi, I.; Sung, J.H. 3D gut-liver chip with a PK model for prediction of first-pass metabolism. Biomed. Microdevices 2017, 19, 100.
- Zakhariants, A.; Burmistrova, O.; Shkurnikov, M.Y.; Poloznikov, A.A.; Sakharov, D.A. Development of a Specific Substrate—Inhibitor Panel (Liver-on-a-Chip) for Evaluation of Cytochrome P450 Activity. Bull. Exp. Biol. Med. 2016, 162, 170–174.
- Kimura, H.; Ikeda, T.; Nakayama, H.; Sakai, Y.; Fujii, T. An on-chip small intestine–liver model for pharmacokinetic studies. J. Lab. Autom. 2015, 20, 265–273.
- Mao, S.; Gao, D.; Liu, W.; Wei, H.; Lin, J.M. Imitation of drug metabolism in human liver and cytotoxicity assay using a microfluidic device coupled to mass spectrometric detection. Lab Chip 2012, 12, 219–226.
- Prot, J.-M.; Bunescu, A.; Elena-Herrmann, B.; Aninat, C.; Snouber, L.C.; Griscom, L.; Razan, F.; Bois, F.Y.; Legallais, C.; Brochot, C. Predictive toxicology using systemic biology and liver microfluidic “on chip” approaches: Application to acetaminophen injury. Toxicol. Appl. Pharmacol. 2012, 259, 270–280.
- Foster, A.J.; Chouhan, B.; Regan, S.L.; Rollison, H.; Amberntsson, S.; Andersson, L.C.; Srivastava, A.; Darnell, M.; Cairns, J.; Lazic, S.E.; et al. Integrated in vitro models for hepatic safety and metabolism: Evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol. 2019, 93, 1021–1037.
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007, 97, 1340–1346.
- Esch, M.B.; Mahler, G.J.; Stokol, T.; Shuler, M.L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014, 14, 3081–3092.
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.A.; Sonntag, F.; Hayden, P.; Ayehunie, S. Chip-based human liver–intestine and liver–skin co-cultures–A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87.
- Li, Z.; Jiang, L.; Zhu, Y.; Su, W.; Xu, C.; Tao, T.; Shi, Y.; Qin, J. Assessment of hepatic metabolism-dependent nephrotoxicity on an organs-on-a-chip microdevice. Toxicol. In Vitro 2018, 46, 1–8.
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip 2010, 10, 446–455.
- Shintu, L.; Baudoin, R.; Navratil, V.; Prot, J.M.; Pontoizeau, C.; Defernez, M.; Blaise, B.J.; Domange, C.; Péry, A.R.; Toulhoat, P.; et al. Metabolomics-on-a-chip and predictive systems toxicology in microfluidic bioartificial organs. Anal. Chem. 2012, 84, 1840–1848.
- De Mello, C.P.P.; Carmona-Moran, C.; McAleer, C.W.; Perez, J.; Coln, E.A.; Long, C.J.; Oleaga, C.; Riu, A.; Note, R.; Teissier, S.; et al. Microphysiological heart–liver body-on-a-chip system with a skin mimic for evaluating topical drug delivery. Lab Chip 2020, 20, 749–759.
- Li, Z.; Su, W.; Zhu, Y.; Tao, T.; Li, D.; Peng, X.; Qin, J. Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics 2017, 11, 034114.
- Liebler, D.C.; Guengerich, F.P. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discoov. 2005, 4, 410–420.
- Jang, K.-J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516.
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829.
- Paul, A. Drug Absorption and Bioavailability. In Introduction to Basics of Pharmacology and Toxicology: Volume 1: General and Molecular Pharmacology: Principles of Drug Action; Raj, G.M., Raveendran, R., Eds.; Springer: Singapore, 2019; pp. 81–88.
- Haste, P.; Tann, M.; Persohn, S.; LaRoche, T.; Aaron, V.; Mauxion, T.; Chauhan, N.; Dreher, M.R.; Johnson, M.S. Correlation of technetium-99m macroaggregated albumin and Yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: A retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J. Vasc. Interv. Radiol. 2017, 28, 722–730.
- NDong, C.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636.
- Petryk, A.A.; Giustini, A.J.; Gottesman, R.E.; Kaufman, P.A.; Hoopes, P.J. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int. J. Hyperth. 2013, 29, 845–851.
- Terentyuk, G.S.; Maslyakova, G.N.; Suleymanova, L.V.; Khlebtsov, B.N.; Kogan, B.Y.; Akchurin, G.G.; Shantrocha, A.V.; Maksimova, I.L.; Khlebtsov, N.G.; Tuchin, V.V. Circulation and distribution of gold nanoparticles and induced alterations of tissue morphology at intravenous particle delivery. J. Biophotonics 2009, 2, 292–302.
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-time quantitative monitoring of in vitro nasal drug delivery by a nasal epithelial mucosa-on-a-chip model. Expert Opin. Drug Del. 2021, 18, 803–818.
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599.
- Cheng, S.; Kourmatzis, A.; Mekonnen, T.; Gholizadeh, H.; Raco, J.; Chen, L.; Tang, P.; Chan, H.K. Does upper airway deformation affect drug deposition? Int. J. Pharm. 2019, 572, 118773.
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157.




