
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ange Booka Ilangala | -- | 4250 | 2022-04-19 10:36:30 | | | |
| 2 | Peter Tang | Meta information modification | 4250 | 2022-04-19 11:10:00 | | |
Video Upload Options
Regenerative medicine is one of the most attractive topics of research worldwide. Different strategies are proposed, and a range of materials of various forms and compositions tailored for tissue engineering are developed, but this approach just started to emerge in clinics. Biodegradable microparticles (MPs) made from degradable and biocompatible polymers, with a mean diameter of ~200 μm, are attractive not only as 3D matrices to multiply cells but also as a scaffold to support tissue rebuilding.
1. Introduction
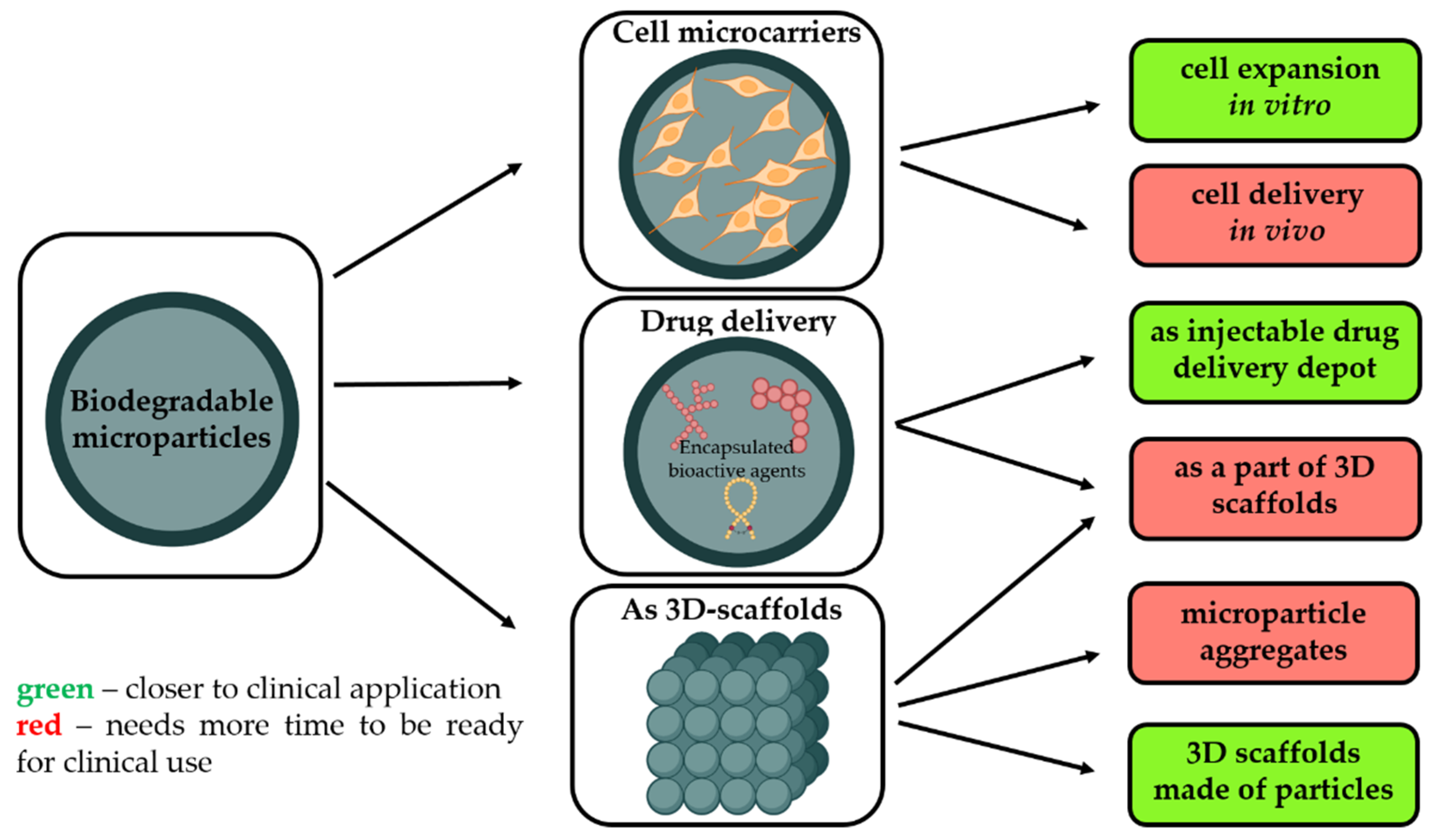
- As a temporary microcarrier to support expansion of cells initially cultivated in vitro [3]. Biodegradability is a crucial characteristic to avoid main technological issues related to cell multiplication on non-degradable microcarriers, i.e., poor yield of cell detachment, contamination related to enzymes requested to harvest cells, and difficulties to separate microparticle debris from free cells. Moreover, as these microcarriers are made from safe and degradable polymers, these cell microcarriers could be injected into the targeted tissues to restore them.
- Without pre-culture with cells in order to provide a sustained and local release of growth factors selected to promote tissue rebuilding while also offering a large surface to enhance in vivo cell adhesion.
- As a part of other types of 3D scaffolds for tissue engineering, including the application of biodegradable microparticles as starting building blocks to generate 3D scaffolds with well-defined architecture, adopting additive technologies or other techniques.
2. Fabrication and Modification of Polymeric-Based Microparticles (MPs)
2.1. Materials for MPs Fabrication
|
Material |
Chemical Nature, Crystallinity, Thermal Properties |
Range of Degradation Rate and the Main Route of Degradation |
Functionality |
Advantages |
Drawbacks |
Approval Status |
Ref. |
|---|---|---|---|---|---|---|---|
|
Synthetic polymers |
|||||||
|
PCL |
Aliphatic polyester; Semi-crystalline; Tg: −60 °C; Tm: 60 °C |
>1 year Ester hydrolysis |
Hydrophobic material; Limited to aliphatic ester functions; Residual organic solvent content; |
Macromolecular features and purity are well-controlled and reliable; Chemical purity is under control; Degradation rate can be easily adjusted in function of the Mw, tacticity, and crystallization %; Easy processability. |
Lack of cell adhesion moieties; Release of acidic by-products during degradation. |
FDA-approved |
[39] |
|
PLA |
Aliphatic polyester; Semi-crystalline or amorphous; Tg: 40 °C; Tm: 180 °C |
>0.6 year Ester hydrolysis |
[40] |
||||
|
PLGA |
Aliphatic polyester; Semi-crystalline or amorphous; Tg: 40 °C; Tm: 180 °C |
>0.3 year Ester hydrolysis |
[41] |
||||
|
Natural polymers |
|||||||
|
Alginates |
Anionic polysaccharides copolymers |
Enzymatic degradation pathway |
Carboxyl groups; Polyelectrolyte. |
Gel-forming ability; Hydrophilicity. |
No cell adhesion characteristics; Lack of control of the macromolecular features (Mw, polydispersity, purity). |
FDA-approved |
[42] |
|
Collagen |
Natural protein present in the extracellular matrices of tissues |
Enzymatic degradation pathway |
Carboxyl and amino groups |
Cell adhesion and proliferation enhancement; Hydrophilicity. |
Risk of allergic reactions; Low mechanical properties. |
FDA-approved |
[13] |
|
Chitosan |
Cationic polysaccharides copolymers. |
Enzymatic degradation pathway |
Primary amino-groups |
Positive charge; Cell adhesion enhancement; Hydrophilicity. |
Lack of control of the macromolecular features (Mw, polydispersity, purity); Difficulty of processing (not soluble in aqueous medium at neutral pH). |
Not approved as pharmaceutical excipient; Under clinical testing as an implant. |
[43] |
|
PHAs |
Polymers with high structural diversity; Semi-crystalline. |
Enzymatic and hydrolytic degradation |
Ester functions |
Cell proliferation stimulation; Hydrophilicity; Controllable mechanical and thermal properties. |
Low mechanical properties |
Not approved |
[44] |
|
Silk fibroin |
Natural protein isolated from animals. |
Enzymatic degradation pathway |
Carboxyl and amino groups |
Cell proliferation stimulation; Hydrophilicity; Gel-forming material |
High risk of allergic reactions. |
Not approved |
[45] |
2.2. Methods of MPs Fabrication
2.3. Microparticle-Based and Microparticle-Contained 3D Structures
- Aggregation of cell-free microparticles via polymer/polymer aggregation or assembly of microparticles with pre-cultured cells through cell/cell interactions;
- Microparticles as filling material to other types of matrices, including the application of them as drug depot, functional fillers to regulate the physico-mechanical properties of the matrix as well as cell-seeded microcarriers within bioinks;
- Microparticles without cells as building blocks for the fabrication of 3D scaffolds.
3. Biomedical Applications of MPs
3.1. Microparticles as Drug Delivery Depot to Promote Tissue Reconstruction
- Their local administration is minimally invasive and feasible into limited accessible sites;
- Their high surface/volume ratio is favorable and reported to be particularly suited as cell supporting microcarriers;
- Being made from well-known biocompatible and biodegradable polyesters, such as PLGA;
- Their degradation rate can be easily adjusted to balance growth factor release kinetics, cell supporting amplification, and mechanical support [55];
- The large scale GMP production of these drug delivery microparticles is already known and applied for several years;
- They are simple products, free of animal cells, and easy to submit to regulatory bodies;
- There is an opportunity to physically combine these microparticles with autologous stem cells just before implantation on a patient.
3.2. Microcarriers for Cell Expansion
3.3. Microparticle-Containing 3D Scaffolds for Regenerative Medicine
References
- Hunsberger, J.G.; Shupe, T.; Atala, A. An Industry-Driven Roadmap for Manufacturing in Regenerative Medicine. Stem Cells Transl. Med. 2018, 7, 564–568.
- Oliveira, M.B.; Mano, J.F. Polymer-Based Microparticles in Tissue Engineering and Regenerative Medicine. Biotechnol. Prog. 2011, 27, 897–912.
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and Detachment Strategies in Microcarrier-Based Cell Culture Technology: A Comprehensive Review. Mater. Sci. Eng. C 2019, 103, 109782.
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the Market: The Evolution of Biodegradable Drug Delivery Systems. Appl. Sci. 2022, 12, 935.
- Ratcliffe, E.; Thomas, R.J.; Williams, D.J. Current Understanding and Challenges in Bioprocessing of Stem Cell-Based Therapies for Regenerative Medicine. Br. Med. Bull. 2011, 100, 137–155.
- Krause, M.; Phan, T.G.; Ma, H.; Sobey, C.G.; Lim, R. Cell-Based Therapies for Stroke: Are We There Yet? Front. Neurol. 2019, 10, 656.
- Laso-García, F.; Diekhorst, L.; Gómez-de Frutos, M.C.; Otero-Ortega, L.; Fuentes, B.; Ruiz-Ares, G.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Cell-Based Therapies for Stroke: Promising Solution or Dead End? Mesenchymal Stem Cells and Comorbidities in Preclinical Stroke Research. Front. Neurol. 2019, 10, 332.
- McKee, C.; Chaudhry, G.R. Advances and Challenges in Stem Cell Culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77.
- Chan, S.W.; Rizwan, M.; Yim, E.K.F. Emerging Methods for Enhancing Pluripotent Stem Cell Expansion. Front. Cell Dev. Biol. 2020, 8, 70.
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810.
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282.
- Demina, T.S.; Akopova, T.A.; Zelenetsky, A.N. Materials Based on Chitosan and Polylactide: From Biodegradable Plastics to Tissue Engineering Constructions. Polym. Sci. Ser. C 2021, 63, 219–226.
- Browne, S.; Zeugolis, D.I.; Pandit, A. Collagen: Finding a Solution for the Source. Tissue Eng. Part A 2013, 19, 1491.
- Zakir Hossain, K.M.; Patel, U.; Ahmed, I. Development of Microspheres for Biomedical Applications: A Review. Prog. Biomater. 2014, 4, 1–19.
- Yao, R.; Zhang, R.; Luan, J.; Lin, F. Alginate and Alginate/Gelatin Microspheres for Human Adipose-Derived Stem Cell Encapsulation and Differentiation. Biofabrication 2012, 4, 025007.
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable Alginate/Hydroxyapatite Gel Scaffold Combined with Gelatin Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 63, 274–284.
- Mahou, R.; Vlahos, A.E.; Shulman, A.; Sefton, M.V. Interpenetrating Alginate-Collagen Polymer Network Microspheres for Modular Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3704–3712.
- Bi, Y.-G.; Lin, Z.-T.; Deng, S.-T. Fabrication and Characterization of Hydroxyapatite/Sodium Alginate/Chitosan Composite Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 100, 576–583.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Francis Suh, J.K.; Matthew, H.W.T. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598.
- Sivashankari, P.R.; Prabaharan, M. Prospects of Chitosan-Based Scaffolds for Growth Factor Release in Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389.
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An Open Multicenter Comparative Randomized Clinical Study on Chitosan. Wound Repair Regen. 2015, 23, 518–524.
- Hong, S.; Hsu, H.J.; Kaunas, R.; Kameoka, J. Collagen Microsphere Production on a Chip. Lab A Chip 2012, 12, 3277–3280.
- Wang, J.; Sun, X.; Zhang, Z.; Wang, Y.; Huang, C.; Yang, C.; Liu, L.; Zhang, Q. Silk Fibroin/Collagen/Hyaluronic Acid Scaffold Incorporating Pilose Antler Polypeptides Microspheres for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2019, 94, 35–44.
- Helary, C.; Browne, S.; Mathew, A.; Wang, W.; Pandit, A. Transfection of Macrophages by Collagen Hollow Spheres Loaded with Polyplexes: A Step towards Modulating Inflammation. Acta Biomater. 2012, 8, 4208–4214.
- Hayashi, K.; Tabata, Y. Preparation of Stem Cell Aggregates with Gelatin Microspheres to Enhance Biological Functions. Acta Biomater. 2011, 7, 2797–2803.
- Basu, A.; Domb, A.J. Recent Advances in Polyanhydride Based Biomaterials. Adv. Mater. 2018, 30, 1706815.
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H.H.; Campos, E. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021.
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A Micro-Ark for Cells: Highly Open Porous Polyhydroxyalkanoate Microspheres as Injectable Scaffolds for Tissue Regeneration. Adv. Mater. 2018, 30, 1802273.
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261.
- Dwivedi, R.; Pandey, R.; Kumar, S.; Mehrotra, D. Poly Hydroxyalkanoates (PHA): Role in Bone Scaffolds. J. Oral Biol. Craniofacial Res. 2020, 10, 389–392.
- Bhardwaj, N.; Rajkhowa, R.; Wang, X.; Devi, D. Milled Non-Mulberry Silk Fibroin Microparticles as Biomaterial for Biomedical Applications. Int. J. Biol. Macromol. 2015, 81, 31–40.
- Mwangi, T.K.; Bowles, R.D.; Tainter, D.M.; Bell, R.D.; Kaplan, D.L.; Setton, L.A. Synthesis and Characterization of Silk Fibroin Microparticles for Intra-Articular Drug Delivery. Int. J. Pharm. 2015, 485, 7–14.
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-Based Microspheres Prepared Using Solid-State Copolymerized Chitosan and D, L -Lactide. Mater. Sci. Eng. C 2016, 59, 333–338.
- Yang, L.; Zhang, J.; He, J.; Zhang, J.; Gan, Z. Fabrication, Hydrolysis and Cell Cultivation of Microspheres from Cellulose-Graft-Poly(l-Lactide) Copolymers. RSC Adv. 2016, 6, 17617–17623.
- Demina, T.S.; Drozdova, M.G.; Sevrin, C.; Compère, P.; Akopova, T.A.; Markvicheva, E.; Grandfils, C. Biodegradable Cell Microcarriers Based on Chitosan/Polyester Graft-Copolymers. Molecules 2020, 25, 1949.
- Fu, H.; Rahaman, M.N.; Brown, R.F.; Day, D.E. Evaluation of BSA Protein Release from Hollow Hydroxyapatite Microspheres into PEG Hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2245.
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Reis, R.L. Materials in Particulate Form for Tissue Engineering. 2. Applications in Bone. J. Tissue Eng. Regen. Med. 2007, 1, 97–109.
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388.
- Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846.
- Cipurković, A.; Horozić, E.; Đonlagić, N.; Marić, S.; Saletović, M.; Ademović, Z. Biodegradable Polymers: Production, Properties and Application in Medicine. Technol. Acta Sci./Prof. J. Chem. Technol. 2018, 11, 25–35.
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823.
- Ahsan, S.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109.
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.M.; Zaharia, C.; Andrei, E.R. Methods of Synthesis, Properties and Biomedical Applications of Polyhydroxyalkanoates: A Review. J. Biomater. Sci. 2019, 30, 695–712.
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16.
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20.
- da Costa, R.C.; Pereira, E.D.; Silva, F.M.; de Jesus, E.O.; Souza, F.G. Drug Micro-Carriers Based on Polymers and Their Sterilization. Chem. Chem. Technol. 2018, 12, 473–487.
- Meng, F.; Jiang, Y.; Sun, Z.; Yin, Y.; Li, Y. Electrohydrodynamic Liquid Atomization of Biodegradable Polymer Microparticles: Effect of Electrohydrodynamic Liquid Atomization Variables on Microparticles. J. Appl. Polym. Sci. 2009, 113, 526–534.
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4.
- Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.C.; Paik, P.; Ficai, D.; et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Appl. Sci. 2021, 11, 5090.
- Bhujel, R.; Maharjan, R.; Kim, N.A.; Jeong, S.H. Practical Quality Attributes of Polymeric Microparticles with Current Understanding and Future Perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102608.
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A Comprehensive Clinical Review of Recombinant Human Bone Morphogenetic Protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734.
- Dimar, J.R.; Glassman, S.D.; Burkus, J.K.; Pryor, P.W.; Hardacker, J.W.; Carreon, L.Y. Clinical and Radiographic Analysis of an Optimized RhBMP-2 Formulation as an Autograft Replacement in Posterolateral Lumbar Spine Arthrodesis. J. Bone Jt. Surg.-Ser. A 2009, 91, 1377–1386.
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469.
- Simitzi, C.; Vlahovic, M.; Georgiou, A.; Keskin-Erdogan, Z.; Miller, J.; Day, R.M. Modular Orthopaedic Tissue Engineering With Implantable Microcarriers and Canine Adipose-Derived Mesenchymal Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 816.
- Perez, R.A.; El-Fiqi, A.; Park, J.H.; Kim, T.H.; Kim, J.H.; Kim, H.W. Therapeutic Bioactive Microcarriers: Co-Delivery of Growth Factors and Stem Cells for Bone Tissue Engineering. Acta Biomater. 2014, 10, 520–530.
- Karam, J. Development of Pharmacologically Active Microcarriers Transporting Stem Cells and Releasing Growth Factors for Cardiac Tissue-Engineering. Ph.D. Thesis, HAL, Université d’Angers, Angers, France, 2014.
- Barcak, E.A.; Beebe, M.J. Bone Morphogenetic Protein: Is There Still a Role in Orthopedic Trauma in 2017? Orthop. Clin. North Am. 2017, 48, 301–309.
- Krishnakumar, G.S.; Roffi, A.; Reale, D.; Kon, E.; Filardo, G. Clinical Application of Bone Morphogenetic Proteins for Bone Healing: A Systematic Review. Int. Orthop. 2017, 41, 1073–1083.
- Seeherman, H.J.; Berasi, S.P.; Brown, C.T.; Martinez, R.X.; Sean Juo, Z.; Jelinsky, S.; Cain, M.J.; Grode, J.; Tumelty, K.E.; Bohner, M.; et al. A BMP/Activin A Chimera Is Superior to Native BMPs and Induces Bone Repair in Nonhuman Primates When Delivered in a Composite Matrix. Sci. Transl. Med. 2019, 11, eaar4953.
- Somers, P.; Cornelissen, R.; Thierens, H.; van Nooten, G. An Optimized Growth Factor Cocktail for Ovine Mesenchymal Stem Cells. Growth Factors 2012, 30, 37–48.
- Ding, Z.-Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel Applications of Platelet Concentrates in Tissue Regeneration (Review). Exp. Ther. Med. 2021, 21, 226.
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22.
- Insights, G.T.; Therapy, G.; Rafiq, Q.A. Toward a Scalable and Consistent Manufacturing Process for the Production of Human MSCs. Cell Gene Ther. Insights 2016, 2, 127–140.
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.Y.; Weng Oh, S.K.; Warkiani, M.E. Large-Scale Production of Stem Cells Utilizing Microcarriers: A Biomaterials Engineering Perspective from Academic Research to Commercialized Products. Biomaterials 2018, 181, 333–346.
- Van Wezel, A.L. Growth of Cell-strains and Primary Cells on Micro-carriers in Homogeneous Culture. Nature 1967, 216, 64–65.
- Chen, X.Y.; Chen, J.Y.; Tong, X.M.; Mei, J.G.; Chen, Y.F.; Mou, X.Z. Recent Advances in the Use of Microcarriers for Cell Cultures and Their Ex Vivo and in Vivo Applications. Biotechnol. Lett. 2020, 42, 1–10.
- Amer, M.H.; Alvarez-Paino, M.; McLaren, J.; Pappalardo, F.; Trujillo, S.; Wong, J.Q.; Shrestha, S.; Abdelrazig, S.; Stevens, L.A.; Lee, J.B.; et al. Designing Topographically Textured Microparticles for Induction and Modulation of Osteogenesis in Mesenchymal Stem Cell Engineering. Biomaterials 2021, 266, 120450.
- Maciel, M.M.; Correia, T.R.; Henriques, M.; Mano, J.F. Microparticles Orchestrating Cell Fate in Bottom-up Approaches. Curr. Opin. Biotechnol. 2022, 73, 276–281.
- Zheng, S.; Liu, Q.; He, J.; Wang, X.; Ye, K.; Wang, X.; Yan, C.; Liu, P.; Ding, J. Critical Adhesion Areas of Cells on Micro-Nanopatterns. Nano Res. 2022, 15, 1623–1635.
- Darge, H.F.; Chuang, S.H.; Lai, J.Y.; Lin, S.Y.; Tsai, H.C. Preparation of Thermosensitive PNIPAm-Based Copolymer Coated Cytodex 3 Microcarriers for Efficient Nonenzymatic Cell Harvesting during 3D Culturing. Biotechnol. Bioeng. 2021, 118, 4076–4091.
- Narumi, Y.; Iwai, R.; Takagi, M. Recovery of Human Mesenchymal Stem Cells Grown on Novel Microcarrier Coated with Thermoresponsive Polymer. J. Artif. Organs 2020, 23, 358–364.
- Li, C.; Qian, Y.; Zhao, S.; Yin, Y.; Li, J. Alginate/PEG Based Microcarriers with Cleavable Crosslinkage for Expansion and Non-Invasive Harvest of Human Umbilical Cord Blood Mesenchymal Stem Cells. Mater. Sci. Eng. C 2016, 64, 43–53.
- Roux, R.; Ladavière, C.; Montembault, A.; Delair, T. Particle Assemblies: Toward New Tools for Regenerative Medicine. Mater. Sci. Eng. C 2013, 33, 997–1007.
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of Tissue Constructs by 3D Bioprinting of Cell-Laden Microcarriers. Biofabrication 2014, 6, 035020.
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid Microscaffold-Based 3D Bioprinting of Multi-Cellular Constructs with High Compressive Strength: A New Biofabrication Strategy. Sci. Rep. 2016, 6, 39140.
- Bhagabati, P.; Bhasney, S.M.; Bose, D.; Remadevi, R.; Setty, M.; Rajkhowa, R.; Katiyar, V. Silk and Wool Protein Microparticle-Reinforced Crystalline Polylactic Acid Biocomposites with Improved Cell Interaction for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 4739–4751.
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D Printing of Silk Microparticle Reinforced Polycaprolactone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111433.
- Liu, S.; Huang, D.; Hu, Y.; Zhang, J.; Chen, B.; Zhang, H.; Dong, X.; Tong, R.; Li, Y.; Zhou, W. Sodium Alginate/Collagen Composite Multiscale Porous Scaffolds Containing Poly(ε-Caprolactone) Microspheres Fabricated Based on Additive Manufacturing Technology. RSC Adv. 2020, 10, 39241–39250.
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of Collagen–Hydroxyapatite Scaffolds Incorporating PLGA and Alginate Microparticles for the Controlled Delivery of RhBMP-2 for Bone Tissue Engineering. J. Control. Release 2015, 198, 71–79.




