
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shabaaz Begum | -- | 3273 | 2022-04-19 06:13:32 | | | |
| 2 | Beatrix Zheng | + 1 word(s) | 3274 | 2022-04-19 08:33:26 | | |
Video Upload Options
Nanoparticles (NPs) are elements derived from a cluster of atoms with one or more dimensions in the nanometer scale in the range of 1–100 nm. The bio nanofabrication of metallic NPs is now an important dynamic area of research, with major significance in applied research. Biogenic synthesis of NPs is more desirable than physical and chemical synthesis due to its eco-friendliness, non-toxicity, lower energy consumption, and multifunctional nature. Plants outperform microorganisms as reducing agents as they contain large secondary biomolecules that accelerate the reduction and stability of the NPs. The produced NPs can then be studied spectroscopically (UV-Visible, XRD, Raman, IR, etc.) and microscopically (SEM, TEM, AFM, etc.). The biological reduction of a metallic ion or its oxide to a nanoparticle is quick, simple, and maybe scaled up at room temperature and pressure. The rise in multi-drug resistant (MDR) microbes due to the immoderate use of antibiotics in non-infected patients is a major cause of morbidity and mortality in humans. The contemporary development of a new class of antibiotics with different mechanisms of action to kill microbes is crucial. Metals and their oxides are extremely toxic to microbes at unprecedentedly low concentrations. In addition, prevailing infections in plants and animals are raising significant concerns across the globe. NPs’ wide range of bioactivity makes them ideal antimicrobial agents in agricultural and medical fields. The present research outlines the synthesis of metallic NPs from botanicals, which enables the metals to be in a stabilized form even after ionization. It also presents a valuable database on the biofunctionalization of synthesized NPs for further drug development.
1. Synthesis of Nanoparticles (NPs)
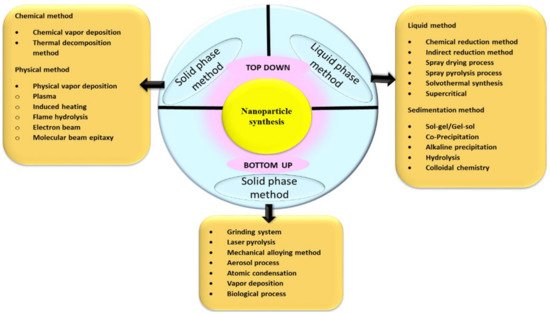
1.1. Perspectives of Nanoparticle Synthesis
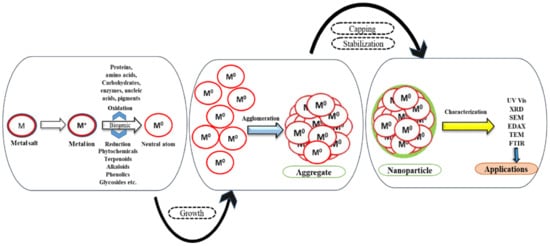
1.2. Secondary Biomolecules for Capping and Stabilization
2. Structural Analysis of NPs
3. Biofunctionalization of NPs
3.1. Gold NPs (Au NPs)
3.2. Silver NPs (Ag NPs)
3.3. Platinum Group of Metals
3.4. Metallic Oxide NPs
| Sr. No. | Botanical Names of Plants | Part Used | Size Range (nm) (SEM/TEM) |
Characterization Tools | Bio-Functionalization | Ref. |
|---|---|---|---|---|---|---|
| Zinc oxide NPs | ||||||
| 1. | Trianthema portulacastrum |
Extract | 25–90 | UV–Vis, XRD, FTIR, SEM, TEM, XPS |
|
[58] |
| 2. | Matricaria chamomilla L., Lycopersicon esculentum M., Olea europaea |
Extract | 40.5–124 | UV–Vis, XRD, FTIR, SEM, TEM, EDS |
|
[59] |
| 3. | Punica granatum | Extract | 32.98–81.84 | UV–Vis, XRD, FTIR, SEM, TEM |
|
[60] |
| 4. | Rheum turketanicum | Extract | 17–20 | UV–Vis, XRD, FTIR, SEM, TEM |
|
[61] |
| 5. | Tecoma castanifolia | Extract | 70–75 | UV–Vis, XRD, FTIR, SEM, TEM |
|
[62] |
| 6. | Silybum marianum | Extract | 31.2 | UV–Vis, XRD, FTIR, SEM, TEM |
|
[63] |
| 7. | Anchusa italic | Flower | ~8–~14 | UV-Vis, EDX XRD, FT-IR, FESEM, TEM |
|
[64] |
| 8. | Aloe vera | Leaves | 8–20 | UV-Vis, EDX, XRD, FT-IR, GC-MS, SEM TEM |
|
[65] |
| 9. | Rosa canina | Fruit | 50–400 | XRD, EDX, DLS, FT-IR, SEM |
|
[66] |
| 10. | Boswellia ovalifoliata | Bark | 20 | UV-Vis, DLS, ZP, FTIR, SEM, TEM |
|
[67] |
| Magnesium oxide NPs | ||||||
| 1. | Emblica officinalis | Fruit | 27 | UV-Vis, XRD, EDX, FT-IR, SEM |
|
[68] |
| 2. | Clitoria ternatea | Whole plant | 50–400 nm | UV-Vis, XRD, PL, FTIR, EDS, FESEM |
|
[4] |
| Copper oxide NPs | ||||||
| 1. | Ocimum tenuiflorum | Extract | 20–30 nm | UV–Vis, XRD, FTIR, SEM, TEM |
|
[69] |
| 2. | Moringa oleifera | Extract | 35–95 nm | UV–Vis, XRD, FTIR, SEM, TEM |
|
[70] |
| 3. | Eichhornia crassipes | Leaves | 28 ± 4 | UV-Vis, XRD, FT-IR, FESEM |
|
[71] |
| 4. | Gloriosa superba | Leaves | 5–10 | UV-Vis, PXRD, SEM TEM |
|
[72] |
| Titanium dioxide NPs | ||||||
| 1. | Artocarpus heterophyllus | Extract | 15–20 nm | UV–Vis, XRD, FTIR, SEM, TEM |
|
[73] |
| 2. | Citrus sinensis | Fruit peel | 20–50 nm | UV–Vis, XRD, FTIR, SEM, EDAX, TEM |
|
[74] |
| 3. | Musa alinsanaya | Fruit peel | 31.5 nm | UV–Vis, XRD, FTIR, SEM, EDAX, TEM |
|
[75] |
| 4. | Psidium guajava | Leaves | 32.58 | XRD, EDX, FT-IR, FESEM |
|
[76] |
| 5. | Vitex negundo | Leaves | 93.33 | UV-Vis, XRD, EDX, FTIR, SEM |
|
[77] |
| Samarium NPs | ||||||
| 1. | Medicago sativa | leaves | 10 | UV-Vis |
|
[78] |
| Neodymium NPs | ||||||
| 1. | Medicago sativa | Leaves | 10 | UV-Vis, RS, PSD, DLS, EDAX, XRD, FT-IR, SEM |
|
[79] |
4. Applications of Phytofabricated NPs
4.1. In Agriculture
4.2. Applications of Phytofabricated NPs as Nanoantibiotics
References
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174.
- Jayaseelan, C.; Ramkumar, R.; Rahuman, A.A.; Perumal, P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod. 2013, 45, 423–429.
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571.
- Sushma, N.J.; Prathyusha, D.; Swathi, G.; Madhavi, T.; Raju, B.D.P.; Mallikarjuna, K.; Kim, H.-S. Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea—characterization and in vitro antioxidant studies. Appl. Nanosci. 2015, 6, 437–444.
- Jyoti, K.; Pattnaik, P.; Singh, T. Green Synthesis of Silver Nanoparticles Using Sustainable Resources and their Use as Antibacterial Agents: A Review. Curr. Mater. Sci. Former. Recent Pat. Mater. Sci. 2021, 14, 40–52.
- Mathew, S.; Victorio, C.P.; Sidhi, J.; Thanzeela, B.H.B. Biosynthesis of silver nanoparticle using flowers of Calotropis gigantea (L.) WT Aiton and activity against pathogenic bacteria. Arab. J. Chem. 2020, 13, 9139–9144.
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2019, 152, 104296.
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374.
- Suresh, J.; Yuvakkumar, R.; Sundrarajan, M.; Hong, S.I. Green synthesis of magnesium oxide nanoparticles. In Advanced Materials Research; Trans Tech Publications Ltd: Bäch, Switzerland, 2014; Volume 952, pp. 141–144.
- Karaiskos, I.; Giamarellou, H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: Current and emerging therapeutic approaches. Expert Opin. Pharmacother. 2014, 15, 1351–1370.
- Komal, R.; Arya, V. Biosynthesis and characterization of silver nanoparticles from aqueous leaf extracts of Carica papaya and its antibacterial activity. Int. J. Nanomater. Biostruct. 2013, 3, 17–20.
- Kumara, P.P.N.V.; Pammib, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameema, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti-bacterial activity. Ind. Crop. Prod. 2014, 52, 562–566.
- Lade, B.D.; Shanware, A.S. Phytonanofabrication: Methodology and factors affecting biosynthesis of nanoparticles. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: London, UK, 2020.
- Farhadi, S.; Ajerloo, B.; Mohammadi, A. Low-cost and eco-friendly phyto-synthesis of Silver nanoparticles by using grapes fruit extract and study of antibacterial and catalytic effects. Int. J. Nano Dimens. 2017, 8, 49–60.
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crop. Prod. 2013, 43, 631–635.
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Palladium Nanoparticles Fabricated by Green Chemistry: Promising Chemotherapeutic, Antioxidant and Antimicrobial Agents. Materials 2020, 13, 3661.
- Devi, G.D.; Murugan, K.; Selvam, C.P. Green synthesis of silver nanoparticles using Euphorbia hirta (Euphorbiaceae) leaf extract against crop pest of cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Biopestic. 2014, 7, 54.
- Luna, C.; Chávez, V.; Barriga-Castro, E.D.; Núñez, N.O.; Mendoza-Reséndez, R. Biosynthesis of silver fine particles and particles decorated with nanoparticles using the extract of Illicium verum (star anise) seeds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 43–50.
- Qiao, J.; Qi, L. Recent progress in plant-gold nanoparticles fabrication methods and bio-applications. Talanta 2020, 223, 121396.
- Zhang, Y.; Zhang, C.; Xu, C.; Wang, X.; Liu, C.; Waterhouse, G.; Wang, Y.; Yin, H. Ultrasmall Au nanoclusters for biomedical and biosensing applications: A mini-review. Talanta 2019, 200, 432–442.
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2019, 206, 120210.
- Shahriari, M.; Hemmati, S.; Zangeneh, A.; Zangeneh, M.M. Biosynthesis of gold nanoparticles using Allium noeanum Reut. ex Regel leaves aqueous extract; characterization and analysis of their cytotoxicity, antioxidant, and antibacterial properties. Appl. Organomet. Chem. 2019, 33, e5189.
- Gharehyakheh, S.; Ahmeda, A.; Haddadi, A.; Jamshidi, M.; Nowrozi, M.; Zangeneh, M.M.; Zangeneh, A. Effect of gold nanoparticles synthesized using the aqueous extract of Satureja hortensis leaf on enhancing the shelf life and removing Escherichia coli O157:H7 and Listeria monocytogenes in minced camel’s meat: The role of nanotechnology in the food industry. Appl. Organomet. Chem. 2020, 34, e5492.
- Valsalam, S.; Agastian, P.; Esmail, G.A.; Ghilan, A.-K.M.; Al-Dhabi, N.A.; Arasu, M.V. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B Biol. 2019, 201, 111670.
- Zhaleh, M.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evalution of cytotocicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properies of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as acapping and reducing agent. Appl. Organomet. Chem. 2019, 33, e5015.
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111715.
- Hemmati, S.; Joshani, Z.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of Thymus vulgaris leaf aqueous extract conjugated gold nanoparticles for the treatment of acute myeloid leukemia in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2019, 34, e5267.
- Ansari, S.; Bari, A.; Ullah, R.; Mathanmohun, M.; Veeraraghavan, V.P.; Sun, Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 2019, 201, 111643.
- Ismail, E.H.; Saqer, A.M.A.; Assirey, E.; Naqvi, A.; Okasha, R.M. Successful Green Synthesis of Gold Nanoparticles using a Corchorus olitorius Extract and Their Antiproliferative Effect in Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2612.
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J. Photochem. Photobiol. B Biol. 2018, 191, 26–37.
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290.
- Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J.; Li, W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019, 14, 4091–4103.
- Liu, Y.; Kim, S.; Kim, Y.J.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.H. Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-kappa B and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomed. 2019, 14, 2945–2959.
- Park, S.Y.; Yi, E.H.; Kim, Y.; Park, G. Anti-neuroinflammatory effects of Ephedra sinica Stapf extract-capped gold nanoparticles in microglia. Int. J. Nanomed. 2019, 14, 2861–2877.
- Zhang, T.; Dang, M.; Zhang, W.; Lin, X. Gold nanoparticles synthesized from Euphorbia fischeriana root by green route method alleviates the isoprenaline hydrochloride induced myocardial infarction in rats. J. Photochem. Photobiol. B Biol. 2019, 202, 111705.
- Ahmeda, A.; Zangeneh, M.M. Novel green synthesis of Boswellia serrata leaf aqueous extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia property in comparison to mitoxantrone in a leukemic mice model: Introducing a new chemotherapeutic drug. Appl. Organomet. Chem. 2019, 34, e5344.
- Yun, Z.; Chinnathambi, A.; Alharbi, S.A.; Jin, Z. Biosynthesis of gold nanoparticles using Vetex negundo and evaluation of pro-apoptotic effect on human gastric cancer cell lines. J. Photochem. Photobiol. B Biol. 2019, 203, 111749.
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elements Med. Biol. 2017, 40, 10–23.
- Acharya, D.; Mohanta, B.; Pandey, P. Green synthesis of Silver and Silver-gold core-shell nanoparticles using Pineapple leaf extract (Ananas comosus) and study of their antibacterial properties. Int. J. Nano Dimens. 2021, 12, 203–210.
- Babu, P.J.; Das, R.K.; Kumar, A.; Bora, U. Microwave-Mediated Synthesis of Gold Nanoparticles Using Coconut Water. Int. J. Green Nanotechnol. 2011, 3, 13–21.
- Francis, G.; Thombre, R.; Parekh, F.; Leksminarayan, P. Bioinspired synthesis of gold nanoparticles using Ficus benghalensis (Indian Banyan) leaf extract. Chem. Sci. Trans. 2014, 3, 470–474.
- Wani, K.; Choudhari, A.; Chikate, R.; Kaul-Ghanekar, R. Synthesis and characterization of gold nanoparticles using Ficus religiosa extract. Carbon Sci. Technol. 2013, 5, 203–210.
- Reddy, G.R.; Morais, A.B.; Gandhi, N.N. Green Synthesis, Characterization and in vitro Antibacterial Studies of Gold Nanoparticles by Using Senna siamea Plant Seed Aqueous Extract at Ambient Conditions. Asian J. Chem. 2013, 25, 8541–8544.
- Amini, S.M. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater. Sci. Eng. C 2019, 103, 109809.
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green Synthesis of Silver Nanoparticles using Achillea biebersteinii Flower Extract and Its Anti-Angiogenic Properties in the Rat Aortic Ring Model. Molecules 2014, 19, 4624–4634.
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137.
- Bindhu, M.; Umadevi, M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabius leaf extract and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 184–190.
- Das, S.; Parida, U.K.; Bindhani, B.K. Green biosynthesis of silver nanoparticles using Moringa oleifera L. leaf. Int. J. Nanotechnol. Appl. 2013, 3, 51–62.
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro Evaluation of Antioxidant and Antibacterial Potential of Green Synthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran. J. Pharm. Res. IJPR 2019, 18, 430–455.
- Gopinath, K.; Gowri, S.; Arumugam, A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J. Nanostruct. Chem. 2013, 3, 68.
- Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; RajeshKumar, S.; Malarkodi, C.; Annadurai, G.; Kannan, C. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J. Nanostruct. Chem. 2013, 3, 67.
- Attar, A.; Yapaoz, M.A. Biosynthesis of palladium nanoparticles using Diospyros kaki leaf extract and determination of antibacterial efficacy. Prep. Biochem. Biotechnol. 2018, 48, 629–634.
- Mavukkandy, M.O.; Chakraborty, S.; Abbasi, T.; Abbasi, S.A. A Clean-Green Synthesis of Platinum Nanoparticles Utilizing a Pernicious Weed Lantana (Lantana Camara). Am. J. Eng. Appl. Sci. 2016, 9, 84–90.
- Narendhran, S.; Manikandan, M.; & Shakila, P. Antibacterial, antioxidant properties of Solanum trilobatum and sodium hydroxide-mediated magnesium oxide nanoparticles: A green chemistry approach. Bull. Mater. Sci. 2019, 42, 1–8.
- Tahir, K.; Nazir, S.; Li, B.; Ahmad, A.; Nasir, T.; Khan, A.U.; Shah, S.A.A.; Khan, Z.U.H.; Yasin, G.; Hameed, M.U. Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2016, 164, 164–173.
- Sathishkumar, M.; Sneha, K.; Yun, Y.S. Palladium nanocrystal synthesis using Curcuma longa tuber extract. Int. J. Mater. Sci. 2009, 4, 11–17.
- Surendra, T.; Roopan, S.M.; Arasu, M.V.; Al-Dhabi, N.A.; Rayalu, G.M. RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B Biol. 2016, 162, 550–557.
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Safi, S.Z.; Khan, F.U.; Imran, M.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157.
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352.
- Sukri, S.N.A.M.; Shameli, K.; Wong, M.M.-T.; Teow, S.-Y.; Chew, J.; Ismail, N.A. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J. Mol. Struct. 2019, 1189, 57–65.
- Nemati, S.; Hosseini, H.A.; Hashemzadeh, A.; Mohajeri, M.; Sabouri, Z.; Darroudi, M.; Oskuee, R.K. Cytotoxicity and photocatalytic applications of biosynthesized ZnO nanoparticles by Rheum turketanicum rhizome extract. Mater. Res. Express 2019, 6, 125016.
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2018, 145, 578–587.
- Hameed, S.; Khalil, A.T.; Ali, M.; Numan, M.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine 2019, 14, 655–673.
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Rahim, R.A.; Ariff, A.; Saad, W.Z. Effect of annealing temperature on antimicrobial and structural properties of bio-synthesized zinc oxide nanoparticles using flower extract of Anchusa italica. J. Photochem. Photobiol. B Biol. 2016, 161, 441–449.
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Al-Khedhairy, A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156.
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302.
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2015, 6, 581–590.
- Ramanujam, K.; Sundrarajan, M. Antibacterial effects of biosynthesized MgO nanoparticles using ethanolic fruit extract of Emblica officinalis. J. Photochem. Photobiol. B Biol. 2014, 141, 296–300.
- Altikatoglu, M.; Attar, A.; Erci, F.; Cristache, C.M.; Isildak, I. Green synthesis of copper oxide nanoparticles using Ocimum basilicum extract and their antibacterial activity. Fresenius Environ. Bull. 2017, 25, 7832–7837.
- Pagar, K.; Ghotekar, S.; Pagar, T.; Nikam, A.; Pansambal, S.; Oza, R.; Sanap, D.; Dabhane, H. Antifungal activity of biosynthesized CuO nanoparticles using leaves extract of Moringa oleifera and their structural characterizations. Asian J. Nanosci. Mater. 2020, 3, 15–23.
- Vanathi, P.; Rajiv, P.; Sivaraj, R. Synthesis and characterization of Eichhornia-mediated copper oxide nanoparticles and assessing their antifungal activity against plant pathogens. Bull. Mater. Sci. 2016, 39, 1165–1170.
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12.
- Ullah, A.M.; Tamanna, A.N.; Hossain, A.; Akter, M.; Kabir, M.F.; Tareq, A.R.; Kibria, A.F.; Kurasaki, M.; Rahman, M.M.; Khan, M.N. In vitro cytotoxicity and antibiotic application of green route surface modified ferromagnetic TiO2 nanoparticles. RSC Adv. 2019, 23, 13254–13262.
- Rueda, D.; Arias, V.; Zhang, Y.; Cabot, A.; Agudelo, A.C.; Cadavid, D. Low-cost tangerine peel waste mediated production of Titanium Dioxide Nanocrystals: Synthesis and characterization. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100285.
- Kirthi, A.V.; Jayaseelan, C.; Rahuman, A. Biosynthesis and characterization of different nanoparticles and its larvicidal activity against human disease vectors. Mar. Biomater. 2013, 25, 273–288.
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.-K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976.
- Ambika, S.; Sundrarajan, M. BF4 ionic liquid-mediated synthesis of TiO2 nanoparticles using Vitex negundo Linn extract and its antibacterial activity. J. Mol. Liquids 2016, 221, 986–992.
- Hu, R.; Beguiristain, T.; De Junet, A.; Leyval, C. Bioavailability and transfer of elevated Sm concentration to alfalfa in spiked soils. Environ. Sci. Pollut. Res. 2020, 27, 44333–44341.
- Rezaee, A. Accumulation and Toxicity of Lanthanum and Neodymium in Horticultural Plants. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2018.
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 411–446.
- Parham, S.; Wicaksono, D.H.B.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial Treatment of Different Metal Oxide Nanoparticles: A Critical Review. J. Chin. Chem. Soc. 2016, 63, 385–393.
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 2021, 334, 116040.
- Saka, R.; Chella, N. Nanotechnology for delivery of natural therapeutic substances: A review. Environ. Chem. Lett. 2020, 19, 1097–1106.
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2018, 103, 881–891.
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427.
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599.
- Sunny, N.E.; Kaviya, A.; Kumar, S.V. Mechanistic approach on the synthesis of metallic nanoparticles from microbes. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 577–602.




