1. Synthesis of Nanoparticles (NPs)
1.1. Perspectives of Nanoparticle Synthesis
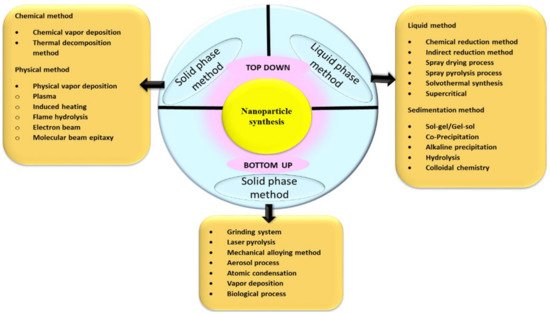
The methodology for making ultrafine NPs from ancient times is generally by the breakdown (top-down), and the build-up (bottom-up) approaches, as illustrated in Figure 1.
Figure 1. Various approaches for the synthesis of NPs.
The breakdown approach of NP synthesis is usually employed during NPs’ physical and chemical synthesis. The size reduction of bulk material is used as a precursor ultimately to the nanosize by applying physical forces such as grinding, pulverization, etc., in the break down method which is also sometimes called the mechanochemical method
[1]. It is challenging to obtain NPs by applying physical forces; usually, microparticles are easily obtained of 3 µm size, which is not significant. The second approach for obtaining NPs is by the build-up process; where major preparation methods for the synthesis of NPs can be achieved in two states of matter, liquid phase and solid phase, without any hazardous chemicals in biogenic synthesis, and remarkable increased use of chemicals in chemical synthesis are used. Biogenic synthesis of NPs falls under the bottom-up approach, where the uses of the biological system or its parts can be seen in the synthesis. To select the best organisms or extracts, one must evaluate their specific properties such as biochemical pathways, phytochemical contents, enzyme activities, cell growth circumstances, and ideal reaction
[2].
1.2. Secondary Biomolecules for Capping and Stabilization
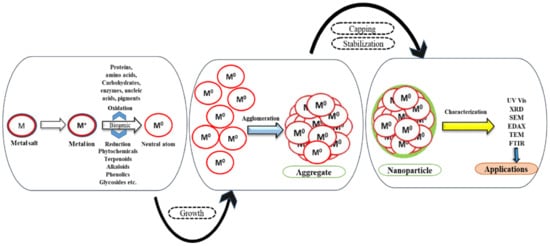
Plant extract not only acts as a reductant but also functions as a capping and stabilizing agent, as depicted in Figure 2.
Figure 2. Biological reduction of NPs.
Prediction of biomolecules acting as capping and stabilizing agents was realistic when IR spectrum of tea extract
[3] showed the involvement of polyphenols, carboxylic acid, polysaccharide, amino acid, and proteins when coordinated with FTIR analysis. Zinc oxide NPs [ZnO NPs] showed peaks in 682–457 cm
−1, indicating the presence of a higher percentage of phenolics. The stability studies of silver NPs (Ag NPs) synthesized from Ziziphora tenuior extract at room temperature revealed that bionanofabrication of Ag NPs was due to some metabolite functional groups such as amines, alcohols, ketones, aldehydes, and carboxylic acid. A peak graph of FTIR between the treated and untreated sample showed significant changes and predicted amide group form of proteins possibly be the covering layer of metal NPs
[4]. The FTIR peak stretches in the OH, CH, C=C ring, and CH2 wagging of ascorbic acid indicated that
Hibiscus cannabis extract comprises ascorbic acid responsible for reducing Ag NPs
[5]. The ferric chloride test of coconut shell extract revealed the presence of phenolic compounds; most importantly, benzoquinone yielded the formation of Au NPs.
Calotropis gigantea, a large shrub, consists of phytoconstituents such as cardiac glycosides, β-sitosterol, saponins, alkaloids, tannins, trisaccharides, and flavanols FTIR spectra denote the interactions of the biomolecules with Ag NPs
[6].
2. Structural Analysis of NPs
The synergistic synthesized metallic NPs are characterized by assessment of their shape, size, morphology, and surface area, using various characterization tools such as Ultraviolet-visible spectrophotometry (UV-Vis), X-ray diffraction (XRD), Energy dispersive X-ray analysis (EDAX), Particle size distribution (PDS), Zeta potential (ZP), Photoluminescence (PL), Dynamic light scattering (DLS), Raman spectroscopy (R), Infrared spectroscopy (IR), Cyclic voltammetry (CV), Nanoparticle tracking analysis (NTA), Fourier transform-infrared spectroscopy (FTIR), Thermal gravimetric analysis (TGA), Selected area electron diffraction (SAED), Atomic field microscopy (AFM), Scanning electron microscope (SEM), field emission scanning electron microscopy (FESEM), Transmission electron microscopy (TEM), High-resolution transmission electron microscopy (HRTEM).
Characterization of a particular biomaterial depends on the complexity of the matrix, the analyte concentration, and the physio-chemical composition
[7].
The UV-visible spectroscopy is primarily used as a characterization technique soon after synthesizing NPs of size from 2–100 nm in the range wavelength of 300–800 nm. The brownish color change of grapefruit extract from yellow due to the formation of silver ion complex was confirmed when a broad surface plasmon resonance band was observed around 450–470 nm
[8]. Magnesium oxide NPs (MgO NPs) cubic structures formed in the presence of reductant Emblica officinalis
[9]. The peak intensity profile was characteristic of NPs calculated with Scherer’s formula. The crystalline size determined for the mean particle size of the MgO NPs was around 27 nm which was well matched with SEM images. Every element in its unblended form will have unique atomic structures with a set of peaks EDAX can identify. A spectrum of Ag NPs observed at ~3 Kev confirmed the presence of silver as a major constituent element
[10]. The elemental composition of Ag NPs synthesized from the plant extract of Boerhaavia diffusa was resolute by SEM equipped with an EDAX detector showing a strong signal in the silver region
[11]. The stability of Ag NPs was evaluated by a zeta potentiometer. It was noted that synthesized NPs were stable in a wide range of pH from 6–12. An increase in the pH increased ZP. At pH 12, Ag NPs were found to be more stable. Raman spectroscopy of Ag NPs was carried out to gain the information of bio components initially for the biosynthesis
[12] such as the polyphenol interactions with S
+ ions during nanoparticle formation. Diluted samples of Ag NPs subjected to NTA reveal minimum and maximum 28 and 22 nm with a standard deviation of ±8 nm, and the results correlated with TEM findings were consistent
[13][14]. Studies of possible biomolecules responsible for capping and stabilization of NPs were carried out by FT-IR and GC-MS analysis. Cocos nucifera coir extract confirmed the presence of biomolecules containing hydrocarbon such as nonacosane and heptacosames, which were predicted in the stabilization of Ag NPs by GC-Ms analysis
[15]. The thermal gravimetric analysis allowed the research of the thermal stability of palladium NPs (Pd NPs) and showed that the phytoconstituents are responsible for reducing Pd
+2 to Pd
o [16]. AFM characterized at ambient temperature exemplified the results of particles of 41 nm
[17]. SEM images of carbon stretches provide a morphologically excellent view of NPs. Only TEM can reveal the exact shape and size of physical, chemical, or bio reduced NPs. TEM pictures of Ag NPs showed that most particles were spherical and measured between 5 and 20 nm
[18].
3. Biofunctionalization of NPs
3.1. Gold NPs (Au NPs)
As the years have passed, gold has become a scarce commodity. Gold, a soft yellow metal with the highest ductility and malleability of any metal, is highly prized for various reasons; however, its physical qualities are critical to modern society’s functioning. In the treatment of rheumatic diseases and discoid lupus erythematosus, restorative dentistry, and other inflammatory skin conditions such as pemphigus urticaria and psoriasis, gold and its compounds were utilized
[19]. Due to their small size, gold NPs (Au NPs) have a much higher surface area and dispersion. In terms of textural qualities, gold has the highest specific surface of any metal.
Plant-based Au NPs are biocompatible and have unique chemical and optical properties, making them useful for photo-thermal treatment, bio-sensing, antioxidants, anti-microbials, and drug delivery
[20][21][22][23][24][25][26][27][28][29][30][31][32][33][34]. Plant-Au NPs with surface modifications have been used in biomedical research and treatment
[35][36][37][38][39]. Plant-Au NPs can interact with bacteria’s biomolecules, altering their structure and causing them to die. Plant-Au NPs, for example, were synthesized using flower extract of Musa acuminate Colla as a stabilizer and reducing agent
[24] and displayed anticancer activity in addition to antibacterial activity against beta-lactamase-producing bacteria. Endocytosis allowed the plant-Au NPs to penetrate the cells after accumulating on the cell surface. On the surface of the Au NPs, free radicals were produced. Electron spin resonance spectroscopy revealed the formation of free radicals on the surface of Au NPs. When Au NPs were cultured with bacteria, the generation of free radicals indicated the ability of the Au NPs to disrupt cell membranes and make cells permeable, eventually leading to cell death. Plant antioxidants play a critical role in their biological competence, including interfering in cancer formation (including instigation, development, advancement, invasion, and metastasis)
[25]. Plant extract-based Au NPs use free radicals and ROS in live cells in an antioxidant application
[26]. When evaluated with 2, 2-diphenyl-1-picrylhydrazyl, the
Thymus vulgaris aqueous extract was used to produce Au NPs
[27], which exhibits antioxidant activity. The therapeutic impact on diabetic and obese rats using Au NPs derived from Smilax glabra was examined
[28], and Smilax glabra rhizome extract capped Au NPs were generated. Histopathological investigations demonstrated the antidiabetic and antiobesity properties of plant based-Au NPs which reinstated the nuclei, inner membrane, and cytoplasm. Furthermore,
Vetex negundo extract-stabilized Au NPs
[37] and Au NPs capped using leaves extract of
Camellia sinensis [31] were employed to cure severe myeloid leukemia in animal samples throughout pro-apoptotic evaluation of human gastric cancer cells, respectively.
A low-cost approach of gold NPs (Au NPs) at room temperature using aqueous seed extract of Abelmoschus esculentus yielded spherical particles, a narrow size range of 45–75 nm with a high antifungal effect against Puccinia graminis and Candida albicans
[2]. As the demand for synthesis for NPs increased, various parts of the plants were utilized. Ananas comosus blended fruit extracts served as an excellent reductant for synthesizing Au NPs, having better antimicrobial activity than standard antibiotics used
[39]. Microwave-mediated synthesis of Au NPs with coconut water is an incredible example of rapid NP synthesis, with an optimum time of 17 s. Furthermore, cytotoxicity was tested on two human cancer cell lines, HeLa (human cervical cancer) and MCF-7 (human breast cancer), and found to be nontoxic
[40]. Leaf extract of
Ficus benghalensis used as a capping agent in synthesis yielding spherical shape of Au NPs; the TEM analysis showed the formation of well-dispersed Au NPs of size 17–50 nm
[41]. Au NPs from
Ficus religiosa extract showed excellent stability and uniform capping due to the presence of polyphenols, amines and carboxylates and were nontoxic to the HEK293 cell lines at 80 µM concentration
[42]. Nanotriangles and nanohexagons Au NPs of ~10 nm obtained from
Gnidia glauca flower extract exhibit chemocatalytic activity in reducing 4-nitrophenol to 4-aminophenol by NaBH4 in the aqueous phase. In vitro antibacterial properties of polyshaped Au NPs synthesized from
Senna siamea showed significant antibacterial activity against Pseudomonas aeruginosa (
P. aeruginosa)
[43].
3.2. Silver NPs (Ag NPs)
In the current situation, numerous publications have been published on the laboratory-scale synthesis of Ag NPs from plants, which have emerged as antibacterial agents due to their unique physical and chemical properties. “Nobel silver NPs” are working to push the boundaries of science and technology, notably in the medical field
[44]. Ag NPs have attracted intensive research in the biomedical, food industry, drug delivery, agriculture, water treatment, textile industries, antimicrobial agent, and anticancer drugs.
Anti-angiogenic properties of Ag NPs in the rat aortic ring model were evaluated. Results showed that Ag NPs at 200 µg/mL led to a 50% reduction in the length and number of vessel-like structures
[45]. Artocarpus heterophyllus assisted Ag NPs reduction, showing excellent antibacterial activity against Staphylococcus aureus (S. aureus) with an inhibition zone of 15 mm diameter compared to the other bacterial strains used
[46]. Antibacterial activity of phytosynthesized Ag NPs from Boerhaavia diffusa L. extract were tested against fish bacterial pathogens Aeromonas hydrophila, Flavobacterium branchiophilum, and Pseudomonas fluorescens; and they demonstrated high antibacterial activity towards Flavobacterium branchiophilum when compared to other two fish bacterial pathogens
[11]. FTIR research revealed that protein fractions acted as reducing and stabilizing agents during the production of Ag NPs using latex from
Calotropis gigantea L.
[6]. In situ green synthesis of Ag NPs was done using Coriandrum sativum L. seed extract, then exposed to synergistic tests. The results showed that the potency of conventional antibiotics could be increased in the presence of Ag NPs. Green Ag NPs reduced using Cocos nucifera coir extract were effective anti-larvicidal agents against C. quinquefasciatus and Anopheles stephensi
[15]. Ag NPs serve as an excellent substitute besides synthetic and chemical insecticides synthesized from Euphorbia hirta L. extract, and administration of it to the crop pest cotton bollworm Helicoverpa armigera impacted its biological parameters such as less consumption of food index due to a decrease in the level of digestive enzymes. Systematic evaluation of antibacterial properties of Ag NPs synthesized from extracts of
Hibiscus cannabis [47],
Moringa oleifera L.
[48],
Prosporis fracta [49],
Pterocarpus santalinus [50], and
Vitis vinifera against common human pathogens such as
Klebsiella pneumoniae (
K. pneumoniae),
Escherichia coli (E. coli),
Enterococcus faecalis, Enterobacter cloacae (E. cloacae), Proteus vulgaris, S. aureus, S. saprophyticus, Bacillus subtilis, and
P. aeruginosa gave a brief insight on eradicating the conventional antibiotics and exploring more therapeutic applications of Ag NPs as nanoantibiotics against MDR bacteria
[51]. Extensive study of bioactivity of Ag NPs against breast cancer cell lines MCF 7 interestingly displayed a decrease in the cell viability. Direct sunlight assisted Ag NPs within five minutes, providing better fungicidal activity against
Candida albicans,
Candida glabrata, and
Aspergillus niger. Ag NPs’ biological activity from
Illicium verum Hook.
[18], was not reported.
3.3. Platinum Group of Metals
Platinum is a silvery-white metal costlier than gold, known for its malleability, durability and ductility. The first research on Pt NPs using leaf extract described the use of an aqueous leaf solution of Diospyros kaki as a bio-reducing agent in the green extracellular synthesis of Pt NPs from an aqueous H2PtCl6.6H2O solution
[52]. With a reaction temperature of 95 °C, platinum ions’ conversion to NPs greater than 90% was accomplished. The mechanism of action was unknown at the time, but the FTIR research indicated that Pt NPs are surrounded by metabolites such as terpenoids, which have functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acids. A single-step technique was proposed for synthesizing Pt NPs using an invasive weed,
Lantana camara, and a moderate reducing agent, ascorbic acid
[53]. The procedure entails mixing adequate amounts of platinum (VI) solution, Lantana leaf extract, and ascorbic acid, then heating the mixture to 95 °C for 8 min. Ascorbic acid and leaf extract combined with decreasing chloroplatinic acid to platinum NPs, with leaf extract also serving as a stabilizing agent for the NPs. The resultant NPs are 35 nm in diameter and crystallize in face-centered cubic symmetry. Palladium is a sister metal of platinum and belongs to the platinum group metals (PGM). It has a white luster finish and is light. However, it is less ductile than platinum.
Palladium NPs (Pd NPs), which are important due to their catalytic characteristics and affinity for hydrogen, have been phyto-sensitized using Solanum trilobatum under contemporary pH and room temperature which exhibited antibacterial and cytotoxic effects
[54]. Pd NPs have also been used as a homogeneous or heterogeneous catalysts in various scientific fields, including hydrogen storage, chemo-optical transducers, and chemical modifiers in ETV-ICP-MS, hydrogen sensor, automotive catalytic converter, plasmonic waveguides, and optical limiting devices. For the production of Pd NPs using diverse concentrations and temperatures, an eco-friendly and cost-effective green technique using water-soluble leaf extract of Sapium sebiferum as a reducing and capping agent was developed. The optimized Pd NPs synthesized using 10 mL leaf extract showed strong bacterial inhibition against
P. aeruginosa (11 ± 0.6 mm), Bacillus subtilis (19 ± 0.6 mm), and S. aureus (29 ± 0.8 mm)
[55]. Uniform-sized palladium NPs were synthesized using Curcuma longa tuber extract with an average size ranging between 10–15 nm
[56]. Liquid flower extracts of
Moringa oleifera flower extracts with 1 mM palladium acetate solution yielded 10–50 nm size bio Pd NPs
[57]. GC-MS analysis showed that Bis-phthalate was mostly the reducing agent. Pd NPs caused significant cytotoxicity to A549 cells and did not induce toxicity in normal healthy peripheral lymphocytes.
3.4. Metallic Oxide NPs
Different types of metal oxides synthesized using the plant parts are useful for bioactivity. The bio-reduction of other metal oxides NPs and their bioactivity is listed in Table 1.
Table 1. Bio-reduction of other metal oxides NPs and their bioactivity.
| Sr. No. |
Botanical Names of Plants |
Part Used |
Size Range (nm)
(SEM/TEM) |
Characterization Tools |
Bio-Functionalization |
Ref. |
| Zinc oxide NPs |
| 1. |
Trianthema
portulacastrum |
Extract |
25–90 |
UV–Vis, XRD,
FTIR, SEM, TEM,
XPS |
-
Cytotoxic
-
Cytotoxic
-
Antibacterial
-
Antifungal
-
Antioxidant
|
[58] |
| 2. |
Matricaria chamomilla
L., Lycopersicon
esculentum M., Olea
europaea |
Extract |
40.5–124 |
UV–Vis, XRD,
FTIR, SEM, TEM,
EDS |
|
[59] |
| 3. |
Punica granatum |
Extract |
32.98–81.84 |
UV–Vis, XRD,
FTIR, SEM, TEM |
|
[60] |
| 4. |
Rheum turketanicum |
Extract |
17–20 |
UV–Vis, XRD,
FTIR, SEM, TEM |
|
[61] |
| 5. |
Tecoma castanifolia |
Extract |
70–75 |
UV–Vis, XRD,
FTIR, SEM, TEM |
-
Antibacterial
-
Antioxidant
-
Anticancer
|
[62] |
| 6. |
Silybum marianum |
Extract |
31.2 |
UV–Vis, XRD,
FTIR, SEM, TEM |
-
Antifungal
-
Antibacterial
-
Cytotoxic
|
[63] |
| 7. |
Anchusa italic |
Flower |
~8–~14 |
UV-Vis, EDX XRD, FT-IR, FESEM, TEM |
|
[64] |
| 8. |
Aloe vera |
Leaves |
8–20 |
UV-Vis, EDX, XRD, FT-IR, GC-MS, SEM TEM |
|
[65] |
| 9. |
Rosa canina |
Fruit |
50–400 |
XRD, EDX, DLS, FT-IR, SEM |
-
Antibacterial
-
Antioxidant
-
Cytotoxic
|
[66] |
| 10. |
Boswellia ovalifoliata |
Bark |
20 |
UV-Vis, DLS, ZP, FTIR, SEM, TEM |
|
[67] |
| Magnesium oxide NPs |
| 1. |
Emblica officinalis |
Fruit |
27 |
UV-Vis, XRD, EDX, FT-IR, SEM |
|
[68] |
| 2. |
Clitoria ternatea |
Whole plant |
50–400 nm |
UV-Vis, XRD, PL, FTIR, EDS, FESEM |
|
[4] |
| Copper oxide NPs |
| 1. |
Ocimum tenuiflorum |
Extract |
20–30 nm |
UV–Vis, XRD,
FTIR, SEM, TEM |
|
[69] |
| 2. |
Moringa oleifera |
Extract |
35–95 nm |
UV–Vis, XRD,
FTIR, SEM, TEM |
|
[70] |
| 3. |
Eichhornia crassipes |
Leaves |
28 ± 4 |
UV-Vis, XRD, FT-IR, FESEM |
|
[71] |
| 4. |
Gloriosa superba |
Leaves |
5–10 |
UV-Vis, PXRD, SEM TEM |
|
[72] |
| Titanium dioxide NPs |
| 1. |
Artocarpus heterophyllus |
Extract |
15–20 nm |
UV–Vis, XRD,
FTIR, SEM, TEM |
-
Anticancer
-
Cytotoxic
-
Antibacterial
|
[73] |
| 2. |
Citrus sinensis |
Fruit peel |
20–50 nm |
UV–Vis, XRD,
FTIR, SEM, EDAX, TEM |
-
Anticancer
-
Cytotoxic
-
Antibacterial
|
[74] |
| 3. |
Musa alinsanaya |
Fruit peel |
31.5 nm |
UV–Vis, XRD,
FTIR, SEM, EDAX, TEM |
|
[75] |
| 4. |
Psidium guajava |
Leaves |
32.58 |
XRD, EDX, FT-IR, FESEM |
-
Antibacterial
-
Antioxidant
|
[76] |
| 5. |
Vitex negundo |
Leaves |
93.33 |
UV-Vis, XRD, EDX, FTIR, SEM |
|
[77] |
| Samarium NPs |
| 1. |
Medicago sativa |
leaves |
10 |
UV-Vis |
|
[78] |
| Neodymium NPs |
| 1. |
Medicago sativa |
Leaves |
10 |
UV-Vis, RS, PSD, DLS, EDAX, XRD, FT-IR, SEM |
|
[79] |
4. Applications of Phytofabricated NPs
4.1. In Agriculture
Antimicrobials based on NPs have an impact because of their extensive physiochemical properties in terms of size, shape, surface area, surface energy, crystallinity, charge, aggregation, agglomeration, and chemical composition.
In previous investigations, the microbicidal activities of several inorganic NPs such as TiO2, Ag, CuO, MgO, S, and ZnO were examined separately or in combination with biopolymer
[80][81][82]. As a result, it is important to create the new green synthesis-based NPs capable of managing fungal phytopathogens through biofunctionalized antimicrobial NPs to protect plants in a cost-effective, environmentally friendly, and long-term manner.
4.2. Applications of Phytofabricated NPs as Nanoantibiotics
Over past decades, resistance to antibiotics has become increasingly widespread and resulted in noteworthy deaths of humans. The emergence and re-emergence of pathogens have become a major public health concern worldwide, and the rapid emergence of antibiotic-resistant Gram-positive and Gram-negative pathogenic germs is a major public health concern
[83][84][85][86][87]. The long list of drug-resistant bacteria includes macrolide-resistant
Streptococcus pyogenes, sulfonamide, penicillin, methicillin-resistant
Staphylococcus aureus (MRSA), vancomycin-resistant
Enterococcus, penicillin-resistant
Streptococcus pneumoniae, multi-drug resistant
Mycobacterium tuberculosis (MDR-M. tuberculosis), penicillin-resistant
Neisseria gonorrhoeae (PRNG),
E. coli, E.
cloacae, K. pneumoniae, Salmonella enterica, Shigella flexneri, Acinetobacter baumannii, Vibrio cholerae, P. aeruginosa, and beta-lactamase-expressing
Haemophilus influenzae.
This entry is adapted from the peer-reviewed paper 10.3390/ph15040455