Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shin Takasawa | -- | 2474 | 2022-04-20 09:12:10 | | | |
| 2 | Peter Tang | -8 word(s) | 2466 | 2022-04-20 10:27:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Takasawa, S. CD38–Cyclic ADP-Ribose Signal System. Encyclopedia. Available online: https://encyclopedia.pub/entry/21979 (accessed on 07 February 2026).
Takasawa S. CD38–Cyclic ADP-Ribose Signal System. Encyclopedia. Available at: https://encyclopedia.pub/entry/21979. Accessed February 07, 2026.
Takasawa, Shin. "CD38–Cyclic ADP-Ribose Signal System" Encyclopedia, https://encyclopedia.pub/entry/21979 (accessed February 07, 2026).
Takasawa, S. (2022, April 20). CD38–Cyclic ADP-Ribose Signal System. In Encyclopedia. https://encyclopedia.pub/entry/21979
Takasawa, Shin. "CD38–Cyclic ADP-Ribose Signal System." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Calcium (Ca2+) is a ubiquitous and fundamental signaling component that is utilized by cells to regulate a diverse range of cellular functions, such as insulin secretion from pancreatic β-cells of the islets of Langerhans. Cyclic ADP-ribose (cADPR), synthesized from NAD+ by ADP-ribosyl cyclase family proteins, such as the mammalian cluster of differentiation 38 (CD38), is important for intracellular Ca2+ mobilization for cell functioning. cADPR induces Ca2+ release from endoplasmic reticulum via the ryanodine receptor intracellular Ca2+ channel complex, in which the FK506-binding protein 12.6 works as a cADPR-binding regulatory protein.
CD38
cADPR
RYR2
FKBP12.6
pancreatic β cells
CD157
1. Introduction
Changes in cytosolic Ca2+ are indispensable for normal cell function, such as secretion, proliferation, neuronal guidance, cell death, and development. Typically, cells respond to a Ca2+ signal that is generated inside the cell in response to the activation of a wide variety of extracellular signals, including those involved in nutrient, neurotransmitter, hormonal, and sensory signaling. In most cases, the initial Ca2+ signal generated in the cell is a specific increase in cytoplasmic Ca2+ concentrations resulting from the release of Ca2+ from internal Ca2+ stores (mainly the endoplasmic reticulum) or the entry of Ca2+ from the external spaces across the plasma membrane. Both routes involve the movement of Ca2+ through Ca2+ channels that are localized within these cellular membranes. While intracellular Ca2+ release from the endoplasmic reticulum occurs via channels activated by inositol 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR), or Ca2+ itself (Ca2+-induced Ca2+ release), Ca2+ influx across the plasma membrane is achieved via numerous types of Ca2+ channels, including voltage-gated Ca2+ channels and store-operated Ca2+ channels as well as a variety of ligand-gated cation channels.
2. cADPR as an Intracellular Ca2+-Releasing Messenger
Changes in cytosolic Ca2+ levels govern a multitude of cellular processes. Many extracellular stimuli mediate complex changes in Ca2+ through the production of second messengers [1]. These molecules effect the release of Ca2+ from the intracellular Ca2+ store. In addition to IP3, cADPR and nicotinic acid adenine dinucleotide phosphate (NAADP) play crucial roles in the generation of agonist-evoked Ca2+ signals [2][3]. IP3 is the best characterized of these molecules [1]. In addition, cADPR and NAADP, which were first discovered in sea urchin eggs [4], also play prominent roles in generating complex Ca2+ signals. cADPR induces Ca2+ release through the activation of the ryanodine receptors (RyRs) located on the endoplasmic reticulum [2][3].
Both cADPR and NAADP are synthesized by the same family of enzymes, the ADP-ribosyl cyclases (EC 3.2.2.6 and EC 2.4.99.20), which therefore play a central role in Ca2+ signaling and have been implicated in a variety of processes ranging from bacterial clearance to social behavior [5]. ADP-ribosyl cyclase (EC 3.2.2.6) activity was originally identified in sea urchin egg homogenates [4], but an enzyme with the activity was later purified [6][7] and cloned [8][9] from Aplysia ovotestes. The enzyme was shown to catalyze the cyclization of NAD+ to form cADPR and the replacement of the nicotinamide moiety in NADP with nicotinic acid to form NAADP. Based on amino acid sequence similarity, it became evident that the mammalian protein cluster of differentiation 38 (CD38; ADP-ribosyl cyclase 1; EC 3.2.2.6) [10][11][12][13] and cluster of differentiation 157 (CD157; ADP-ribosyl cyclase 2; EC 3.2.2.6) [14][15] were also ADP-ribosyl cyclase in mammalian cells.
3. cADPR in Insulin Secretion from Pancreatic β-Cells
Glucose induces an increase in intracellular Ca2+ concentration in pancreatic islet β-cells and induces the secretion of insulin. Ashcroft et al. first explained the importance of the increase in the Ca2+ concentration in 1984 [16]. The millimolar concentration of ATP is produced by the process of glucose metabolism in pancreatic β-cells. As a result, there is the closure of ATP-sensitive potassium (KATP) channels, which results in membrane depolarization, the influx of Ca2+ through voltage-dependent Ca2+ channels, and an increase in the cytosolic Ca2+ concentration that triggers the exocytosis of insulin granules. In 1993, another model of insulin secretion by glucose via cADPR-mediated Ca2+ mobilization from an intracellular Ca2+ pool, the endoplasmic reticulum, was proposed [12][17], as shown in Figure 1. According to this model, ATP inhibits the cADPR hydrolase of CD38, causing the accumulation of cADPR, which acts as a second messenger for Ca2+ mobilization from the endoplasmic reticulum for insulin secretion [18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34].
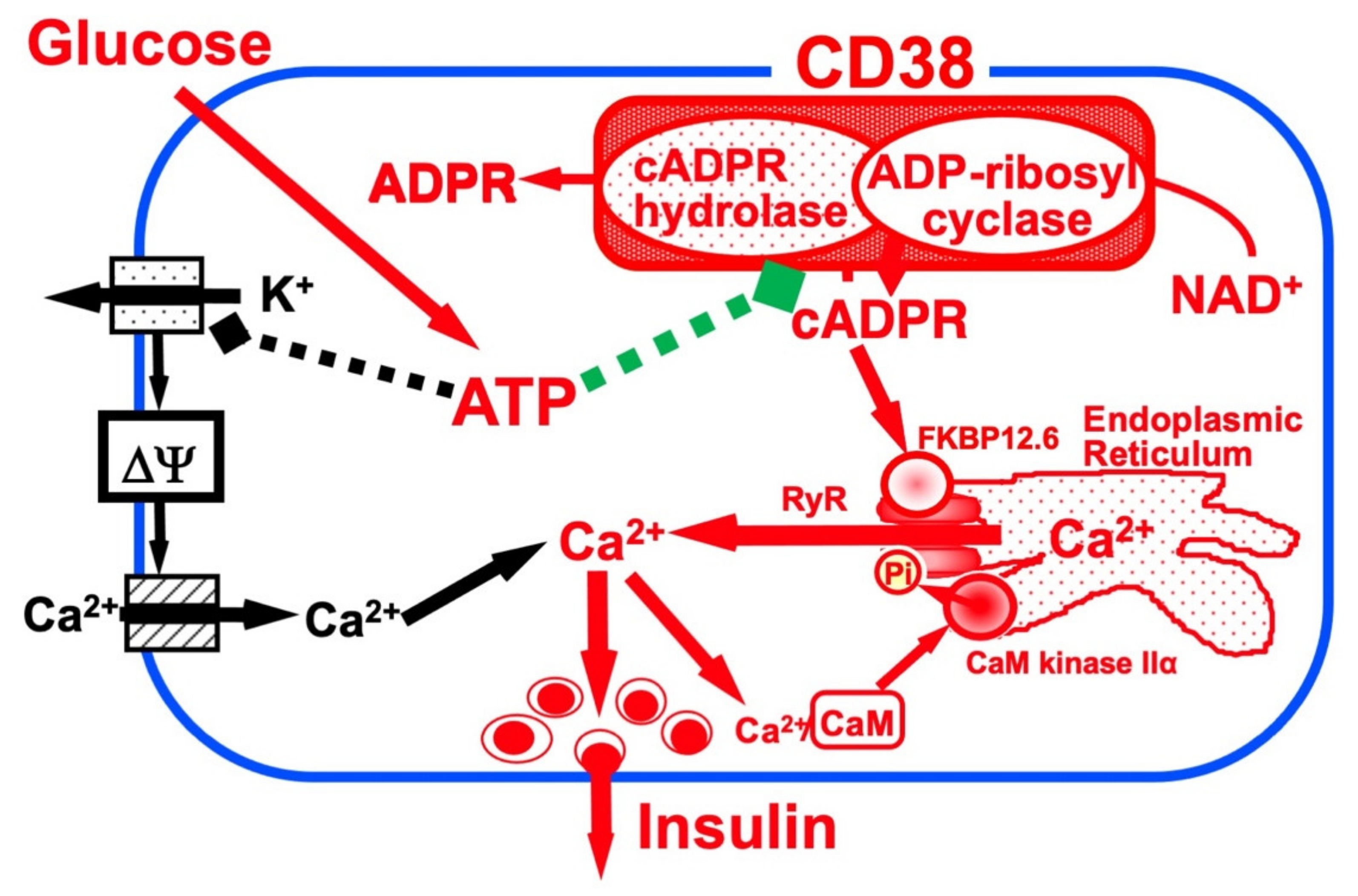
Figure 1. Insulin secretion by glucose stimulation in pancreatic β-cells [33]. The insulin secretion via the CD38-cADPR signal system is shown in red. cADPR, produced from NAD+ by the ADP-ribosyl cyclase of CD38, binds to FKBP12.6 to release Ca2+, dissociating FKBP12.6 from RyR [25]. CaM kinase II phosphorylates RyR to sensitize and activate the Ca2+ channel (Pi, phosphorylation of RyR by CaM kinase II) [24]. Ca2+, released from intracellular stores and/or supplied from extracellular sources, further activates CaM kinase II and amplifies the process. In this way, Ca2+-induced Ca2+ release (CICR) can be explained in glucose-induced insulin secretion in pancreatic β-cells. The conventional insulin secretion mechanism by Ca2+ influx from extracellular sources [16] is shown in black.
The first important issue is whether the accumulation of cADPR is actually caused by glucose stimulation in pancreatic islets. In the laboratory, the researchers assayed the cADPR content in islets isolated from normal rats (Wistar) and mice (C56BL/6J) that were incubated with low (2.8 mM) and high (20 mM) glucose by radioimmunoassay using an anti-cADPR antibody. The cADPR content in the high glucose-treated islets was rapidly increased within 5 min. In contrast, the cADPR content in the low glucose-treated islets was not [28].
Using the rat islet microsome Ca2+ releasing system, fluo 3 (2,2′-{[2-(2-{2-[Bis(caboxymethyl)amino]-5-}2,7-dichloro-6-hydroxy-3-oxo-3H-xanthen-9-yl)phenoxy]ethoxy}-4-methylphenyl]azanediyl}diacetic acid) fluorescence showed cADPR released Ca2+ [17][24][28]. IP3 did not cause the release of Ca2+, and after the addition of IP3, the islet microsomes were still responsive to cADPR. In contrast, IP3 caused a release of Ca2+ from cerebellum microsomes, and cADPR also caused a release of Ca2+. Heparin, an inhibitor of IP3 binding to its receptor, blocked the IP3-induced Ca2+ release from cerebellum microsomes but did not block the cADPR-induced Ca2+ release. These results indicate that islet microsomes respond to cADPR but not to IP3. In contrast, cerebellum microsomes respond to both cADPR and IP3, but cADPR induces the Ca2+ release via a different mechanism than that utilized by IP3.
The effect of cADPR on insulin secretion was examined using digitonin-permeabilized rat pancreatic islets. cADPR as well as Ca2+ induced insulin secretion, but IP3 did not. The combined addition of cADPR and Ca2+ did not induce significantly more insulin secretion than the addition of cADPR or Ca2+ alone. The cADPR-induced insulin secretion was inhibited by the addition of EGTA. These results indicated cADPR induced Ca2+ release from islet microsomes and the increment of Ca2+ resulted in insulin secretion from pancreatic islets [17].
From these results, glucose-induced insulin secretion via cADPR formation from NAD+ and cADPR-induced Ca2+ mobilization from the endoplasmic reticulum was proposed. Concerning the second messenger role of cADPR for glucose-induced insulin secretion, some researchers reported that cADPR-induced intracellular Ca2+ release was not observed in ob/ob mouse islets and rat insulinoma-derived RINm5F cells [19][35][36][37][38]. Malaisse et al. [39][40] measured the cADPR content in rat islets and reported that it appeared not to be significantly affected by glucose concentrations. On the other hand, the fasting of the rats before the isolation of islets and the usage of Hanks’ solution containing 2.8 mM glucose during the islet isolation may account for the rapid and significant increase in cADPR content in the islets in response to glucose stimulation. Furthermore the researchers determined the cADPR content by assessing the recovery of cADPR in the extraction and concentration procedures, and they did not. Differences in the experimental conditions may be responsible for the different results [19][28]. Nevertheless, the reports that Balb/c mouse islets showed increases in glucose-induced cADPR production, the intracellular concentration of Ca2+, and insulin secretion [41] and that human insulin promoter-SV40 large T transgene-introduced C57BL/6 mouse pancreatic β-cell-derived MIN6 cells, which show glucose-induced insulin secretion, showed a dramatic Ca2+ mobilization in response to cADPR via the ryanodine receptor (RyR) [42][43] despite the lack of response to IP3 [43]. These results indicate that the CD38-cADPR signal system is functioning in glucose-induced insulin secretion in pancreatic β-cells.
4. CD38 as a Major Enzyme for the Synthesis of cADPR
CD38 is a 300-amino-acid protein and was first recognized as a human leukocyte surface antigen. CD38 was found to express in a variety of tissues and cells, including pancreatic β-cells [12][20][44]. The researchers and others have found that CD38 has both ADP-ribosyl cyclase for cADPR synthesis from NAD+ and cADPR hydrolase for the hydrolysis of cADPR to form ADP-ribose [11][12][13][21][45]. Using purified human CD38 protein, it was found that millimolar concentrations of ATP inhibit the cADPR hydrolase activity of CD38, competing with the substrate, cADPR [12][26]. The competitive inhibition of the cADPR hydrolysis by ATP suggests that ATP and cADPR bind to the same site of CD38. The researchers purified human recombinant CD38, incubated with an ATP analogue, 5′-p-fluorosulfonylbenzoyladenosine, and identified the binding site for ATP and/or cADPR as the lysine-129 of CD38 [26]. From these results and other available evidence, the researchers proposed that CD38 catalyzes the formation of cADPR from NAD+ and also the hydrolysis of the cADPR to ADP-ribose. As shown in Figure 2, lysine-129 of CD38 is the cADPR binding site for hydrolysis to ADP-ribose and ATP competes with cADPR in the site, resulting in the inhibition of the hydrolysis of cADPR and then in the accumulation of cADPR by ATP [12][21][34].
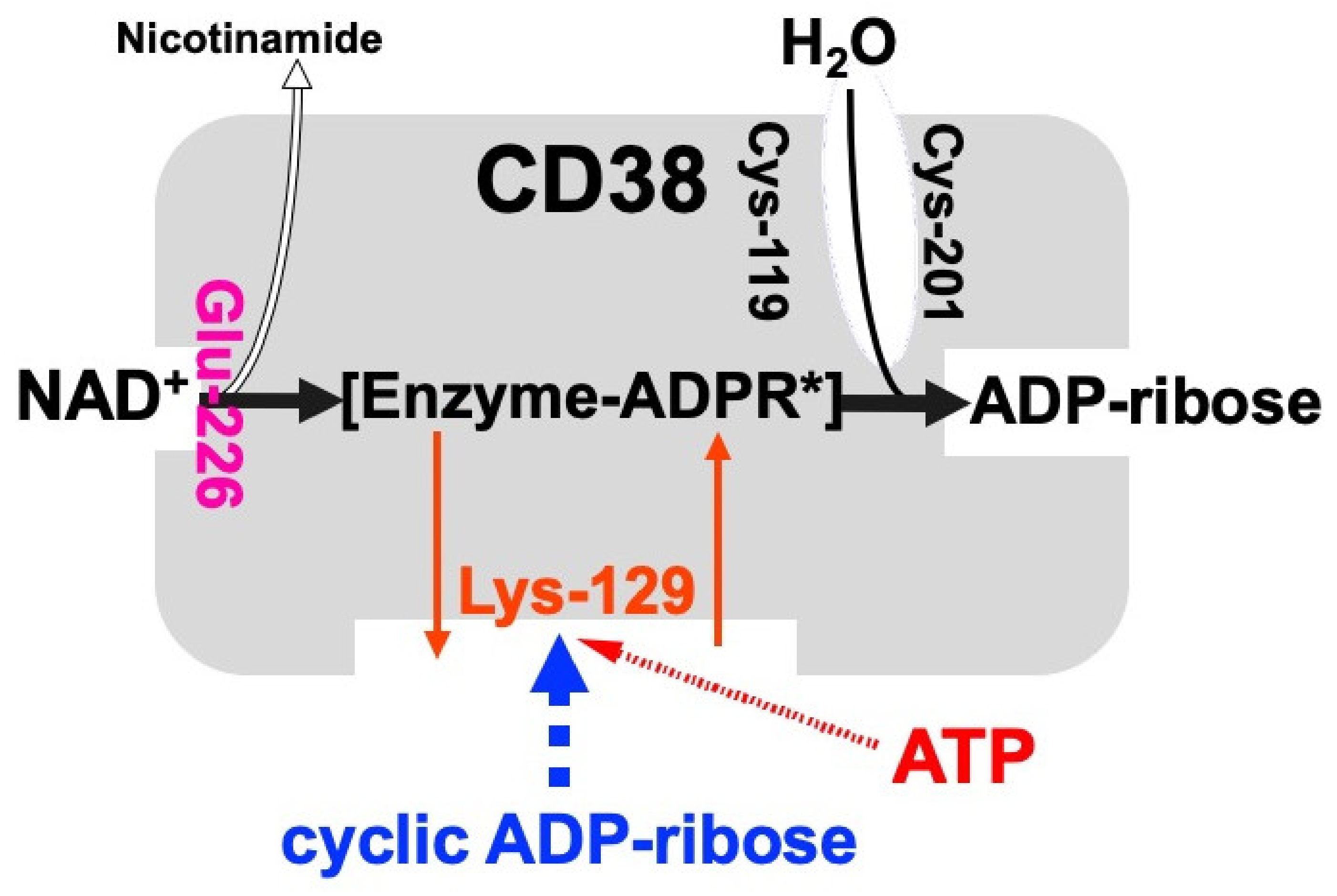
Figure 2. Schematic representation of human CD38 in enzyme activities for the synthesis and hydrolysis of cADPR [26]. Glu-226 in human CD38 is essential for cADPR synthesis from NAD+ (ADP-ribosyl cyclase activity) [46]. Cys-119 and Cys-201 are essential for cADPR hydrolysis to form ADP-ribose (cADPR hydrolase activity). Lys-129 is the cADPR binding site and is indispensable for cADPR hydrolysis (cADPR hydrolase) [21]. ATP, produced by glucose metabolism, competes with cADPR for the binding site (Lys-129), inhibiting the cADPR hydrolysis by cADPR hydrolase of CD38, which causes the accumulation of cADPR in the cell [12]. [Enzyme-cADPR*] is proposed as an enzyme-stabilized ADP-ribosyl oxocarbonium ion intermediate.
The human CD38 gene was assigned to band p15 of chromosome four as a single copy gene by in situ fluorescence hybridization [22]. The researchers then isolated the human CD38 gene and determined its primary structure [27]. The human CD38 gene as well as the Xenopus Cd38 gene extending ~70 kb [27][44] contained eight exons (Figure 3). The human and Xenopus Cd38 promoter regions have no TATA box but do have a CpG island, a methylation-controlled region more frequently associated with housekeeping than tissue-specific genes. Exon one encoded the 5′-untranslated region, translational start site, putative transmembrane domain and N-terminal region of Cd38. Exons two to eight encoded the remainder of Cd38. Cycsteines-119 and -201 of human CD38, which are indispensable for the hydrolysis of cADPR to form non-Ca2+ mobilizing ADPR [21], were encoded in exons two and five, respectively. Lysine-129, which is involved in cADPR binding to CD38 [26], was encoded in exon three. Glutamate-226 and Trp-185, which seem to play important roles in catalytic activities [46][47], were encoded in exons six and four, respectively. The 10 cysteine residues conserved among human, rat, mouse, chicken, and frog Cd38s; human, rat, mouse, chicken, and frog Cd157s; Aplysia ADP-ribosyl cyclases, purple sea urchin ADP-ribosyl cyclase, and blood fluke ADP-ribosyl cyclase were encoded into six exons [27][32][44]. The translational termination codon and 3′-untranslated region were located in exon eight.
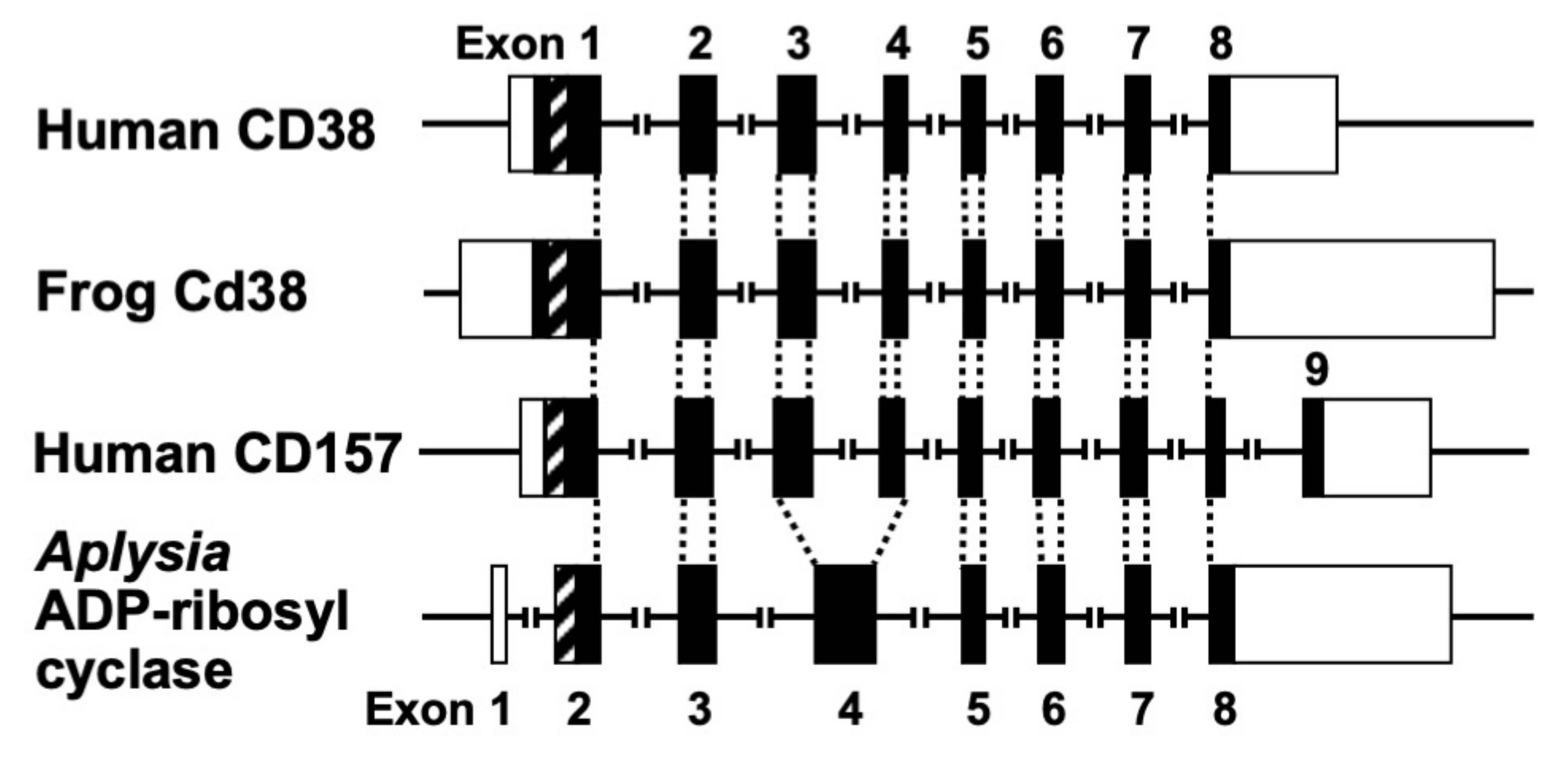
Figure 3. Structural organization of human CD38, frog Cd38, human CD157, and Aplysia kurodai ADP-ribosyl cyclase genes [9][27][44]. The exons are depicted as boxes; filled and open boxes represent protein-coding regions and untranslated regions, respectively. Recently, primate-specific exon 1b, located between exon 1 and 2 of human CD157, that encodes 15 additional amino acids, was reported [48]. Hatched boxes represent transmembrane (CD38)- or signal peptide (CD157 and Aplysia ADP-ribosyl cyclase)-coding regions. The corresponding exon-intron junctions are indicated as broken lines. Exons are numbered.
Isolation and determination of the primary structure of the Aplysia kurodai ADP-ribosyl cyclase gene [9] and the frog (Xenopus laevis) Cd38 gene [44] as well as the human CD38 gene [27] were performed. The exon–intron organization of the Aplysia ADP-ribosyl cyclase gene and the frog Cd38 gene is very similar to that of the human CD38 gene, suggesting that they evolved from a common ancestral gene [27][44] (Figure 3).
Kaisho et al. [49] found that the amino acid sequence of bone marrow stromal antigen 1 (BST-1/CD157) had significant sequence homology (~30% identity) with those of CD38 and Aplysia ADP-ribosyl cyclase. They determined the gene structure of human CD157 [50]. The gene extended for ~30 kb, very close to its paralogue CD38, and consisted of nine exons. Comparison of the gene organization reveals that the human CD157 gene has an exon–intron organization similar to that of human CD38 and Aplysia ADP-ribosyl cyclase genes (Figure 3). However, the human CD157 gene has an additional exon, exon nine, that encodes a peptide that is removed upon attachment of the glycosyl phosphatidylinositol anchor. In addition to the similar exon–intron structures of CD38, CD157 and Aplysia ADP-ribosyl cyclase genes and human CD38 and CD157 genes are located on the subregion of the human chromosome 4p15 as a next neighbor; the fact that mouse Cd157 (known as BP-3) is very close to the map site of the Cd38 gene on mouse chromosome 5 [51] strongly suggests that the three genes (CD38, CD157, and Aplysia ADP-ribosyl cyclase) have evolved from a common ancestor, and CD38 and CD157 genes were created by gene duplication before human and rodent divergence (Figure 4).
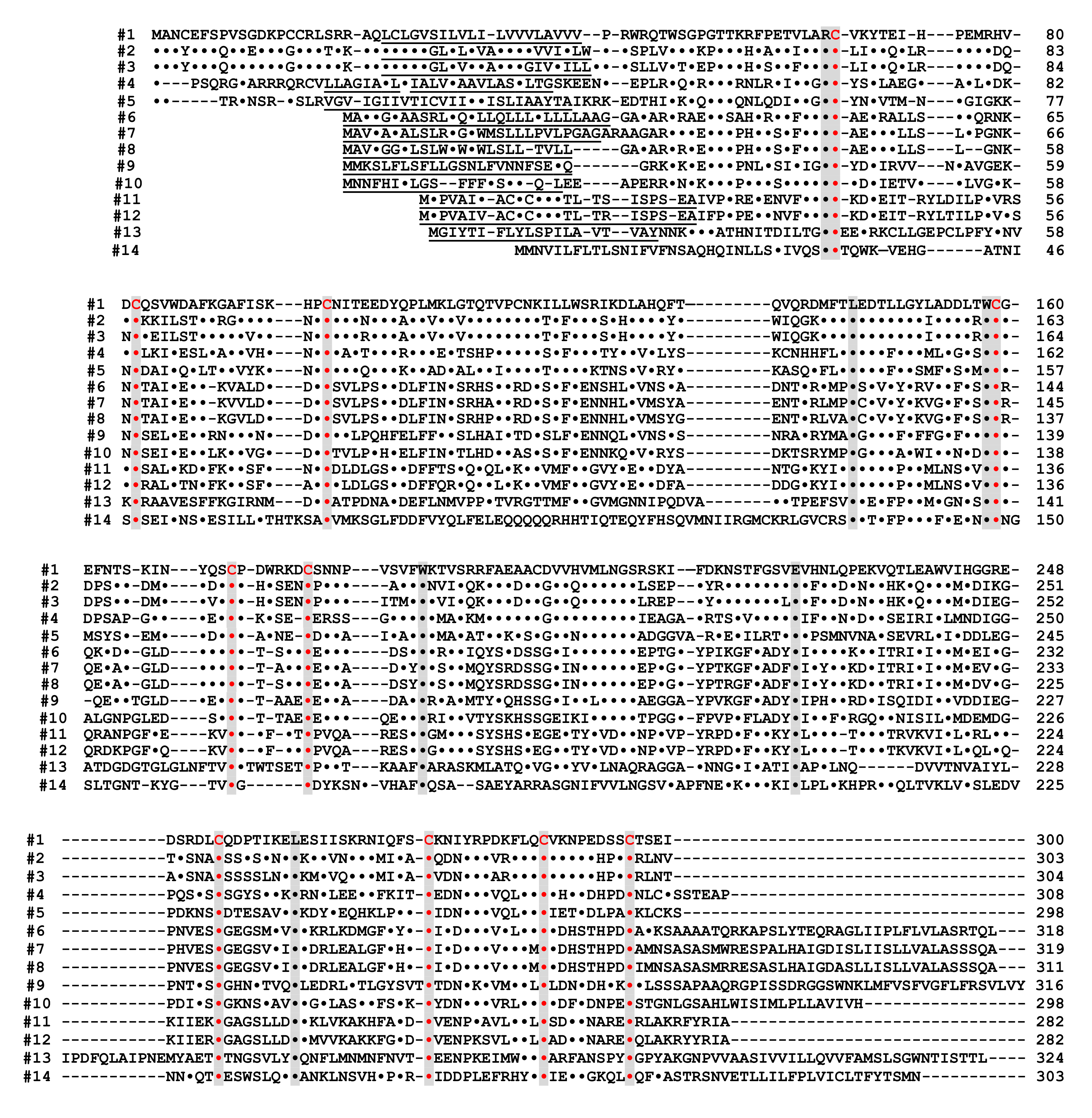
Figure 4. Alignment of the amino acid sequences of the CD38/CD157/ADP-ribosyl cyclase family based on the primary structures of the encoded proteins [44]. Dots indicate amino acids identical to human CD38. Dashes indicate gaps for maximal alignment. Ten cysteine and five amino acid residues conserved among all the proteins are highlighted in red and gray, respectively. The underlined amino acid residues are a putative transmembrane domain in CD38 and signal sequence in CD157 and ADP-ribosyl cyclase. The sequences used in alignment are #1: human CD38 (AAA68482 [52]), #2: rat CD38 (BAA06129 [20]), #3: mouse CD38 (AAA03163 [53]), #4: chicken CD38 (NM_001201388), #5: Xenopus laevis CD38 (AB194899 [44]), #6: human BST1 (CD157) (BAA04885 [47]), #7: rat BST1 (CD157) (Q63072 [51]), #8: mouse BST1 (CD157) (BAA06597 [54]), #9: chicken CD157 (NM_001200043), #10: Xenopus laevis CD157 (AB194901 [44]), #11: Aplysia californica ADP-ribosyl cyclase (AAA65698 [8]), #12: Aplysia kurodai ADP-ribosyl cyclase (BAA06284 [9]), #13: Strongylocentrotus purpuratus ADP-ribosyl cyclase (AM494973 [55]), and #14: Schistoma mansoni ADP-ribosyl cyclase (AAX35328 [56]). Although XP_005162637 (Danio rerio ADP-ribosyl cyclase 1-like [57]) showed significant homology with CD38/CD157/ADP-ribosyl cyclase family, the 8th and 10th cysteines in the conserved 10 cysteine residues (red), which are essential for the enzyme activities, are not conserved. It may be important to measure ADP-ribosyl cyclase activity in zebrafish ADP-ribosyl cyclase 1-like for understanding the involvement of the two cysteines in ADP-ribosyl cyclase activity.
The expression of Cd157 was reported in rat and mouse pancreatic β-cells [58][59]. However, no diabetic features were observed in Cd157 knockout mice [60]. Furthermore, CD157 exhibits neither cADPR-synthesizing (ADP-ribosyl cyclase) nor -hydrolyzing (cADPR hydrolase) activity in physiological conditions [14], suggesting that CD157 may mainly play a role as a surface antigen rather than a major enzyme for the synthesis of cADPR in response to glucose stimulation in pancreatic β-cells.
References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility of calcium signalling. Nat. Rev. Mol Cell Biol. 2000, 1, 11–21.
- Lee, H.C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 317–345.
- Guse, A.H.; Lee, H.C. NAADP: A universal Ca2+ trigger. Sci. Signal. 2008, 1, re10.
- Clapper, D.L.; Walseth, T.F.; Dargie, P.J.; Lee, H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987, 262, 9561–9568.
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45.
- Hellmich, M.R.; Strumwasser, F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991, 2, 193–202.
- Lee, H.C.; Aarhus, R. ADP-ribosyl cyclase: An enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991, 2, 203–209.
- Glick, D.L.; Hellmich, M.R.; Beushausen, S.; Tempst, P.; Bayley, H.; Strumwasser, F. Primary structure of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991, 2, 211–218.
- Nata, K.; Sugimoto, T.; Tohgo, A.; Takamura, T.; Noguchi, N.; Matsuoka, A.; Numakunai, T.; Shikama, K.; Yonekura, H.; Takasawa, S.; et al. The structure of the Aplysia kurodai gene encoding ADP-ribosyl cyclase, a second-messenger enzyme. Gene 1995, 158, 213–218.
- States, D.J.; Walseth, T.F.; Lee, H.C. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human leukocyte antigen CD38. Trends. Biochem. Sci. 1992, 17, 495.
- Howard, M.; Grimaldi, J.C.; Bazan, J.F.; Lund, F.E.; Santos-Argumedo, L.; Parkhouse, R.M.; Walseth, T.F.; Lee, H.C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993, 262, 1056–1059.
- Takasawa, S.; Tohgo, A.; Noguchi, N.; Koguma, T.; Nata, K.; Sugimoto, T.; Yonekura, H.; Okamoto, H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J. Biol. Chem. 1993, 268, 26052–26054.
- Summerhill, R.J.; Jackson, D.G.; Galione, A. Human leukocyte antigen CD38 catalyzes the production of cyclic ADP-ribose. FEBS Lett. 1993, 335, 231–233.
- Hirata, Y.; Kimura, N.; Sato, K.; Ohsugi, Y.; Takasawa, S.; Okamoto, H.; Ishikawa, J.; Kaisho, T.; Ishihara, K.; Hirano, T. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 1994, 356, 244–248.
- Chini, E.N.; Chini, C.C.; Kato, I.; Takasawa, S.; Okamoto, H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem. J. 2002, 362, 125–130.
- Ashcroft, F.M.; Harrison, D.E.; Ashcroft, S.J.H. Glucose induced closure of single potassium channels in isolated rat pancreatic β-cells. Nature 1984, 312, 446–448.
- Takasawa, S.; Nata, K.; Yonekura, H.; Okamoto, H. Cyclic ADP-ribose in insulin secretion from pancreatic β cells. Science 1993, 259, 370–373.
- Okamoto, H.; Takasawa, S.; Tohgo, H. New aspects of the physiological significance of NAD, poly ADP-ribose and cyclic ADP-ribose. Biochimie 1995, 77, 356–363.
- Okamoto, H.; Takasawa, S.; Nata, K. The CD38-cyclic ADP-ribose signalling system in insulin secretion: Molecular basis and clinical implications. Diabetologia 1997, 40, 1485–1491.
- Koguma, T.; Takasawa, S.; Tohgo, A.; Karasawa, T.; Furuya, Y.; Yonekura, H.; Okamoto, H. Cloning and characterization of cDNA encoding rat ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (homologue to human CD38) from islets of Langerhans. Biochim. Biophys. Acta 1994, 1223, 160–162.
- Tohgo, A.; Takasawa, S.; Noguchi, N.; Koguma, T.; Nata, K.; Sugimoto, T.; Furuya, Y.; Yonekura, H.; Okamoto, H. Essential cysteine residues for cyclic ADP-ribose synthesis and hydrolysis by CD38. J. Biol. Chem. 1994, 269, 28555–28557.
- Nakagawara, K.; Mori, M.; Takasawa, S.; Nata, K.; Takamura, T.; Berlova, A.; Tohgo, A.; Karasawa, T.; Yonekura, H.; Takeuchi, T.; et al. Assignment of CD38, the gene encoding human leukocyte antigen CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase), to chromosome 4p15. Cytogenet. Cell Genet. 1995, 69, 38–39.
- Kato, I.; Takasawa, S.; Akabane, A.; Tanaka, O.; Abe, H.; Takamura, T.; Suzuki, Y.; Nata, K.; Yonekura, H.; Yoshimoto, T.; et al. Regulatory role of CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) in insulin secretion by glucose in pancreatic β cells. Enhanced insulin secretion in CD38-expressing transgenic mice. J. Biol. Chem. 1995, 270, 30045–30050.
- Takasawa, S.; Ishida, A.; Nata, K.; Nakagawa, K.; Noguchi, N.; Tohgo, A.; Kato, I.; Yonekura, H.; Fujisawa, H.; Okamoto, H. Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization. J. Biol. Chem. 1995, 270, 30257–30259.
- Noguchi, N.; Takasawa, S.; Nata, K.; Tohgo, A.; Kato, I.; Ikehata, F.; Yonekura, H.; Okamoto, H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J. Biol. Chem. 1997, 272, 3133–3136.
- Tohgo, A.; Munakata, H.; Takasawa, S.; Nata, K.; Akiyama, T.; Hayashi, N.; Okamoto, H. Lysine 129 of CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) participates in the binding of ATP to inhibit the cyclic ADP-ribose hydrolase. J. Biol. Chem. 1997, 272, 3879–3882.
- Nata, K.; Takamura, T.; Karasawa, T.; Kumagai, T.; Hashioka, W.; Tohgo, A.; Yonekura, H.; Takasawa, S.; Nakamura, S.; Okamoto, H. Human gene encoding CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase): Organization, nucleotide sequence and alternative splicing. Gene 1997, 186, 285–292.
- Takasawa, S.; Akiyama, T.; Nata, K.; Kuroki, M.; Tohgo, A.; Noguchi, N.; Kobayashi, S.; Kato, I.; Katada, T.; Okamoto, H. Cyclic ADP-ribose and inositol 1,4,5-trisphosphate as alternate second messengers for intracellular Ca2+ mobilization in normal and diabetic β-cells. J. Biol. Chem. 1998, 273, 2497–2500.
- Yagui, K.; Shimada, F.; Miura, M.; Hashimoto, N.; Suzuki, Y.; Tokuyama, Y.; Nata, K.; Tohgo, A.; Ikehata, F.; Takasawa, S.; et al. A missense mutation in the CD38 gene, a novel factor for insulin secretion: Association with Type II diabetes mellitus in Japanese subjects and evidence of abnormal function when expressed in vitro. Diabetologia 1998, 41, 1024–1028.
- Ikehata, F.; Satoh, J.; Nata, K.; Tohgo, A.; Nakazawa, T.; Kato, I.; Kobayashi, S.; Akiyama, T.; Takasawa, S.; Toyota, T.; et al. Autoantibodies against CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) that impair glucose-induced insulin secretion in noninsulin- dependent diabetes patients. J. Clin. Investig. 1998, 102, 395–401.
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, i, and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872.
- Okamoto, H.; Takasawa, S.; Nata, K.; Kato, I.; Tohgo, A.; Noguchi, N. Physiological and pathological significance of the CD38-cyclic ADP-ribose signaling system. Chem. Immunol. 2000, 75, 121–145.
- Takasawa, S.; Okamoto, H. Pancreatic β-cell death, regeneration and insulin secretion: Roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. Int. J. Diabetes Res. 2002, 3, 79–96.
- Okamoto, H.; Takasawa, S. Recent advances in the Okamoto model: The CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in β-cells. Diabetes 2002, 51 (Suppl. S3), S462–S473.
- Islam, M.S.; Larsson, O.; Berggren, P.O.; Takasawa, S.; Nata, K.; Yonekura, H.; Okamoto, H.; Galione, A. Cyclic ADP-ribose in β cells. Science 1993, 262, 584–586.
- Rutter, G.A.; Theler, J.-M.; Wollheim, C.B. Ca2+ stores in insulin-secreting cells: Lack of the effect of cADP ribose. Cell Calcium 1994, 16, 71–80.
- Webb, D.-L.; Islam, M.S.; Efanov, A.M.; Brown, G.; Köhler, M.; Larsson, O.; Berggren, P.-O. Insulin exocytosis and glucose-mediated increase in cytoplasmic free Ca2+ concentration in the pancreatic β-cell are independent of cyclic ADP-ribose. J. Biol. Chem. 1996, 271, 19074–19079.
- Islam, M.S.; Berggren, P.O. Cyclic ADP-ribose and the pancreatic beta cell: Where do we stand? Diabetologia 1997, 40, 1480–1484.
- Malaisse, W.J.; Kanda, Y.; Inageda, K.; Scruel, O.; Sener, A.; Katada, T. Cyclic ADP-ribose measurements in rat pancreatic islets. Biochem. Biophys. Res. Commun. 1997, 231, 546–548.
- Scruel, O.; Wada, T.; Kontani, K.; Sener, A.; Katada, T.; Malaisse, W.J. Effects of D-glucose and starvation upon the cyclic ADP-ribose content of rat pancreatic islets. Biochem. Mol. Biol. Int. 1998, 45, 783–790.
- An, N.H.; Han, M.K.; Um, C.; Park, B.H.; Park, B.J.; Kim, H.K.; Kim, U.H. Significance of ecto-cyclase activity of CD38 in insulin secretion of mouse pancreatic islet cells. Biochem. Biophys. Res. Commun. 2001, 282, 781–786.
- Varadi, A.; Rutter, G.A. Dynamic imaging of endoplasmic reticulum Ca2+ concentration in insulin-secreting MIN6 cells using recombinant targeted cameleons: Roles of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2 and ryanodine receptors. Diabetes 2002, 51, S190–S201.
- Mitchell, K.J.; Pinton, P.; Varadi, A.; Tacchetti, C.; Ainscow, E.K.; Pozzan, T.; Rizzuto, R.; Rutter, G.A. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J. Cell Biol. 2001, 155, 41–51.
- Ikeda, T.; Takasawa, S.; Noguchi, N.; Nata, K.; Yamauchi, A.; Takahashi, I.; Yoshikawa, T.; Sugawara, A.; Yonekura, H.; Okamoto, H. Identification of a major enzyme for the synthesis and hydrolysis of cyclic ADP-ribose in amphibian cells and evolutional conservation of the enzyme from human to invertebrate. Mol. Cell. Biochem. 2012, 366, 69–80.
- Zocchi, E.; Franco, L.; Guida, L.; Benatti, U.; Bagellesi, A.; Malavasi, F.; Lee, H.C.; De Flora, A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993, 196, 1459–1465.
- Munshi, C.; Thiel, D.J.; Mathews, I.I.; Aarhus, R.; Walseth, T.F.; Lee, H.C. Characterization of the active site of ADP-ribosyl cyclase. J. Biol. Chem. 1999, 274, 30770–30777.
- Okamoto, H.; Takasawa, S. CD38. In Encyclopedia of Molecular Medicine; Creighton, T.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 601–604.
- Ferrero, E.; Lo Buono, N.; Morone, S.; Parrotta, R.; Mancini, C.; Brusco, A.; Giancomino, A.; Augeri, S.; Rosal-Vela, A.; García-Rodríguez, S.; et al. Human canonical CD157/Bst1 is an alternatively spliced isoform masking a previously unidentified primate-specific exon included in a novel transcript. Sci. Rep. 2017, 7, 15923.
- Kaisho, T.; Ishikawa, J.; Oritani, K.; Inazawa, J.; Muraoka, O.; Ochi, T.; Hirano, T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc. Natl. Acad. Sci. USA 1994, 91, 5325–5329.
- Muraoka, O.; Tanaka, H.; Itoh, M.; Ishihara, K.; Hirano, T. Genomic structure of human BST-1. Immunol. Lett. 1996, 54, 1–4.
- Dong, C.; Willerford, D.; Alt, F.W.; Cooper, M.D. Genomic organization and chromosomal localization of the mouse BP3 gene, a member of the CD38/ADP-ribosy cyclase family. Immunogenetics 1996, 45, 35–43.
- Jackson, D.G.; Bell, J.I. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J. Immunol. 1990, 144, 2811–2815.
- Harada, N.; Santos-Arugumedo, L.; Chang, R.; Grimaldi, J.C.; Lund, F.E.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; Heath, A.W.; Parkhouse, R.M. Expression cloning of a cDNA encoding a novel murine B cell activation marker. Homology to human CD38. J. Immunol. 1993, 151, 3111–3118.
- Itoh, M.; Ishihara, K.; Tomizawa, H.; Tanaka, H.; Kobune, Y.; Ishikawa, J.; Kaisho, T.; Hirano, T. Molecular cloning of murine BST-1 having homology with CD38 and Aplysia ADP-ribosyl cyclase. Biochem. Biophys. Res. Commun. 1994, 203, 1309–1317.
- Churamani, D.; Boulware, M.J.; Geach, T.J.; Martin, A.C.R.; Moy, G.W.; Su, Y.-H.; Vacquier, V.D.; Marchant, J.S.; Dale, L.; Patel, S. Molecular characterization of a novel intracellular ADP-ribosyl cyclase. PLoS ONE 2007, 2, e797.
- Goodrich, S.P.; Muller-Steffner, M.; Osman, A.; Moutin, M.-J.; Kusser, K.; Roberts, A.; Woodland, D.L.; Randall, T.D.; Kellenberger, E.; LoVerde, P.T.; et al. Production of calcium-mobilizing metabolites by a novel member of the ADP-ribosyl cyclase family expressed in Schistosoma mansoni. Biochemistry 2005, 44, 11082–11097.
- Kelu, J.J.; Web, S.E.; Galione, A.; Miller, A.L. Characterization of ADP-ribosyl cyclase 1-like (ARC1-like) activity and NAADP signaling during slow muscle cell development in zebrafish embryos. Dev. Biol. 2019, 445, 211–225.
- Furuya, Y.; Takasawa, S.; Yonekura, H.; Tanaka, T.; Takehara, J.; Okamoto, H. Cloning of a cDNA encoding rat bone marrow stromal cell antigen 1 (BST-1) from the islets of Langerhans. Gene 1995, 165, 329–330.
- Kajimoto, Y.; Miyagawa, J.; Ishihara, K.; Okuyama, Y.; Fujitani, Y.; Itoh, M.; Yoshida, H.; Kaisho, T.; Matsuoka, T.; Watada, H.; et al. Pancreatic islets cells express BST-1, a CD38-like surface molecule having ADP-ribosyl cyclase activity. Biochem. Biophys. Res. Commun. 1996, 219, 941–946.
- Itoh, M.; Ishihara, K.; Hiroi, T.; Lee, B.O.; Maeda, H.; Iijima, H.; Yanagita, M.; Kiyono, H.; Hirano, H. Deletion of bone marrow stromal cell antigen-1 (CD157) gene impaired systemic thymus independent-2 antigen-induced IgG3 and mucosal TD antigen-elicited IgA responses. J. Immunol. 1998, 161, 3974–3983.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
20 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




