Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | KONSTANTINOS TSIANTAS | -- | 5377 | 2022-04-20 13:48:36 | | | |
| 2 | Rita Xu | -3 word(s) | 5374 | 2022-04-21 05:48:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsiantas, K.; Zoumpoulakis, P.; Konteles, S.; Kritsi, E.; Sinanoglou, V.; Tsiaka, T. Lipids and Gut Microbiota Alterations. Encyclopedia. Available online: https://encyclopedia.pub/entry/22020 (accessed on 07 February 2026).
Tsiantas K, Zoumpoulakis P, Konteles S, Kritsi E, Sinanoglou V, Tsiaka T. Lipids and Gut Microbiota Alterations. Encyclopedia. Available at: https://encyclopedia.pub/entry/22020. Accessed February 07, 2026.
Tsiantas, Konstantinos, Panagiotis Zoumpoulakis, Spyros Konteles, Eftichia Kritsi, Vassilia Sinanoglou, Thalia Tsiaka. "Lipids and Gut Microbiota Alterations" Encyclopedia, https://encyclopedia.pub/entry/22020 (accessed February 07, 2026).
Tsiantas, K., Zoumpoulakis, P., Konteles, S., Kritsi, E., Sinanoglou, V., & Tsiaka, T. (2022, April 20). Lipids and Gut Microbiota Alterations. In Encyclopedia. https://encyclopedia.pub/entry/22020
Tsiantas, Konstantinos, et al. "Lipids and Gut Microbiota Alterations." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Advances in sequencing technologies over the past 15 years have led to a substantially greater appreciation of the importance of the gut microbiome to the health of the host. Recent outcomes indicate that aspects of nutrition, especially lipids (exogenous or endogenous), can influence the gut microbiota composition and consequently, play an important role in the metabolic health of the host. Thus, there is an increasing interest in applying holistic analytical approaches, such as lipidomics, metabolomics, (meta)transcriptomics, (meta)genomics, and (meta)proteomics, to thoroughly study the gut microbiota and any possible interplay with nutritional or endogenous components.
nutrition
gut microbiota
phytosterols
fat-soluble vitamins
carotenoids
1. Introduction
Currently, more and more researchers are embracing the view that microbes are equally as important for the human body as cells. Among the systems that harbor microbes, the gut comprises the densest populated microenvironment, consisting of more than 3.8 × 1013 microorganisms [1], while the collected genetic material of all gut microorganisms constitutes the gut microbiome (GM). In addition, the human diet contains compounds (i.e., carotenoids, polyphenols, and dietary fibers), that are not digested by human enzymes, reaching the gut intact, where they are further catabolized by the microbiome, resulting in the production of unique metabolites. Interestingly, these gut-produced metabolites, along with the host’s other metabolites, shape the metabolic signature of the host, which can be mapped through the analyses of various biological fluids, such as urine, plasma, and feces. Taking into account the complexity of the GI tract, it is quite apparent that it is almost impossible to identify or quantify all the metabolites present in a biological sample. To date, state-of-the-art technological platforms (i.e., metabolomics, metagenomics, and transcriptomics) can be used in order to monitor and describe the unique and highly dynamic metabolic processes or pathways that occur in the human gut. Moreover, the implementation of -omics approaches enable the detection of a wide spectrum of different metabolites in various tissues [2][3].
One of the most important factors shaping the composition and, consequently, the properties of the intestinal microbiome is dietary lipids [4]. For instance, high-fat diets are suspected to play a role in the promotion of gut dysbiosis, which is defined as the imbalance of microbial populations in favor of pathogenic communities, while several dietary lipids (i.e., phytosterols and carotenoids) may reverse these effects [5][6]. Lipids are organic bio-molecules, which play a variety of important biological roles, such as energy saving, maintaining the integrity of membranes, and transporting and degrading other compounds. The term “lipidome” refers to (a) either the lipids originating from anabolic and catabolic pathways (endogenous lipids) or (b) the uptake of exogenous lipids through diet (dietary lipids), while “lipidomics” is a term used to present the current analytical framework applied in order to explain alterations that involve the lipidome [7].
Lipidomics provides new approaches to screen the metabolic pathways of lipids and therefore helps to understand lipid metabolism and its role in health and disease through the detection of lipid metabolites or other nutritional biomarkers [2][8]. In addition, considering the significant impact of diet in lipid metabolism, clinical lipidomics is a new integrative biomedicine field focused on the combination of lipid science with clinical medicine and nutrition [9]. This type of lipidomics is considered to be the answer to why certain types of diets, foods or even nutrients promote or inhibit the development of various gut-related diseases.
Application-wise, the combination of -omics techniques with high-throughput lipidomics can maximize their potential by developing tools which will help to achieve the desired comprehensive lipid analysis. However, it is essential to overcome specific limitations that may arise during experimental design or analysis. For example, the isomeric diversity of specific lipids (mostly fatty acids) as well as the differences between mass spectrometer ion sources need to be addressed in order to allow lipidomics to rapidly progress [10]. In addition, the lack of corresponding internal standards can be a real setback and may lead to quantitative inaccuracies due to the high sample complexity [11]. This is why an integrated, multifocal lipidomics platform must be very carefully designed in order to provide useful, reliable, and reproducible results and to extract as much information as possible. For that reason, targeted (determination of specific compounds) and untargeted (holistic) approaches, using GC/LC-MS2 techniques, are combined in metabolomics studies [10]. Regarding endogenous lipids, a new analytical field, known as lipid mediator (LM) metabolomics or metabololipidomics, is gaining ground. The expansion and implementation of this promising field will: (a) shed light on the pathways (biosynthesis or a biological role in inflammation) of bioactive lipids, suggesting novel pre-resolving mechanisms by which the host responds during inflammation, tissue damage, or the disturbance of homeostasis (gut dysbiosis) [12], (b) establish a benchmark for novel active resolution pharmacology approaches to control or even treat gut-related diseases, and (c) allow the direct correlation and assessment of the personalized metabolome with medicine and nutrition without the need for conjectures.
Despite the conflicting views that prevailed for years, lipids are now classified into eight major groups (1: fatty acyls-FA, 2: Glycerolipids-GL, 3: glycerophospholipids-GP, 4: sphingolipids-SP, 5: sterol lipids-ST, 6: prenol lipids-PR, 7: saccharolipids-SL, and 8: polyketides-PK) and several sub-classes (fatty acids, mono-, di-, or triglycerides, ceramides, isoprenoids, and acrylaminosugars). Assaying the impact of different dietary habits on configuring the intestinal microbial profile, the key role of lipid nutrients in host health management and in disease prevention must be underscored. Due to the Westernization of the human diet [13], researchers have scrutinized the effect of polar lipid intake, mainly fatty acids (i.e., ω-3, ω-6 PUFAs, MUFAs, etc.) and phospholipids, on the modification of gut microflora and on the maintenance of intestinal immunity and homeostasis [13]. The overall impact of an unhealthy nutritional lifestyle includes the increase in non-commensal (i.e., Firmicutes and Proteobacteria) bacteria, intestinal barrier dysfunctions, the decrease in gut microbiota diversity and intestinal immunity, the reduction in the mucus layer, the lower levels of bacteria-generated butyrate, and the stimulation of chronic inflammation pathways [14]. On the other hand, the balanced supplementation of phospholipids and the ω-3/ω-6 PUFAs ratio (in favor of the ω-3 fatty acids) increase the abundance of commensal bacteria (i.e., Bifidobacterium, Akkermansia) and reduce the Firmicutes-to-Bacteroidetes ratio (F/B ratio) [15], precluding the onset of various non-communicable gut-related disorders [16][17][18][19][20][21][22][23][24][25][26][27][28][29].
However, only non-polar dietary or amphoteric endogenous lipids were examined (Figure 1). This decision was based: (a) on the already existing huge amount of published data regarding gut-related interactions with more polar lipid categories, such as fatty acids, phospholipids, and short-chain fatty acids or cholesterol, and at the same time (b) on the lack of collective knowledge regarding the interrelationship of the lipids under study, gut microbiota, and host’s health state, which underlined the need for further investigation [13][15][30].
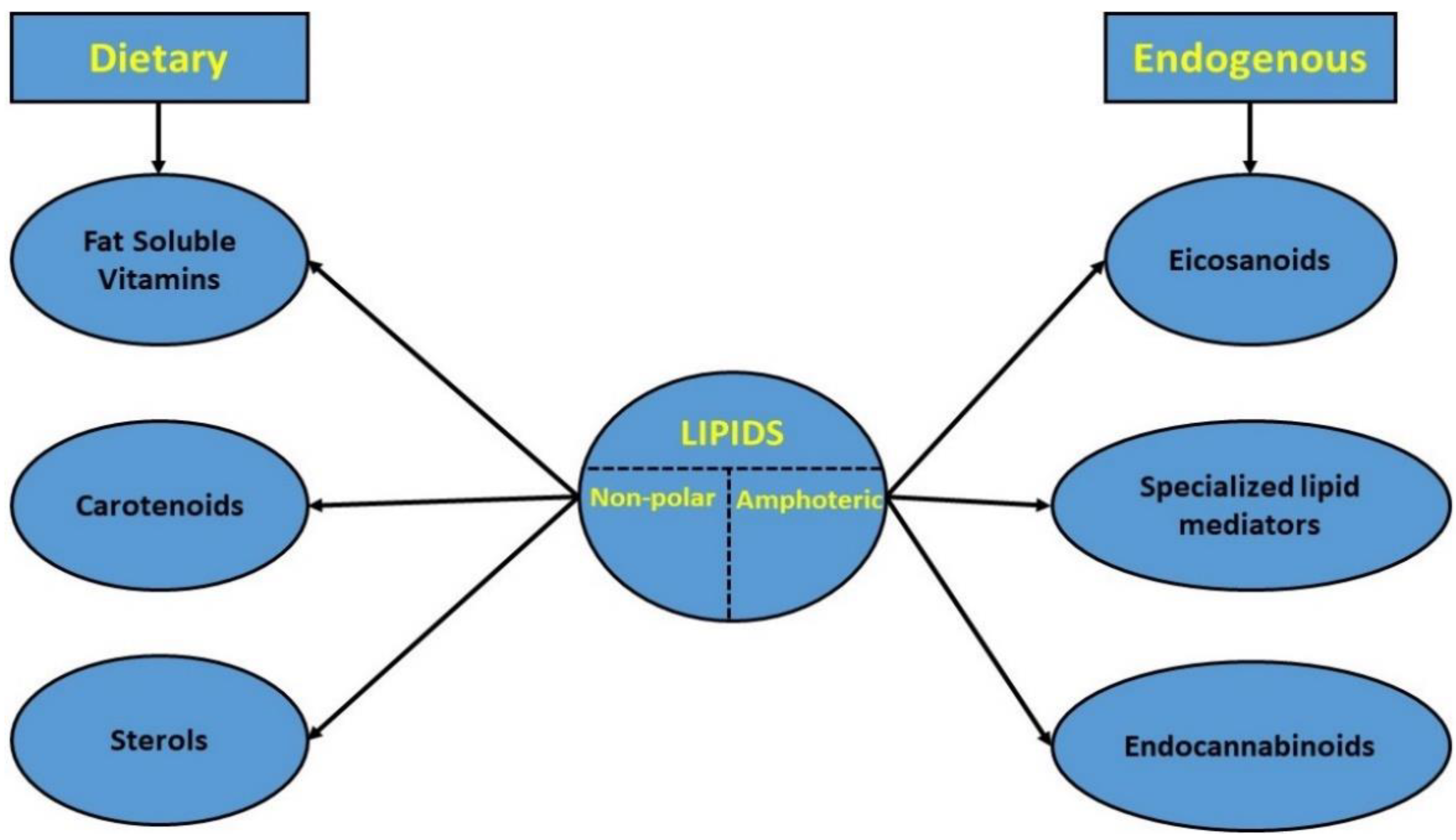
Figure 1. Classification of the studied lipid categories.
In particular, the dietary intake of these lipids could serve as a modulation strategy of gut microbiota functional ecology, to counteract any possible adverse health-related outcomes [30]. Nonetheless, data from both animal models and human interventions are still elusive and the effects of these nutrients are understudied. For example, despite the strong evidence that sterols (in particular phytosterols) affect the intestinal microbiome and the metabolism of the host by regulating microbiota composition (i.e., increase in Bacteroides, Coprococcus, Oscillospira, Lactobacillus and Akkermansia and decrease in Desulfovibrio genus, in a dose-dependent manner in the sterol-fed group), and cholesterol synthesis [31][32], the involved mechanisms and interactions have not been fully elucidated. Additionally, the metabolic fate and the effect on the intestinal microflora (and vice versa) of fat-soluble vitamins (FSVs) is still unclear. Recent findings show that this bidirectional relationship enhances important biological processes that take place in the gut (regulation, activation, and production of FSVs in the gut). In turn, these processes trigger many pivotal FSV-related functions, such as (i) the improvement of intestinal barrier integrity, (ii) the modulation of gut microbiota composition (i.e., increased Proteobacteria in the case of a high intake of vitamin D or increased Sutterella in the case of a lower intake of vitamin E), and (iii) the regulation of the immune and inflammatory response [33][34]. The landscape is similar for carotenoids. So far, carotenoids’ effect on gut microbiota composition has been investigated through (mainly) animal and human interventions focusing on specific metabolic diseases (i.e., obesity, diabetes type 2, etc.) or on diseases associated with metabolic syndromes, such as nonalcoholic fatty liver disease (NAFLD)).
At the same time, even less is known regarding the interplay between amphoteric endogenous lipids (i.e., eicosanoids, endocannabinoids, and specialized pro-resolving lipid mediators (SPMs)), the gut microbiota, and nutrition patterns. At present, the research interest in such molecules is mainly focused on their ability to act as “mediators” during the manifestation of various inflammatory conditions, related to either the intestine or the various axes where gut microbiota participate (gut–brain, gut–retinal, gut–kidney, and gut–liver). In any case, nutrition remains the most important factor that regulates this bidirectional relationship. Therefore, the employment of high-throughput lipidomics is crucial in order to further investigate the role of endogenous lipids in the prο- and anti-inflammatory pathways, as well as to mark novel prognostic markers of gut function.
2. Characteristics of the GI Microbiota
2.1. An Insight into Gut: What We Have Learnt So Far?
Although the definitions of the terms “microbiome” and “microbiota” are clearly different, these terms are commonly used interchangeably [35]. Nowadays, the study of the composition, structure, and functional properties of the human microbiome is a rapidly evolving scientific field. It is worth mentioning that the relationship between commensal bacterial and the host is an extremely dynamic system in which an intricate and mutually beneficial relationship, also known as symbiosis, is established [36]. The importance of this dynamic ecosystem is inextricably linked to various basic primary, as well as secondary functions, including the metabolism, immune system protection, the structural integrity of the epithelial barrier, and gut–brain axis communication [37] (Figure 2).
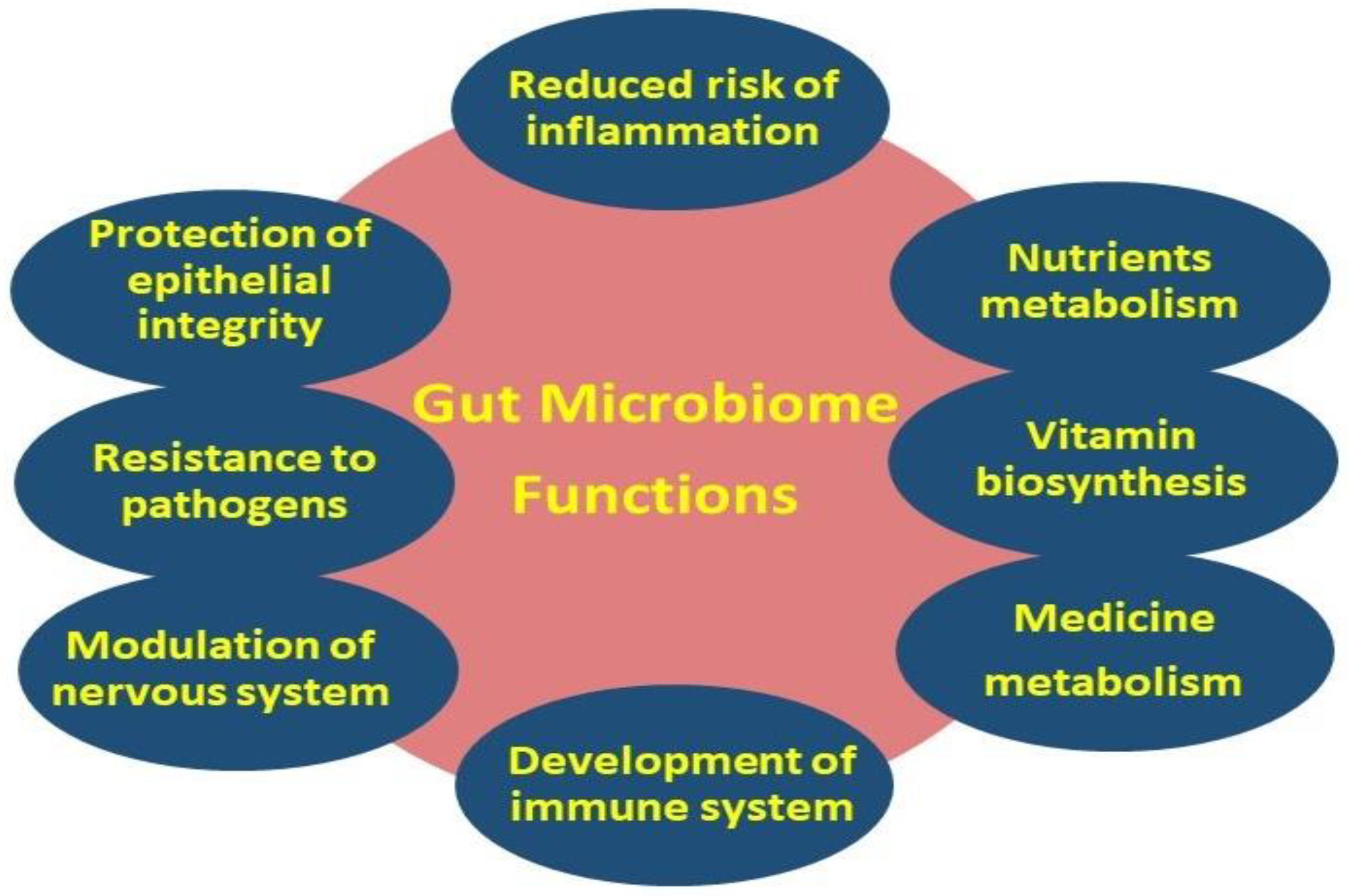
Figure 2. Primary (i.e., metabolism, gut–brain axis, and protection of epithelial integrity) and secondary (i.e., nutrients, vitamin and medicine metabolism, regulation of the immune and nervous systems, and resistance to pathogens) gut microbiome functions.
There is growing evidence that several gut disorders involve not only the GI system but distant organs as well [38]. Through a complex communication that includes the central nervous system as well as the autonomic and the intestinal nervous system, two-way interactions are created which affect both the gut microbiome and the lipids. Moreover, intestinal immune cells as well as the enteric nervous system affect the metabolism, absorption, and distribution of lipids, since they are key regulators of gut homeostasis [39]. Most recent studies link the gut with brain function (gut–brain axis), the host immune response, cell proliferation and vascularization, the regulation of intestinal endocrine functions, the modulation of energy biogenesis, the vitamin biosynthesis, and bile salts metabolism [40][41][42][43][44]. Focusing especially on lipid constituents, the gut–brain axis has the ability to regulate endogenous lipids (i.e., endocannabinoids, and SPMs) making them act “on demand” by exerting various bioactive properties, such as pro- or anti-inflammatory activities on the gut microbiota and immune system.
2.2. Gut Microbiota Stability and Composition: A Key Player in Various Gut-Related Diseases
As already stated, the gut microbiota presents a dynamic equilibrium that has adapted to harmoniously colonize the GI tract (symbiosis) [45]. Alteration in gut microbiota homeostasis can lead to undesirable situations, generally known as dysbiosis and abnormalities in the immune response of the intestinal microbiome. Gut dysbiosis is related to several chronic inflammatory conditions, also known as inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s Disease (CD). Moreover, various multi-factorial diseases or metabolic disorders (e.g., duodenum cancer, obesity, diabetes, and metabolic and immune-mediated disorders) are linked to microbial imbalances, which are also associated with the intake of lipids and their interactions with certain bacterial populations, highlighting the need to further investigate the underlying mechanisms [46].
Taking into account some unquestionable data regarding the structure, functionality, and anatomy of the GI system, it is widely accepted that the latter is divided into the stomach, small intestine, which is further divided into (a) duodenum, (b) jejunum, and (c) ileum, and large intestine (LI), which includes the colon and cecum. Every “compartment” is characterized by different conditions, such as pH, nutrient availability, or oxygen availability, and thus, each organ promotes the growth of specific microbes. Despite the fact that the gut environment favors the growth of bacteria from seven predominant phyla (e.g., Firmicutes, Bacteroides, Actinobacteria, Fusobacteria, Proteobacteria, Verrucomicrobia, and Cyanobacteria), its diversity is limited since more than 85% of the total population is constituted by Bacteroides and Firmicutes [47]. More specifically, the species of Bacteroides and Firmicutes phyla belong to the genera (a) Bacteroides and Prevotella and (b) Clostridium, Eubacterium and Ruminococcus, respectively. The major genus belonging to the phylum Actinobacteria in the human gut is Bifidobacterium, while Actinobacteria contribute to a small fraction of the total bacteria [48]. In Table 1, the different major phyla and bacterial genera that colonize each organ of the GI system are summarized.
Table 1. Bacteria phyla and genera in the GI tract.
| Major phyla | Stomach | Duodenum | Jejunum | Ileum | Cecum | Colon | Refs. |
|---|---|---|---|---|---|---|---|
| Firmicutes | √ | √ | √ | √ | √ | [49][50] | |
| Bacteroides | √ | √ | √ | √ | |||
| Actinobacteria | √ | √ | √ | √ | |||
| Fusobacteria | √ | √ | |||||
| Proteobacteria | √ | √ | √ | ||||
| Bacterial genera | Stomach | Duodenum | Jejunum | Ileum | Cecum | Colon | Refs. |
| Lactobacillus | √ | √ | [51][52] | ||||
| Enterococcus | √ | √ | |||||
| Streptococcus | √ | √ | |||||
| Bacteroides | √ | ||||||
| Bifidobacterium | √ | ||||||
| Actinomycinae | √ | ||||||
| Peptostreptococcus | √ | ||||||
| Prevotella | √ | ||||||
| Veillonella | √ | ||||||
| Rothia | √ | ||||||
| Haemophilus | √ | ||||||
| Mucosa genera | Stomach | Duodenum | Jejunum | Ileum | Cecum | Colon | Refs. |
| Lactobacillus | √ | [53] | |||||
| Akkermansia | √ | ||||||
| Clostridium | √ | √ | |||||
| Enterobacteriaceae | √ | √ | √ | √ |
However, despite the various bacteria that colonize the GI system, even pathogen microorganisms can be found within it (i.e., E. coli, H. pylori, C. jejuni, S. enterica, and B. fragili) [54]. Furthermore, the fact that Firmicutes and Bacteroides are the predominant bacteria should not be considered as an infallible view, since significant differences can be observed in other phyla because of: (a) the current physio-pathological conditions, (b) the age (i.e., the microbiota is enriched during lactation and early years) and (c) the genetic background of the host, (d) the role of nutrition, and (e) geographical factors (i.e., levels of both Firmicutes and Proteobacteria were higher in European children, while Firmicutes were absent in West African children) [55].
3. Dietary (Exogenous) Non-Polar Lipids
As has already been stated, lipid supplementation through the diet can affect (a) gut microbiota composition, (b) the metabolic end products, (c) other enzymatic indicators (i.e., alkaline phosphate (ALP), aspartate transaminase (AST), alanine transaminase (ALT), and high- or low-density lipoproteins (HDL-LDL)), and thus (d) the fate of gut-related diseases [5][14][48]. In this direction, a thorough review of the literature was conducted in order to evaluate the relationship between the intestinal microbiome and dietary non-polar lipids, such as (phyto)sterols, fat-soluble vitamins, and carotenoids.
3.1. Dietary Sterols: Are They an Inducer of Gut Dysbiosis?
Sterols, similarly to cholesterol, play an important role in the structure, integrity and properties of membranes. Phytosterols, which are plant-derived sterols, are found in abundance in Mediterranean diet models that promote beneficial changes in bacterial communities, while they are not present in a Western diet (high fat and cholesterol) [56]. In total, 20–80% of the cholesterol consumed daily (average recommended intake of 300 mg cholesterol per day) is absorbed, while the microbial absorption of phytosterols is only 2–3% (average intake of phytosterols is less than 500 mg per day) [57][58], which means that non-absorbed sterols can be further processed by the gut microbiome.
In particular, phytosterols are naturally occurring structural analogues of cholesterol, involved in altering certain lipid metabolic pathways. Thus, they are strongly related to the regulation of intestinal ecosystem and to the reduction in high hepatic cholesterol levels, which promotes gut dysbiosis in various liver abnormalities, such as steatosis, cirrhosis, liver failure, NASH, NAFLD, and hepatocellular carcinoma [59][60]. The manifestation of these pathologies is associated with the depletion of Bacteroides and Bifidobacterium and the increased richness of Mucispirillum, Desulfovibrio, Anaerotruncus, and Desulfovibrionaceae. Updated evidence has confirmed the detrimental effect of dietary cholesterol in microbial populations and in gut bacterial metabolites (taurocholic acid (TCA) and 3-indolepropionic acid (IPA)) [61].
Nonetheless, according to estimations, the dietary intake of PS (150–400 mg phytosterols/day) does not reach the necessary established levels (1500–3100 mg phytosterols/day) in order to exert its hypocholesterolemic effect (and receive the corresponding health claim). Consequently, the above-mentioned levels can only be achieved in the daily diet through PS-enriched foods, such as dairy products (PS-enriched milk, cheese, and fermented milk products). Focusing on in vitro studies, Cuevas-Tena et al. [62] investigated the impact of plant sterol enrichment dose on the gut microbiota of lean and obese subjects using an in vitro fermentation model, also known as TIM-2. In this entry, the “PS-enriched” supplement, but also β-sitosterol alone, was able to increase the proportion of the genera belonging to the Firmicutes phylum. This increase suggested a potential modification of the short-chain fatty acids (SCFAs) and of the microbial profile of both lean and obese populations. However, the authors suggest that the daily intake of PS over several weeks and the different fecal inocula may lead to different effects on gut microbiota composition. One year earlier, the same research team revealed that the presence of PS during batch-culture fermentation led to a decrease in Erysipelotrichaceae species and an increment in Eubacterium hallii [63].
Meanwhile, another in vitro dynamic model was used in order to examine the impact of plant-sterol- and galactooligosaccharide-enriched beverages on colonic metabolism and composition [64]. According to the authors, a higher diversity in the gut microbiome was found in the transverse and descending colon, where the production of sterol metabolites (coprostanol, methylcoprostanol, and sitostenone) also took place. In addition, despite the fact that the prebiotic effect of galactooligosaccharides was not detected, alterations in gut microbiota (an increase in the Parabacteroides genus and the Synergistaceae and Lachnospiraceae families) denoted an enhancement of sterol metabolism.
Furthermore, recent in vitro and in vivo studies confirmed that phytosterols, mainly β-sitosterol and stigmasterol, promoted gut symbiosis in cases of morbid obesity and hypercholesterolemia, by reducing the levels of the bacterial family Erysipelotrichaceae [65]. The supplementation of β-sitosterol in ruminants (sheep) lowered the abundance of the family Lachnospiraceae and increased the proportion of the genera Prevotella (Bacteroidetes phylum), presumably through the consequent increase in ruminal pH incited by the enrichment of the genus Selenomonas [66]. Although high-fat diets shift the F/B ratio toward the Firmicutes phylum in hamster models, plant sterols (i.e., soybean sterols) significantly attenuated this imbalance and improved gut microbiota diversity and richness of bacterial microenvironment (increase in Bacteroides, Coprococcus, Oscillospira, Lactobacillus, Coprobacillus, Akkermansia, and Allobaculum genera levels). The increased populations of these genera may present alleviating effects against high-fat-diet-related diseases, such as hypercholesterolemia and dyslipidemia [31][67].
Further intervention studies highlighted the potential modulating activity not only of free phytosterols, but also of their esters and their fully saturated derivatives, known as phytostanols. Namely, the relative abundance of Anaerostipes and Bacteroidetes species was increased in a high-dose diet of phytosterol esters (i.e., steryl esters). Phytosterol esters’ regulatory action was intertwined, via bile acid metabolism, with hepatic steatosis prevention in adult participants [68]. Sitostanol also increased the levels of Bacteroidetes communities, while campestanol uptake reduced the quantity of SCFA butyrate, produced by Firmicutes species in human clinical studies [5][68][69]. Apart from being dietary derivatives of phytosterols, 5α/β stanols (coprostanol, cholestenol, 5α/β-sitostanol, 5α/β-campestanol), detected in human feces, can also be gut-produced metabolites of sterols and, thus, potential biomarkers of bacterial metabolism [70].
In summary, although the exact associations of (phyto)sterols and the intestinal microbiome are still under study, there is enough evidence showing that these compounds are excellent regulators of cholesterol and potential modifiers of the gut microbiota composition. At the same time, even though the body of evidence regarding the impact of phytosterols on gut microbiota alterations and on diet-induced health or disease conditions is growing, there are a limited number of well-designed and controlled human studies. Since the current knowledge concerning the use of phytosterols as new therapeutic targets remains quite an unexplored domain, further focus is required to classify phytosterols as phyto-therapeutics in the foreseeable future [5][65].
3.2. Fat-Soluble Vitamins (FSVs): The Master Player in Nutrition–Gut Microbiome Tug-of-War
According to an increasingly large body of clinical findings, malnutrition, especially the low supply of non-energy-delivering micronutrients, such as vitamins, is negatively affecting the configuration of gut microbiota diversity and the intestinal health. Vitamin deficiency plays an important role in the pathogenesis of several diseases, namely neuropsychiatric disorders (depression, autism, Parkinson disease, schizophrenia, and multiple sclerosis), cardiometabolic disorders, complications of lipid metabolism (metabolic syndrome, obesity, and hepatic disease), and child development impairments in different age groups [14][71][72]. Of note, vitamins also manipulate the communities of the micro-ecosystems of mothers during pregnancy and of their offspring, both postpartum and during early childhood. For instance, vitamin D and retinol favor the growth of Actinobacteria and Proteobacteria, while vitamin E depleted them (mainly Proteobacteria) [73]. To date, mostly water-soluble vitamins (primarily those of B-group) have been in the spotlight of extensive research. However, many questions are left to be answered regarding the links between the intake or deficiency of fat-soluble vitamins, the resulting modification of the gut microbial ecosystem, and the contingent manifestation of various pathologies.
The Mediterranean diet is recommended as the ideal nutritional pattern in order to cope with the lack of FSVs, which are present in food items, such as vegetables, fruits, nuts, olive oil, dairy products, and fishes. The mutualistic interaction between vitamin uptake and gut microbiota composition is outlined with two different, yet firmly interrelated notions: (a) the impact of vitamins on shaping the microbial profile of pathogenic and nonpathogenic bacteria and (b) the role of microbiota in the synthesis, shuttling, and metabolism of vitamins and their metabolites [72]. Based on a brief overview of the impact of FSVs on microbial populations and health status control, the current data are quite controversial. On one hand, the administration of vitamins D, A, and K favored the prevalence of Lactobacillus. Nonetheless, in some cases, the intake of FSVs led to the increase of opportunistic pathogens or the depletion of synergistic bacteria belonging to several bacterial categories, such as Proteobacteria, Deferribacteres, Enterobacteriacae, Clostridiaceae, Ruminococcus, and Odoribacter, or Verrucomicrobia, Bifidobacterium, and symbiotic Bacteroidetes, respectively [14].
3.2.1. Vitamin A
Vitamin A (retinol) and its enzymatic oxidation product (retinoic acid) play a key role in the intestinal immune response through interactions with the intestinal microbiome [74]. A sheep model confirmed the potential of vitamin A as a putative diagnostic indicator for male infertility. The abnormalities in its absorption were linked to the deregulation of bile acid metabolism, which is related to lower levels of Ruminococcaceae [75]. The inclusion of vitamin A in obesogenic diet patterns in three-week-old male C57BL/6J mice precluded changes in microbiota α-diversity and enriched the abundance of Lachnospiraceae [76]. Another study, targeting the investigation of gut microbiota alterations at different lifetime points, demonstrated that vitamin A insufficiency played a pivotal role in the embryonic but also in the early-stage development of four-week-old healthy rats. Especially in the periods of gestation, lactation, and weaning, the populations of Diaphorobacter and Psychrobacter (increase) or Propionibacterium, Ochrobactrum, Enterobacter, and Staphylococcus (increase) were affected. Τhe effect of vitamin A was imprinted in the serum metabolome by the presence of retinol, which presented a positive and a negative correlation with Faecalibacterium and Staphylococcus, respectively [77].
3.2.2. Vitamin E
Vitamin E is considered a group of fat-soluble compounds and includes two main sub-categories: (a) α-, β-, γ-, and δ-tocopherols (TOHs) and (b) α-, β-, γ-, and δ-tocotrienols (T3), which are mainly presented in edible oils and several nuts [78]. Among these, a-tocopherol is one of the most important fat-soluble antioxidants of cellular membranes as it is the most biologically active form retrieved from human tissues. Additionally, it accounts for approximately 90% of the total vitamin E of the body [79].
In an experimental model, where five-week-old C57BL/6 male mice followed a high- and low-vitamin E diet, the phyla Bacteroidetes and Verrucomicrobia (Akkermansia muciniphila species) were related to lower body weight. More specifically, a dose-dependent relationship was highlighted between α-tocopherol and different gut microbial compositions, as the authors observed an increase in Proteobacteria and a decrease in Verrucomicrobias phylum [80]. Another study revealed that α-tocopherol supplementation was associated with changes in gut microbiota composition. Particularly, it was shown that a-tocopherol can reduce levels of Bacteroides and Lactobacillaceae, as well as the F/B ratio in humans [81]. δ-Tocotrienol, and its hydrogenated metabolite present in human feces, δTE-13′-carboxychromanol, can be considered as starting points against tumor growth [82]. Although they showed no significant effect on bacterial richness, they exhibited a modulating role in gut microbiota composition, by promoting the increase in health-promoting Lactococcus and Bacteroides. Focusing on δTE-13′-carboxychromanol, this tocotrienol metabolite counterbalanced the reduction in Roseburia in IBD patients and uniquely facilitated the elevation of Eubacterium coprostanoloi gene levels [82].
3.2.3. Vitamin K
Vitamin K consists of vitamin K1 (phylloquinone, PKs) and vitamin K2 (menaquinone, MKs). Vitamin K1 is a naturally occurring compound in green leafy vegetables, as it is directly related to photosynthesis, while vitamin K2 is found in animal products. Apart from their intake through diet, menaquinones (ΜΚs) are also bacterial products of vitamin K, able to be remodeled in vivo. As proved by certain studies, vitamin K deficiency mostly affects female microbial composition with increased levels of Lachnospiraceae and Ruminococcaceae families [83]. A metagenomic analysis of the gut microbiota profiles of healthy volunteers and type 2 diabetes mellitus patients underlined the vital role of the phyla Actinobacteria, Bacteroidetes, and Firmicutes, mainly the Erysipelotrichaceae and Corynebacterium taxa, in the metabolic functionality of the diabetic gut microbiome related to the production of menaquinones [84]. According to the results of the aforementioned study, vitamin K2 emerged as a novel biomarker in the treatment of diabetes mellitus, also exerting other beneficial activities, such as enabling insoluble fiber digestion and refining immunomodulatory and nutritive molecules, such as SCFAs. Notably, MKs play a key role in gut microbiota homeostasis, promoting the growth of symbiotic bacteria. MK-7, one of the most studied vitamin K-related compounds, was reported to have protective effects against colon cancer during a study in male C57BL/6J mice [85]. In particular, the authors noticed a reduction in bacterial species promoting colorectal cancer, such as Helicobacter apodemus, Helicobacter mesocricetorum, Allobaculum stercoricanis, and Adlercreutzia equolifaciens.
3.2.4. Vitamin D
Despite the well-known contribution of vitamin D to calcium homeostasis and bone health [86], the forms of this vitamin (calcitriol, cholecalciferol-vit-D3, and ergocalciferol-vit-D2) also participate in the regulation of: (a) blood pressure, (b) inflammation, (c) immune response, and, most recently, (d) gut microbiota [87][88][89][90]. Unlike vitamins A, E, and K, which were supplemented mainly in animal studies, vitamin D has a leading role, among lipid-soluble vitamins, in human clinical interventions. The aligned data in the literature provide a comprehensive insight into the crosstalk of the gut microbiota and vitamin D, primarily concerning the downregulation of inflammatory pathways. Though the effect of the gut microbiota signature on vitamin D metabolism is relatively established knowledge, the impact of vitamin D on gut microbial populations is still quite an uncharted field [91].
The administration of vitamin D in Crohn’s disease patients in remission positively affected bacterial taxa and the abundance of Megasphaera and Lactobacillus. However, no changes were observed in the gut microbiota diversity of ulcerative colitis (UC) patients, despite the major increase in Enterobacteriaceae [92]. Oral supplementation of vitamin D3 in a study including twenty adults resulted in a dose-dependent increase in serum D3 metabolite, 25-hydroxyvitamin D [25(OH)D]. Consequently, this led to the enrichment of Bacteroides and Parabacteroides abundance, which was associated with the alleviation of IBD symptoms [93]. However, seasonal sunshine variability (winter vs. summer) is responsible for the fluctuations in the levels of circulating 25-hydroxyvitamin D in IBD patients. Thus, a cohort study that evaluated the effect of seasons on the relationship between vitamin D levels and gut microbiota, covarying in intestinal metabolic derangements, suggested that higher levels of sunshine reduced pathogenic genera, such as Fusobacterium, Collinsella aerofaciens, Eggerthella lenta, Bacteroides, Helicobacter, Faecalibacterium prausnitzii, and Rhodococcus, and increased species of Pediococcus, Clostridium, and Escherichia/Shigella [94]. Faecalibacterium and Akkermansia species, which were increased after D3 intake, also influenced the immune responses and health status in autoimmune intestinal pathologies, such as UC syndromes [92].
As proved in in vivo studies (three-week-old male C57/bl6 mice) related to the microbiota–pain interrelationship, suboptimal levels of vitamin D resulted in a restricted microbial diversity and in an increase in F/B ratio [95]. A multi-vitamin dietary supplement, including vitamin D and vitamin B, was administrated in overweight individuals. Shifts were observed in one phylum (Actinobacteria decrease) and three families (Actinomycetaceae, Bifidobacteriaceae, and Corynebacteriaceae decrease) after vitamin D supplementation, and in three phyla (Bacteroidetes increase, Cyanobacteria and Proteobacteria decrease) and three families (Christensenellaceae, Lachnospiraceae, and Enterobacteriaceae decrease) after a combined vitamin D and B supplementation [96]. A cirrhotic rat model suggested that calcitriol, the active form of vitamin D3, controlled bacterial translocation and gut permeability and enriched the populations of Bacteroidales, Allobaculum, Ruminococcaceae, Muribaculaceae, and Anaerovorax [97]. Recent studies in NAFLD subjects verified the impact of vitamin D in the delay of cell death caused by inflammation, through the remodeling of the relative bacterial abundances in favor of Lactobacillus and against Acetatifactor, Oscillibacter, and Flavonifractor [98].
Based on official guidelines, vitamin D is an essential nutrient in pre- and post-natal maternal diet and infant formulas, as the infant microbiome is rapidly evolving and altering up till early childhood years. According to the results of the CHILD (Canadian Healthy Infant Longitudinal Development) cohort study, the supplementation of vitamin D to both formula-fed and exclusively or partially breastfed infants negatively affected the concentrations of the Megamonas genus. In the group of exclusive breastfeeding, a diet rich in vitamin D during pregnancy was related to higher populations of Haemophilus and lower populations of Bilophila and Lachnospiraceae, while no compositional changes in the gut microbiota of partially breastfed or formula-fed infants were observed. Even though vitamin D supplementation of the mother or infant was not directly linked to Clostridioides difficile colonization, the maternal intake of vitamin-D-fortified milk minimized the risk of C. difficile colonization in infants [99]. Aligned data from the current literature highlight the importance of the feeding regimen in the foundation and constitution of the gut ecosystem in infants. The additional supplementation of vitamin D in the breastfed group stimulated the farming of Bifidobacterium, which are known to act as probiotics. On the contrary, no significant differences were noted in the gut taxonomy of formula-fed infants with or without vitamin D supplementation [100].
Additionally, the lack of vitamin D, which induced the abundance of Erysipelotrichaceae and Veillonellaceae, is the most common marker in the cases of osteoporosis in postmenopausal women. Nonetheless, it was intriguing that the presence of vitamin D in serum disclosed a negative correlation with Enterobacteriaceae and Erwinia. In addition, higher concentrations of vitamin D were affiliated with the amino acid metabolism, particularly with higher levels of the metabolites alanine, proline, tyrosine, valine, and leucine [101]. While the focus of current dietary interventions concerns chronic disease cases, little is known about the gut-regulated individualized responsiveness of healthy female subjects to vitamin D intake. The fact that the deficiency of vitamin D can be responsible for fragile bone health is a common observation. According to studies related to the effect of vitamin D on women, the dominating commensal phylum Bacteroidetes and taxa Akkermansia and Bifidobacterium were more abundant after vitamin D supplementation. Moreover, the variations in the gut microbiota diversity of bacterial genera were more prominent in the group of individuals who responded to vitamin D supplements than in the non-responders group, where the concentrations of Bacteroides acidifaciens were decreased [102].
Furthermore, several studies pointed out that the administration of FSVs, in total, yielded beneficial outcomes with regard to the state of the health of neuropsychiatric patients, by orchestrating the balance between bad and good microbes, through their biosynthesis and their interaction with gut microbiota [71]. Based on the results of a pilot study in an older Australian population, all vitamins (hydrophilic and lipophilic) are colon-delivered micronutrients, which instigate modifications in (a) the phyla of Actinobacteria (increase with vitamin A) and Bacteroidetes (reduction with vitamin D3), (b) the families of Coriobacteriaceae (increase with vitamin A), Ruminococcaceae, Peptostreptococcacea (increase with vitamin D3), and Desulfovibrionaceae (slight decrease with vitamin D3), (c) the genera of Collinsella, species aerofaciens (slight increase with vitamin A and D3) and Bilophila (slight decrease with D3), and (d) the species Collinsella aerofaciens (slight increase with vitamin E) and Eubacterium hallii, Coprococcus comes, and Dorea longicatena (increase with vitamin D3) [103].
In light of the dietary interventions under review, FSVs are wielded in the manipulation and restoration of gut microbiota, compared with the other two non-polar nutrients included in the present entry. Nonetheless, the elucidation of the reciprocal interactions between lipid-soluble micronutrients and the gut microenvironment merits further research, in order to entrench specific guidelines for FSV supplementation and implementation in novel therapeutic strategies.
References
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533.
- Ibáñez, C.; Mouhid, L.; Reglero, G.; Ramírez de Molina, A. Lipidomics insights in health and nutritional intervention studies. J Agric. Food Chem. 2017, 65, 7827–7842.
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Oresic, M.; Bertram, H.C. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods 2018, 149, 3–12.
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of dietary nutrients in the modulations of gut microbiota: A narrative review. Nutrients 2020, 12, 381.
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471.
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat died fed-mice. J. Agric. Food Chem. 2020, 68, 5118–5128.
- Yang, K.; Han, X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem. Sci. 2016, 41, 954–969.
- Smilowitz, J.T.; Zivkovic, A.M.; Wan, Y.J.Y.; Watkins, S.M.; Nording, M.L.; Hammock, B.D.; German, J.B. Nutritional lipidomics: Molecular metabolism, analytics and diagnostics. Mol. Nutr. Food Res. 2013, 57, 1319–1335.
- Zhang, J.L.; Yan, F.; Wang, X. Clinical lipidomics: A new way to diagnose human diseases. Clin. Trans. Med. 2018, 7, e12.
- De Castro, M.D.L.; Quiles-Zafra, R. Lipidomics: An omics discipline with a key role in nutrition. Talanta 2020, 219, 121197.
- O’Donnell, V.B.; Ekroos, K.; Liebisch, G.; Wakelam, M. Lipidomics: Current state of the art in a fast-moving field. Rev. Syst. Biol. Med. 2019, 12, e1466.
- Serhan, C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Asp. Med. 2017, 58, 1–11.
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among dietary Omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat. Inflamm. 2021, 2021, 8879227.
- Stacchiotti, V.; Rezzi, S.; Eggersdorfer, M.; Galli, F. Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit. Rev. Food. Sci Nutr. 2020, 61, 3211–3232.
- Ye, Z.; Xu, Y.J.; Liu, Y. Influences of dietary oils and fats, and the accompanied minor content of components on the gut microbiota and gut inflammation: A review. Trends Food Sci. Technol. 2021, 113, 255–275.
- Shama, S.; Liu, W. Omega-3 fatty acids and gut microbiota: A reciprocal Interaction in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2020, 65, 906–910.
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelanm, M.; d’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha supplementation on NAFLD: The MAST4HEALTH randomised, controlled trial. Mol. Nutr. Food Res. 2021, 65, 2001178.
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Gunther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2018, 8, 1363–1369.
- Serini, S.; Calviello, G. Omega-3 PUFA responders and non-responders and the prevention of lipid dysmetabolism and related diseases. Nutrients 2020, 12, 1363.
- Liu, Y.; Ding, W.; Wang, H.L.; Dai, L.L.; Zong, W.H.; Wang, Y.Z.; Bi, J.; Han, W.; Dong, G.J. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265.
- Weir, T.L.; Trihka, S.; Thompson, H.J. Diet and cancer risk reduction: The role of diet-microbiota interactions and microbial metabolites. Semin. Cancer Biol. 2020, 70, 53–60.
- Bojkova, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary fat and cancer-which is good, which is bad, and the body of evidence. Int. J. Mol. Sci. 2020, 21, 4114.
- Wu, W.K.; Ivanova, E.; Orekhov, A.N. Gut microbiome: A possible common therapeutic target for treatment of atherosclerosis and cancer. Semin. Cancer Biol. 2021, 70, 85–97.
- Fan, Y.; Zhang, J. Dietary modulation of intestinal microbiota: Future opportunities in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Microbiol. 2019, 10, 740.
- Kendig, M.D.; Leigh, S.J.; Morris, M.J. Unravelling the impacts of western-style diets on brain, gut microbiota and cognition. Neurosci. Biobehav. Rev. 2021, 128, 233–243.
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193.
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37.
- Vander Wyst, K.B.; Ortega-Santos, C.P.; Toffoli, S.N.; Lahti, C.E.; Whisner, C.M. Diet, adiposity, and the gut microbiota from infancy to adolescence: A systematic review. Obes. Rev. 2021, 22, e13175.
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microb. 2021, 13, 1880221.
- Moszak, M.; Szulinska, M.; Walczak-Galezewska, M.; Bogdanski, P. Nutritional approach targeting gut microbiota in NAFLD-To date. Int. J. Environ. Res. Public Health 2021, 18, 1616.
- Li, X.; Zhang, Z.; Cheng, J.; Diao, C.; Yan, Y.; Liu, D.; Wang, H.; Zheng, F. Dietary supplementation of soybean-derived sterols regulates cholesterol metabolism and intestinal microbiota in hamsters. J. Funct. Foods 2019, 59, 242.
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary supplementation of black rice anthocyanin extract regulates cholesterol metabolism and improves gut gicrobiota dysbiosis in C57BL/6J mice fed a high-fat and cholesterol diet. Mol. Nutr. Food Res. 2020, 64, 1900876.
- Steinert, R.E.; Lee, Y.K.; Sybesma, W. Vitamins for the gut microbiome. Trends Mol. Med. 2020, 26, 137–140.
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-soluble vitamin intestinal absorption: Absorption sites in the intestine and interactions for absorption. Food Chem. 2015, 172, 155–160.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 1–22.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2020, 474, 1823–1836.
- Cryan, F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussoto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013.
- Schroeder, J.B.; Backhead, F. Signals from gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089.
- Bessac, A.; Cani, P.D.; Meunier, E.; Dietrich, G.; Knauf, C. Inflammation and gut-brain-axis during type 2 diabetes: Focus on the crosstalk between intestinal immune cells and enteric nervous system. Front. Neurosci. 2018, 12, 725.
- Fulde, M.; Hornef, M.W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 2014, 260, 21–34.
- Kamada, N.; Chen, G.Y.; Inohara, N.; Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690.
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521.
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276.
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017, 32, 300–313.
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet and disease. Microorganisms 2019, 7, 14.
- Sanchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as regulator of gut microbiota and its role in health and disease. Arch. Med. Res. 2019, 50, 259–268.
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012, 16, 559–564.
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–590.
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernades, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 12, 174–180.
- Evgenik, A.; Evgenik, M. Exploring the impact of intestinal ion transport on the gut microbiota. Comput. Struct. Biotechnol. J. 2021, 19, 134–144.
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270.
- Holister, B.; Gao, C.; Versalovic, J. Compositional and functional features of Gastrointestinal Microbiome and Their Effects on Human Health. Gastroenterology 2014, 146, 1449–1458.
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502.
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699.
- Lecerf, J.M.; de Lorgeril, M. Dietary cholesterol: From physiology to cardiovascular risk. Br. J. Nutr. 2021, 106, 6–14.
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E. Dose effects of dietary phytosterols on cholesterol metabolism: A control feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38.
- García-Llatas, G.; Rodríguez-Estrada, M.T. Current and new insights on phytosterols oxides in plant sterol-enriched food. Chem. Phys. Lipids 2021, 164, 607–624.
- Feng, S.M.; Dai, Z.Q.; Liu, A.N.; Wang, H.; Chen, J.S.; Luo, Z.S.; Yang, C.S. Beta-Sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat Westernstyle diet. Food Funct. 2017, 8, 4179–4186.
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.; Wei, H.; Yang, X.; Sung, J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774.
- Cuevas-Tena, M.; Alegria, A.; Lagarda, M.J.; Venema, K. Impact of plant sterols enrichment dose on gut microbiota from lean and obese subjects using TIM-2 in vitro fermentation model. Funct. Foods 2019, 54, 164–174.
- Cuevas-Tena, M.; del Pulgar, E.M.G.; Benítez-Páez, A.; Sanz, Y.; Alegría, A.; Lagarda, J.M. Plant sterols and human gut microbiota relationship: An in vitro colonic fermentation study. J. Funct. Foods 2018, 44, 322–329.
- Blanco-Morales, V.; Garcia-Llatas, G.; Yebra, M.J.; Sentandreu, V.; Lagarda, M.J.; Alegría, A. Impact of a Plant Sterol- and Galactooligosaccharide-Enriched Beverage on Colonic Metabolism and Gut Microbiota Composition Using an in vitro Dynamic Model. J. Agric. Food Chem. 2020, 68, 1884–1895.
- Vezza, T.; Canet, F.; de Maranon, A.M.; Banuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional health players in the management of obesity and its related disorders. Antioxidants 2020, 9, 1266.
- Xia, G.L.; Sun, J.; Fan, Y.T.; Zhao, F.F.; Ahmed, G.; Jin, Y.; Zhang, Y.; Wang, H. β-Sitosterol attenuates high grain diet-induced inflammatory stress and modifies rumen fermentation and microbiota in sheep. Animals 2020, 10, 171.
- Li, X.; Wang, H.; Wang, T.; Zheng, F.; Wang, H.; Wang, C. Dietary wood pulp-derived sterols modulation of cholesterol metabolism and gut microbiota in high-fat-diet-fed hamsters. Food Funct. 2019, 10, 775–785.
- Song, L.; Zhao, X.G.; Ouyang, P.L.; Guan, Q.; Yang, L.; Peng, F.; Du, H.; Yin, F.; Yan, W.; Yu, W.J.; et al. Combined effect of n3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2020, 123, 1148–1158.
- Baumgartner, S.; Mensink, R.P.; Smet, E.D.; Konings, M.; Fuentes, S.; de Vos, W.; Plat, J. Effects of plant sterol ester consumption on fasting plasma oxy(phyto)sterol concentrations as related to fecal microbiota characteristics. J. Steroid Biochem. 2017, 169, 46–53.
- Schott, H.F.; Krautbauer, S.; Horing, M.; Liebisch, G.; Matysik, S.A. Validated, fast method for quantification of sterols and gut microbiome derived 5α/β-stanols in human feces by isotope dilution LC-high-resolution MS. Anal. Chem. 2018, 90, 8487–8494.
- Tong, J.; Satyanarayanan, S.K.; Su, H. Nutraceuticals and probiotics in the management of psychiatric and neurologicaldisorders: A focus on microbiota-gut-brain-immune axis. Brain Behav. Immun. 2020, 90, 403–419.
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64.
- Maher, S.E.; O’Brien, E.C.; Moore, R.L.; Byrne, D.F.; Geragthy, A.A.; Saldova, R.; Murphy, E.F.; Sinderen, D.V.; Cotter, P.D. The association between the maternal diet and the maternal and infant gut microbiome: A systematic review. Br. J. Nutr. 2020, 4, 1–29.
- Xiao, L.; Cui, T.; Liu, S.; Chen, B.; Wang, Y.; Yang, T.; Li, T.; Chen, J. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition 2019, 57, 97–108.
- Zhang, T.; Sun, P.; Geng, Q.; Fan, H.; Gong, Y.; Hu, Y.; Shan, L.; Sun, Y.; Shen, W.; Zhou, Y. Disrupted spermatogenesis in a metabolic syndrome model: The role of vitamin A metabolism in the gut–testis axis. Gut 2022, 71, 78–87.
- Biyong, E.F.; Alfos, S.; Dumetz, F.; Helbling, J.C.; Aubert, A.; Brossaud, J.; Foury, A.; Moisan, M.P.; Laye, S.; Richard, E.; et al. Dietary vitamin A supplementation prevents early obesogenic diet-induced microbiota, neuronal and cognitive alterations. Int. J. Obes. 2021, 45, 588–598.
- Chen, B.; Liu, S.; Feng, D.; Xiao, L.; Yang, T.; Li, T.; Sun, W.; Chen, J. Vitamin A Deficiency in the Early-Life Periods Alters a Diversity of the Colonic Mucosal Microbiota in Rats. Front. Nutr. 2020, 4, 580780.
- Azzi, A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103.
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Ozer, N.K. Vitamin E: Emerging Aspects and New Directions. Free Radical Bio. Med. 2017, 102, 16–36.
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-tocopherol) consumption influences gut microbiota composition. Int. J. Food Sci. Nutr. 2020, 71, 221–225.
- Kim, D.J.; Yoon, S.; Ji, S.C.; Yang, J.; Kim, Y.K.; Lee, S.; Yu, K.S.; Jang, I.J.; Chung, J.Y.; Cho, J.Y. Ursodeoxycholic acid improves liver function via phenylalanine/tyrosine pathway and microbiome remodeling in patients with liver dysfunction. Sci. Rep. 2018, 8, 11874.
- Yang, C.; Zhao, Y.; Im, C.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567.
- Ellis, J.L.; Karl, P.; Oliverio, A.M.; Fu, X.; Soares, J.W.; Wolfe, E.B.; Hernandez, C.J.; Mason, J.B.; Booth, S.L. Dietary vitamin K is remodeled by gut microbiota and influences community composition. Gut Microbes 2021, 13, 1887721.
- Dash, N.R.; Al Bataineh, M.T. Metagenomic analysis of the gut microbiome reveals enrichment of menaquinones (vitamin K2) pathway in diabetes mellitus. Diabetes Metab. J. 2021, 45, 77–85.
- Zhang, Y.; Ma, C.; Zhao, J.; Xu, H.; Hou, Q.; Zhang, H. Lactobacillus casei and vitamin K2 prevent intestinal tumorigenesis in mice via adiponectin-elevated different signaling pathways. Oncotarget 2017, 8, 24719–24727.
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36–45.
- Beveridge, L.A.; Struthers, A.D.; Khan, F.; Jorde, R.; Scragg, R.; Macdonald, H.M.; Alvarez, J.A.; Boxer, R.S.; Dalbeni, A.; Gepner, A.D.; et al. Effect of Vitamin D Supplementation on blood pressure: A systematic review and meta-analysis incorporating individual patient data. JAMA Intern. Med. 2015, 175, 745–754.
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS ONE 2015, 10, e0141770.
- Clark, A.; Mach, N. Role of vitamin D in the hygiene hypothesis: The interplay between Vitamin D, Vitamin D receptors, gut microbiota, and immune response. Front. Immunol. 2016, 7, 627.
- Fakhoury, H.M.A.; Peter, K.R.; AlKattan, W.; Al Anouti, F.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Ster. Biochem. Mol. Biol. 2020, 200, 105663.
- Garcia-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 2468–2481.
- Yamamoto, E.A.; Jørgensen, T.N. Relationships between Vitamin D, gut microbiome, and systemic autoimmunity. Front. Immunol. 2020, 10, 3141.
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097.
- Soltys, K.; Stuchlikova, M.; Hlavaty, T.; Gaalova, B.; Budis, J.; Gazdarica, J.; Krajcovicova, A.; Zelinkova, Z.; Szemes, T.; Kuba, D.; et al. Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients. Sci Rep. 2020, 10, 6024.
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; de Filippis, F.; Paino, S.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immun. 2018, 67, 230–245.
- Jiang, S.; Zhu, Q.; Mai, M.; Yang, W.; Du, G. Vitamin B and vitamin D as modulators of gut microbiota in overweight individuals. Int. J. Food Sci. Nutr. 2020, 71, 1001–1009.
- Lee, P.; Hsieh, Y.; Huo, T.; Yang, U.; Lin, C.; Li, C.; Huang, Y.; Hou, M.; Lin, H.; Lee, K. Active Vitamin D 3 Treatment Attenuated Bacterial Translocation via Improving Intestinal Barriers in Cirrhotic Rats. Mol. Nutr. Food Res. 2020, 65, 2000937.
- Zhang, X.; Shang, X.; Jin, S.; Ma, Z.; Wang, H.; Na, A.O.; Yang, J.; Du, J. Vitamin D ameliorates high-fat-diet-induced hepatic injury via inhabiting pyroptosis and alters gut microbiota in rats. Arch. Biochem. Biophys. 2021, 705, 108894.
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, T.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: Implications for viral respiratory infections. Gut Microbes 2020, 12, 1799734.
- Lei, W.T.; Huang, K.Y.; Jhong, J.H.; Chen, C.H.; Weng, S.L. Metagenomic analysis of the gut microbiome composition associated with vitamin D supplementation in Taiwanese infants. Sci. Rep. 2021, 11, 2856.
- Palacios-Gonzalez, B.; Ramirez-Salazar, E.G.; Rivera-Paredez, B.; Quiterio, M.; Flores, Y.N.; Macias-Kauffer, L.; Moran-Ramos, S.; Denova-Gutierez, E.; Ibarra-González, I.; Vela-Amieva, M.; et al. A multi-omic analysis for low bone mineral density in postmenopausal women suggests a relationship between diet, metabolites and microbiota. Microorganisms 2020, 8, 1630.
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 1–14.
- Pham, H.; Waterhouse, M.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; Kimlin, M.G.; et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: An analysis of data from the D-Health Trial. Lancet Diabetes Endocrinol. 2021, 9, 69–81.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




