
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrew W. Taylor-Robinson | -- | 1726 | 2022-04-21 06:10:39 | | | |
| 2 | Lindsay Dong | Meta information modification | 1726 | 2022-04-22 03:41:02 | | | | |
| 3 | Andrew W. Taylor-Robinson | Meta information modification | 1726 | 2022-04-22 07:12:00 | | |
Video Upload Options
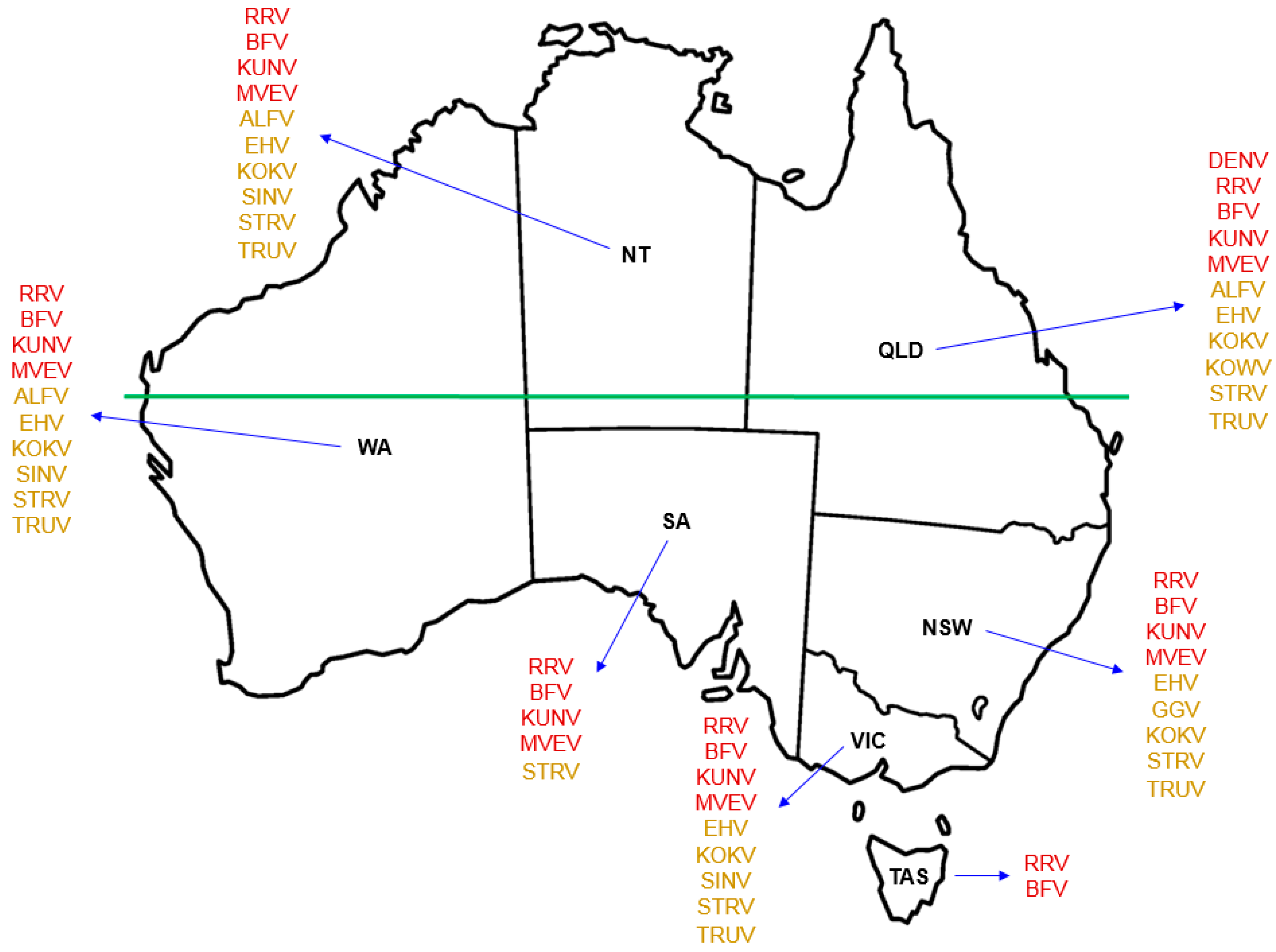
In excess of 75 arboviruses have been identified in Australia, some of which are now well established as causative agents of debilitating diseases. These include Ross River virus, Barmah Forest virus, and Murray Valley encephalitis virus, each of which may be detected by both antibody-based recognition and molecular typing. For most of the remaining arboviruses that may be associated with pathology in humans, routine tests are not available to diagnose infection. A number of these so-called ‘neglected’ or ‘orphan’ arboviruses are considered likely to have infected humans at a regular rate for decades. Some may be associated with undifferentiated febrile illness — fever, the cause of which is not obvious — for which around half of all cases each year remain undiagnosed. Ongoing research aims to better understand the distribution, epidemiology, and transmission ecology of these mosquito-transmitted viruses that are currently unique to Australia.
1. Introduction
2. Arbovirus Ecology and Epidemiology
3. Arboviruses in Australia
4. Transmission Cycles of Australian Arboviruses
References
- World Health Organization. Arboviruses and Human Disease: Report of a WHO Scientific Group; Technical Report Series No. 369; WHO: Geneva, Switzerland, 1967.
- Centers for Disease Control and Prevention. Arbovirus Catalog. Available online: https://wwwn.cdc.gov/arbocat/ (accessed on 22 April 2022).
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS) — Arboviral Diseases, Neuroinvasive and Non-neuroinvasive 2015 Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ (accessed on 22 April 2022).
- Beckham, J.D.; Tyler, K.L. Arbovirus infections. Continuum (Minneapolis, Minn) 2015, 21, 1599–1611.
- Gyawali, N.; Bradbury, R.S.; Aaskov, J.G.; Taylor-Robinson, A.W. Neglected Australian arboviruses: Quam gravis? Microbes Infect. 2017, 19, 388–401.
- Australian Government Department of Health. National Notifiable Diseases Surveillance System. Notifications for All Diseases by State & Territory and Year. Available online: http://www9.health.gov.au/cda/source/rpt_2_sel.cfm (accessed on 22 April 2022).
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801.
- Young, P.R.; Ng, L.F.P.; Hall, R.A.; Smith, D.W.; Johansen, C.A. Arbovirus infection. In Manson’s Tropical Diseases, 23rd ed.; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 129–161.
- Morens, D.M.; Fauci, A.S. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog. 2013, 9, e1003467.
- Cao-Lormeau, V.-M.; Musso, D. Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572.
- Gubler, D.J. Dengue viruses: Their evolution, history and emergence as a global public health problem. In Dengue and Dengue Hemorrhagic Fever; Gubler, D.J., Ooi, E., Vasudevan, S., Farrar, J., Eds.; CAB International: Wallingford, UK, 2014; pp. 1–29.
- Mackenzie, J.S.; Gubler, D.J.; Peterson, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109.
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika and now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e0005462.
- Sutherst, R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004, 17, 136–173.
- Russell, R.C. Mosquito-borne disease and climate change in Australia: Time for a reality check. Aust. J. Entomol. 2009, 48, 1–7.
- Fraser, J.R. Epidemic polyarthritis and Ross River virus disease. Clin. Rheum. Dis. 1986, 12, 369–388.
- Vale, T.G.; Carter, I.W.; McPhie, K.A.; James, G.; Cloonan, M.J. Human arbovirus infections along the south coast of New South Wales. Aust. J. Exp. Biol. Med. Sci. 1986, 64, 307–309.
- Russell, R.C. Arboviruses and their vectors in Australia: An update on the ecology and epidemiology of some mosquito-borne arboviruses. Rev. Med. Vet. Entomol. 1995, 83, 141–158.
- Gyawali, N.; Bradbury, R.S.; Taylor-Robinson, A.W. The epidemiology of dengue infection: Harnessing past experience and current knowledge to support implementation of future control strategies. J. Vector Borne Dis. 2016, 53, 293–304.
- Naish, S.; Tong, S. Hot spot detection and spatio-temporal dynamics of dengue in Queensland, Australia. In Proceedings of the ISPRS Technical Commission VIII Symposium, Hyderabad, India, 9–12 December 2014; Dadhwal, V.K., Diwakar, P.G., Seshasai, M.V.R., Raju, P.L.N., Hakeem, A., Eds.; International Society of Photogrammetry and Remote Sensing. pp. 197–204.
- Gyawali, N.; Bradbury, R.S.; Taylor-Robinson, A.W. Knowledge, attitude and recommendations for practice regarding dengue among the resident population of Queensland, Australia. Asian Pac. J. Trop. Biomed. 2016, 6, 360–366.
- Hawkes, R.A.; Boughton, C.R.; Naim, H.M.; Wild, J.; Chapman, B. Arbovirus infections of humans in New South Wales. Seroepidemiology of the flavivirus group of togaviruses. Med. J. Aust. 1985, 143, 555–561.
- Mackenzie, J.; Lindsay, M.; Coelen, R.; Broom, A.; Hall, R.; Smith, D. Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol. 1994, 136, 447–467.
- Guard, R.W.; McAuliffe, M.; Stallman, N.; Bramston, B. Haemorrhagic manifestations with Sindbis infection. Case report. Pathology 1982, 14, 89–90.
- Aaskov, J.G.; Phillips, D.A.; Wiemers, M.A. Possible clinical infection with Edge Hill virus. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 452–453.
- Boughton, C.R.; Hawkes, R.A.; Naim, H.M. Illness caused by a Kokobera-like virus in south-eastern Australia. Med. J. Aust. 1986, 145, 90–92.
- Australian Government Department of Health. Australian National Notifiable Diseases by Disease Type. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-distype.htm (accessed on 22 April 2022).
- Colmant, A.M.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Suen, W.W.; O’Brien, C.A.; van den Hurk, A.F.; Hall, R.A. A newly discovered flavivirus in the yellow fever virus group displays restricted replication in vertebrates. J. Gen. Virol. 2016, 97, 1087–1093.
- Johansen, C.A.; Williams, S.H.; Melville, L.F.; Nicholson, J.; Hall, R.A.; Bielefeldt-Ohmann, H.; Prow, N.A.; Chidlow, G.R.; Wong, S.; Sinha, R.; et al. Characterization of Fitzroy River virus and serologic evidence of human and animal infection. Emerg. Infect. Dis. 2017, 23, 1289–1299.
- Doherty, R.L.; Gorman, B.M.; Whitehead, R.H.; Carley, J.G. Studies of arthropod-borne virus infections in Queensland. V. Survey of antibodies to group A arboviruses in man and other animals. Aust. J. Exp. Biol. Med. Sci. 1966, 44, 365–377.
- Inglis, T.J.; Bradbury, R.S.; McInnes, R.L.; Frances, S.P.; Merritt, A.J.; Levy, A.; Nicholson, J.; Neville, P.J.; Lindsay, M.; Smith, D.W. Deployable molecular detection of arboviruses in the Australian Outback. Am. J. Trop. Med. Hyg. 2016, 95, 633–638.
- Vale, T.G.; Spratt, D.M.; Cloonan, M.J. Serological evidence of arbovirus infection in native and domesticated mammals on the south coast of New South Wales. Aust. J. Zool. 1991, 39, 1–7.
- Anderson, S.G. Murray Valley encephalitis and Australian X disease. Epidemiol. Infect. 1954, 52, 447–468.
- Doherty, R. Arboviruses of Australia. Aust. Vet. J. 1972, 48, 172–180.
- Inglis, T.J. Climate change and infectious diseases in Australia. Aust. Prescr. 2009, 32, 58–59.
- Van den Hurk, A.F.; Craig, S.B.; Tulsiani, S.M.; Jansen, C.C. Emerging tropical diseases in Australia. Part 4. Mosquito-borne diseases. Ann. Trop. Med. Parasitol. 2010, 104, 623–640.




