Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Carrasco García | -- | 1712 | 2022-04-20 13:00:00 | | | |
| 2 | Dean Liu | -8 word(s) | 1704 | 2022-04-21 04:21:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carrasco García, A.; Moral-Vico, J.; Abo Markeb, A.; Sánchez, A. Conversion of Carbon Dioxide into Methanol. Encyclopedia. Available online: https://encyclopedia.pub/entry/22011 (accessed on 07 February 2026).
Carrasco García A, Moral-Vico J, Abo Markeb A, Sánchez A. Conversion of Carbon Dioxide into Methanol. Encyclopedia. Available at: https://encyclopedia.pub/entry/22011. Accessed February 07, 2026.
Carrasco García, Anna, Javier Moral-Vico, Ahmad Abo Markeb, Antoni Sánchez. "Conversion of Carbon Dioxide into Methanol" Encyclopedia, https://encyclopedia.pub/entry/22011 (accessed February 07, 2026).
Carrasco García, A., Moral-Vico, J., Abo Markeb, A., & Sánchez, A. (2022, April 20). Conversion of Carbon Dioxide into Methanol. In Encyclopedia. https://encyclopedia.pub/entry/22011
Carrasco García, Anna, et al. "Conversion of Carbon Dioxide into Methanol." Encyclopedia. Web. 20 April, 2022.
Copy Citation
As an energy source, methanol presents the advantage of being liquid at environmental temperature, which makes it easier to transport and store.
carbon dioxide hydrogenation
methanol
nanomaterials
1. Introduction
Climate change stands as one of the greatest issues and challenges that humanity faces, and emissions of greenhouse gases produced by the use of fossil fuels for energy production are identified as the main cause of global warming. Gases such as carbon dioxide and methane are the most important representatives. The alarming increase in atmospheric carbon dioxide concentration caused by anthropogenic emissions has become a serious threat to the planet, causing global warming [1]. During 2018, 33 gigatonnes of carbon dioxide emitted into the atmosphere resulted in an increase in its concentration from 280 to 410 ppm [2]. To tackle this issue, two strategies have been proposed: carbon capture and storage (CCS), whose main drawback is economical justifiability, since it is a non-profitable option [3], and carbon capture and utilisation (CCU) [2], which intends to transform waste carbon dioxide into useful products, such as chemical feedstocks and renewable fuels. There currently exist several technologies that achieve the conversion of carbon dioxide into other value-added products, such as carbon dioxide hydrogenation, electrochemical reduction, photochemical reduction, and photoelectrochemical reduction. Specifically, this project focuses on the catalytic hydrogenation of carbon dioxide, since it is considered as the most commonly used and simplest process [4]. Several chemical products can be obtained from carbon dioxide hydrogenation, among which methanol stands out for two main reasons: its use as a renewable energy source and its utility as a feedstock for obtaining other chemical products. As an energy source, methanol presents the advantage of being liquid at environmental temperature, which makes it easier to transport and store. Moreover, 85% of methanol is used in the chemical industry as a solvent or as a reagent for the synthesis of formaldehyde and acetic acid [4], which are valuable products in industrial terms. In the early 1920s, BASF successfully produced methanol from syngas during the ammonia synthesis process using the ZnO-Cr2O3 catalyst at a temperature of 320–450 °C and a pressure of 250–350 bar [5]. In the 1960s, ICI (Imperial Chemical Industries) developed a route for methanol synthesis in which syngas containing a high proportion of carbon dioxide reacted with highly selective copper and zinc oxide catalysts, resulting in the first commercially developed Cu/ZnO/Al2O3 catalyst [6]. This material is currently the most commonly used for the conversion of carbon dioxide into methanol, which means that heterogeneous catalysts are preferable to homogeneous systems due to their high stability and easy use.
2. Catalytic Activity of Nanomaterials
The space–time yield (STY) evaluated the amount of methanol produced per mass unit of catalyst and time. It was determined for each catalyst used at different temperatures, as shown in Figure 1. In general, the production of methanol increased with temperature. Nevertheless, for some materials, it was observed that the same values were obtained for the STY at 260 and at 280 °C; even in the case of Cu/ZnO/PN, a decrease in the STY was observed in this range. This could be related to the selectivity of the reaction, which can favour the production of carbon monoxide compared to methanol at these high temperatures. If researchers consider the results of the STY analysis when classified by types of materials, monometallic materials should be the first to be analysed. In particular, the results indicate that methanol was hardly obtained with copper—only at high temperatures and with non-significant concentrations of methanol. The lower activity of the copper was suggested to result from formate poisoning of the copper surface, which is an intermediate of the hydrogenation of carbon dioxide into methanol [7]. Furthermore, copper was found to be ineffective as a catalyst material for its low surface area [8]. Regarding zinc oxide, the methanol concentrations were slightly higher than those of copper. However, these concentrations were small compared with those of the other catalysts. The difference in methanol concentrations between zinc oxide and copper and the role that these two metals played in methanol synthesis was previously analysed, where it was discussed that zinc oxide is fundamental for stabilising reaction intermediates and reducing energy barriers for the production of methanol [8]. For these two reasons, more methanol was produced with zinc oxide than with copper. Zinc oxide synthesised with and without PVP was compared in terms of methanol STY, with better results for the case synthesised with PVP.
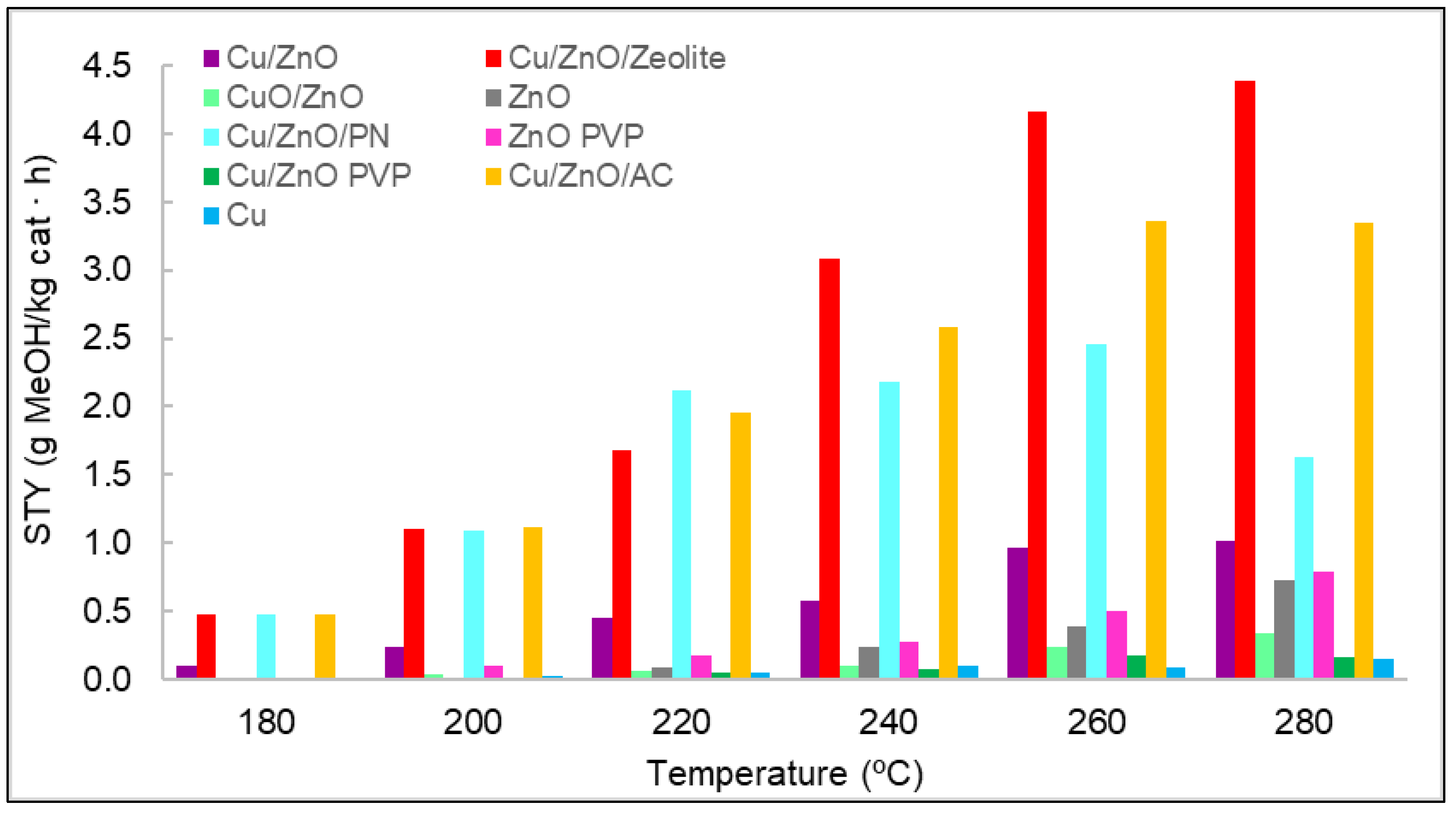
Figure 1. STY values for each catalyst at different temperatures and at a pressure of 15 bar.
As observed, adding PVP entailed the obtention of smaller nanoparticles with more regular morphology, which made the specific surface higher. The bimetallic materials were compared as well. It was observed that with CuO/ZnO, the amount of methanol produced was lower than with Cu/ZnO. It was found in the literature that the active component is Cu0, and although Cu2+ can also act as an active component, it offers less catalytic activity [9][10].
Moreover, several publications reported that copper nanoparticles are an active phase for carbon dioxide hydrogenation, and zinc oxide helps to disperse these copper nanoparticles on the surface of the catalyst [11][12]. Thus, the presence of zinc oxide increases the exhibition of copper active sites, as it is a physical spacer between these nanoparticles. Nevertheless, A literature suggests that Zn atoms at the edges of copper nanoparticles are also active sites [13]. Thus, metallic Zn on the copper surface increases the binding energy of oxygen-bound intermediates, that is, formate, and this facilitates the hydrogenation pathway of carbon dioxide. Therefore, zinc oxide also catalyses the reaction, although in smaller quantities than Cu/ZnO because the active sites for carbon dioxide hydrogenation are found in both metals.
A complete study was performed on Cu/ZnO, and the effect of adding PVP was also compared. In the case of this material, the results clearly showed that adding PVP to a bimetallic system is not a viable alternative. The possible advantages of the use of supports in the production of methanol were analysed. For this purpose, the catalyst Cu/ZnO without PVP was chosen due to its good results, and three types of supports were chosen. The first supports tested were polypyrrole nanotubes in order to solve the problem of nanoparticle agglomeration and, thus, to increase the exposure of active sites. Figure 1 shows the obvious difference between Cu/ZnO and Cu/ZnO with polypyrrole nanotubes, confirming the effect of the support in terms of methanol production. The other two supports used were zeolite and activated carbon, which are both porous materials. The best results of conversion from carbon dioxide to methanol were achieved with the use of these porous materials, thanks to the synergy between the carbon dioxide adsorption capacity of the supports and the active sites of the Cu/ZnO catalyst. Nevertheless, the Cu/ZnO/zeolite material produced the best results, probably due to zeolite’s higher carbon dioxide adsorption capacity per gram of material [14][15].
These three supports were analysed to verify whether they catalysed methanol by themselves. Therefore, a catalytic test was performed at the same conditions of temperature, pressure, and flow as with the other catalyst. The results indicated that none of them had catalytic activity.
The methanol selectivity of all of the studied catalysts is shown in Figure 2. In all of the cases, from 180 to 220 °C, the methanol selectivity obtained was 100%, which means that no carbon monoxide was produced at these temperatures; thus, only the methanol selectivity values from 240 to 280 °C are plotted in Figure 2. Almost all catalysts had 100% methanol selectivity at 240 °C, but as the temperature increased, in general, all materials lost selectivity. This was due to the standard enthalpy of the reaction of methanol being negative; hence, it is an exothermic reaction that is favoured at low temperatures. However, in three catalysts, the amount of carbon monoxide obtained was zero for temperatures up to 280 °C. These catalysts were zinc oxide with and without PVP and Cu/ZnO with PVP. As stated in the literature [7], the catalytic activity and methanol selectivity can be correlated with the increase in Zn0 concentration. It was discovered that a zinc enrichment in the catalyst leads to greater activity and methanol selectivity due to two factors: improving the Cu and ZnO interactions that increase carbon dioxide hydrogenation and inhibiting the RWGS reaction, which produces carbon monoxide [16]. In the case of materials synthesised using PVP, this lack of carbon monoxide was possibly due to the poor distribution of zinc oxide and copper nanoparticles, which were also agglomerated. As expected, the active sites of copper were poisoned by formate, and only the active sites of zinc oxide were available.
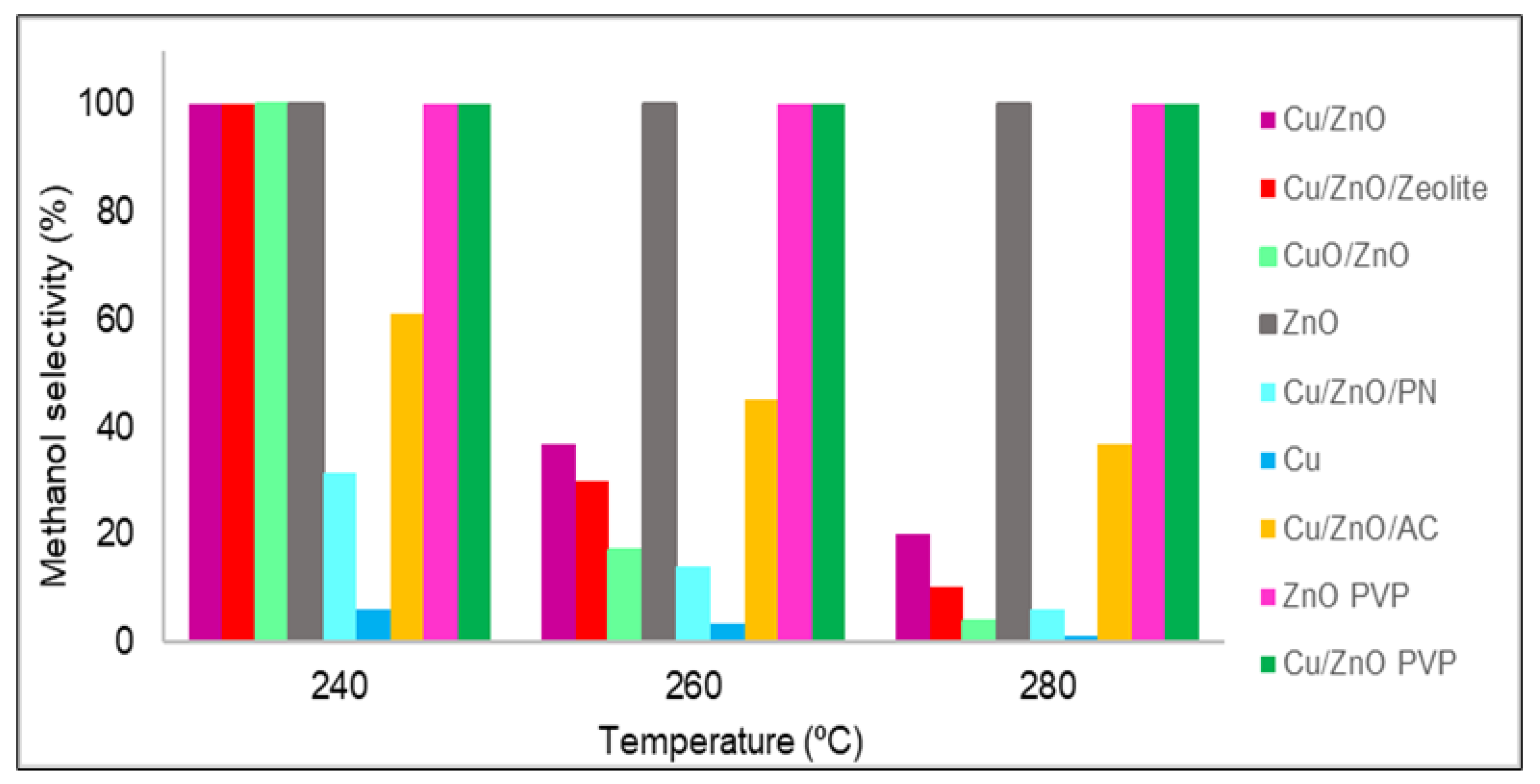
Figure 2. Methanol selectivity (%) values of all catalysts.
The relationships between the methanol selectivity and the CO2 conversion for some catalysts with good STY results are shown in Figure 3. The displayed values correspond to the methanol selectivity and conversion of carbon dioxide from 240 to 280 °C. As observed, the general behaviour was that as selectivity decreased, conversion and temperature increased. Figure 3 shows that the catalyst with the highest carbon dioxide conversion was Cu/ZnO/Zeolite, similarly to the STY results seen in Figure 1. The yield and conversion data were low compared with the literature. For example, using Cu/ZnO plates, the maximum CO2 conversion reported was 8% [17][18]. However, the operating conditions of pressure in these studies were higher (30 bar) than those of the current work (15 bar). Even though many existing publications studied the catalytic properties of similar materials, it is very rare to find documents with studies operating at pressures lower than 20 bar [19][20].
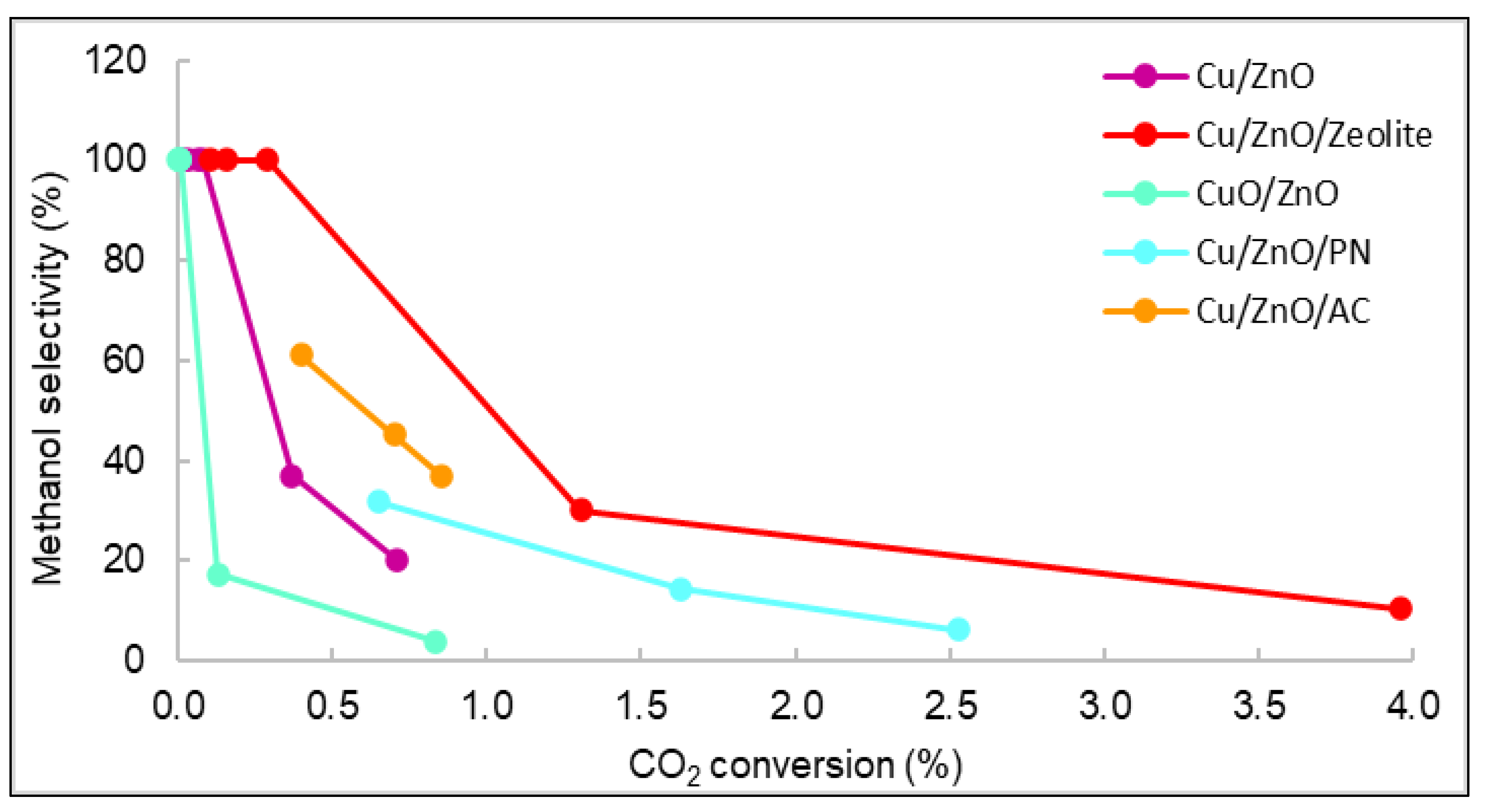
Figure 3. Representation of the selectivity vs. carbon dioxide conversion of all catalysts.
Finally, the STY values and selectivity towards methanol of all the catalysts at 260 °C and the temperature with the maximum STY values are plotted in Figure 4.
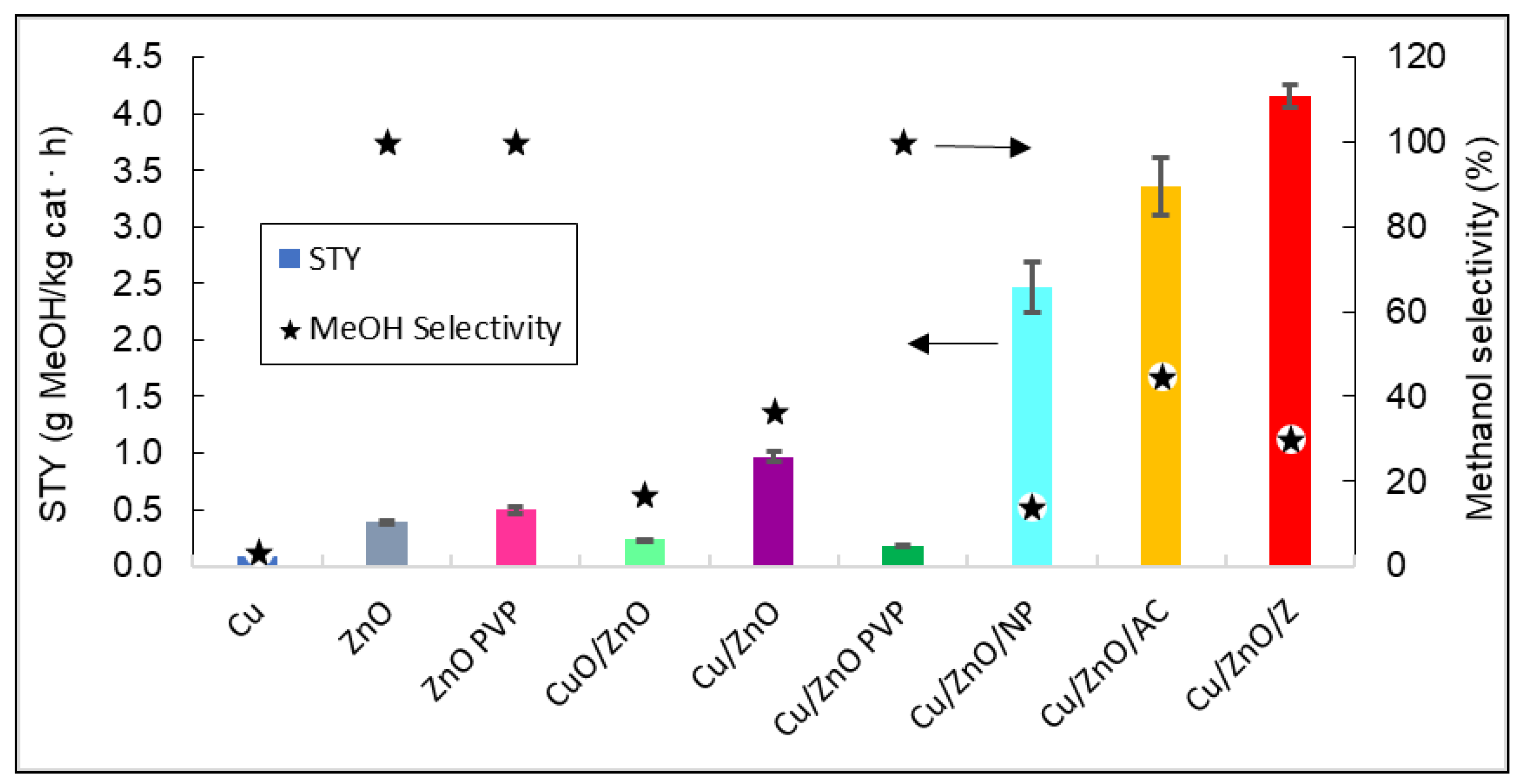
Figure 4. Representation of the STY and the methanol selectivity of all catalysts. The arrows indicate that methanol selectivity values can be read in the right vertical axis, and STY in the left vertical axis.
Figure 4 shows that the catalyst that produced the highest yield of methanol was Cu/ZnO/zeolite. As expected, by adding a support such as zeolite to the Cu/ZnO bimetallic system, the STY values obtained were 76.75% higher than without the support. It can also be stated that bimetallic systems have a better catalytic efficiency than ZnO and Cu0 by themselves, with the latter having no methanol production.
References
- Alotaibi, M.A.; Din, I.U.; Alharthi, A.I.; Bakht, M.A.; Centi, G.; Shaharun, M.S.; Naeem, A. Green methanol synthesis by catalytic CO2 hydrogenation, decipehring the role of metal-metal interaction. Sustain. Chem. Pharm. 2021, 21, 100420.
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905.
- Li, M.M.J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464.
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol-An Industrial Review. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH GmbH: Weinheim, Germany, 2012.
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH GmbH: Weinheim, Germany, 2008; Volumes 1–5.
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykima, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838.
- Stangeland, K.; Li, H.; Yu, Z. CO2 hydrogenation to methanol: The structure-activity relationships of different catalyst systems. Energ. Ecol. Environ. 2020, 5, 272–285.
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.; et al. The Active Site of Methanol Synthesis over Cu/ZnO/Al2O3 Industrial Catalysts. Science 2012, 759, 893–898.
- Dasireddy, V.D.B.C.; Likozar, B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew. Energy 2019, 140, 452–460.
- Dong, X.; Li, F.; Zhao, N.; Xiao, F.; Wang, J.; Tan, Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B Environ. 2016, 191, 8–17.
- Schumann, J.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Cu, Zn-based catalysts for methanol synthesis: On the effect of calcination conditions and the part of residual carbonates. Appl. Catal. A Gen. 2016, 516, 117–126.
- Lei, H.; Nie, R.; Wu, G.; Hou, Z. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 2015, 154, 161–166.
- Studt, F.; Behrens, M.; Kunkes, E.L.; Thomas, N.; Zander, S.; Tarasov, A.; Schumann, J.; Frei, E.; Varley, J.B.; Abild-Pedersen, F.; et al. The Mechanism of CO and CO2 Hydrogenation to Methanol over Cu-Based Catalysts. ChemCatChem 2015, 7, 1105–1111.
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 9, 796–854.
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, T.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total Environ. 2017, 595, 51–62.
- Martin, O.; Mondelli, C.; Curulla-Ferré, D.; Drouilly, C.; Hauert, R.; Pérez-Ramírez, J. Zinc-Rich Copper Catalysts Promoted by Gold for Methanol Synthesis. ACS Catal. 2015, 5, 5607–5616.
- Huang, C.; Wen, J.; Sun, Y.; Zhang, M.; Bao, Y.; Zhang, Y.; Liang, L.; Fu, M.; Wu, J.; Ye, D.; et al. CO2 hydrogenation to methanol over Cu/ZnO plate model catalysts: Effects of reducing gas induced Cu nanoparticle morphology. Chem. Engin. J. 2019, 374, 221–230.
- Sun, Y.; Huang, C.; Chen, L.; Zhang, Y.; Fu, M.; Wu, J.; Ye, D. Active site structure study of Cu/Plate ZnO model catalysts for CO2 hydrogenation to methanol under the real reaction conditions. J. CO2 Util. 2020, 37, 55–64.
- Niu, J.; Liu, H.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200.
- Xiuyi, X.; Zhang, H. Kinetically relevant variation triggered by hydrogen pressure: A mechanistic case study of CO2 hydrogenation to methanol over Cu/ZnO. J. Catal. 2022, 406, 145–156.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




