
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Paula Marques Boaventura | -- | 2392 | 2022-04-18 11:56:01 | | | |
| 2 | Vivi Li | + 1 word(s) | 2393 | 2022-04-19 03:47:49 | | |
Video Upload Options
COVID-19 is currently considered a systemic infection involving multiple systems and causing chronic complications. Compared to other post-viral fatigue syndromes, these complications are wider and more intense. The most frequent symptoms are profound fatigue, dyspnea, sleep difficulties, anxiety or depression, reduced lung capacity, memory/cognitive impairment, and hyposmia/anosmia. Risk factors for this condition are severity of illness, more than five symptoms in the first week of the disease, female sex, older age, the presence of comorbidities, and a weak anti-SARS-CoV-2 antibody response. Different lines of research have attempted to explain these protracted symptoms; chronic persistent inflammation, autonomic nervous system disruption, hypometabolism, and autoimmunity may play a role. Due to thyroid high ACE expression, the key molecular complex SARS-CoV-2 uses to infect the host cells, thyroid may be a target for the coronavirus infection. Thyroid dysfunction after SARS-CoV-2 infection may be a combination of numerous mechanisms, and its role in long-COVID manifestations is not yet established. The presence of post-COVID symptoms deserves recognition of COVID-19 as a cause of post-viral fatigue syndrome. It is important to recognize the affected individuals at an early stage so researchers can offer them the most adequate treatments, helping them thrive through the uncertainty of their condition.
1. Introduction
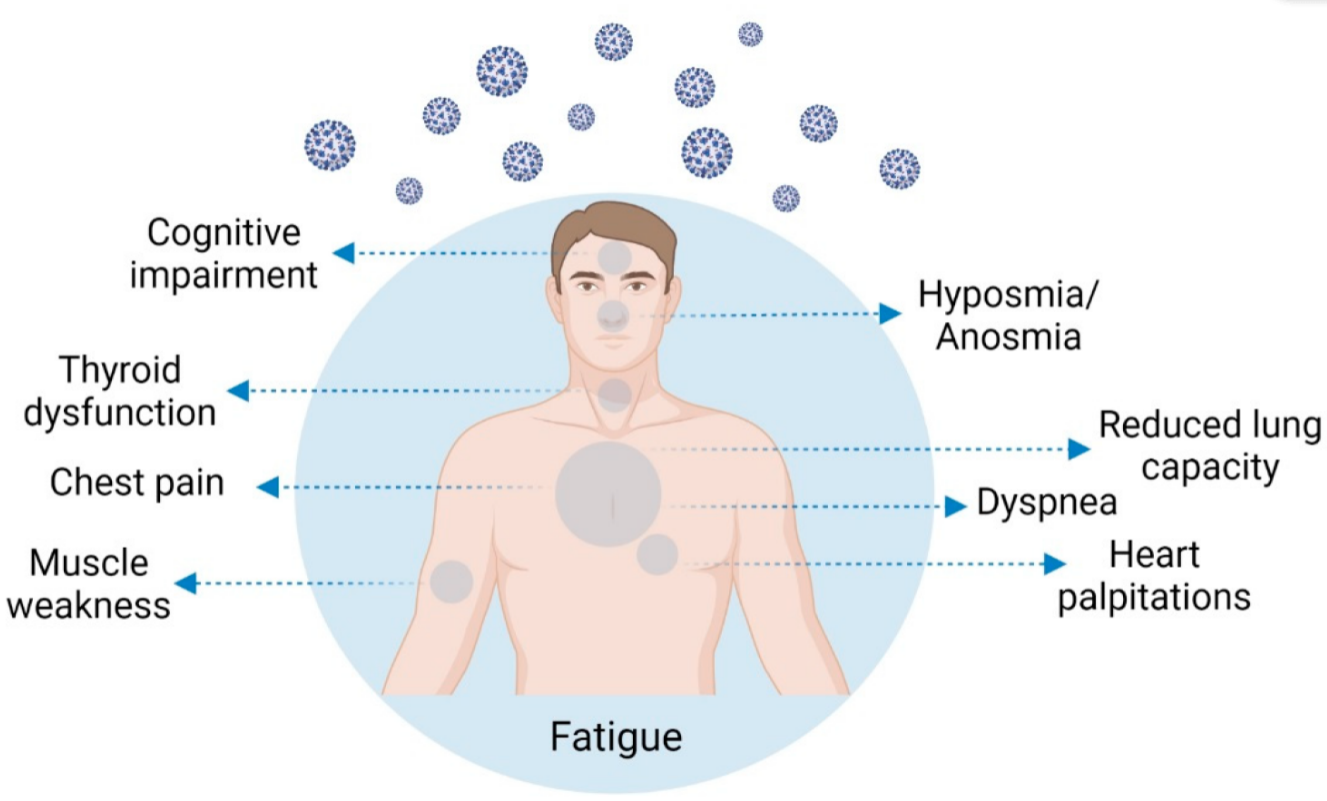
2. Post-COVID-19 Condition Symptomatology and Prevalence
| Post-COVID-19 Symptoms | Number of Patients Included in the Study | % Patients Suffering from Symptom/References |
|---|---|---|
| Fatigue | 596, 177, 538, 270, 138, 3065, 134, 242, 115, 143, 96, 1733, 5440, 384, 287 | 13.1% [34], 13.6% [20], 28.3% [7], 34.8% [25], 39.0% [27][30], 39.6% [6], 41.7% [35], 47% [26], 53.1% [36], 56.3% [28], 63% [19], up to 65% [16], 69% [37], 72.8% [38] |
| Persistent breathlessness /dyspnea | 596, 3065, 287, 270, 96, 138, 143, 384, 134, 5440, 35 | 6.0% [34], 23.2% [30], 28.2% [38], 34.0% [25], 37.5% [28], 40.0% [27], 43.4% [36], 53.0% [37], 60% [6], up to 61% [16], 80% [23] |
| Myalgia /muscle weakness | 277, 242, 134, 1733 | 19.6% [25], 35,1% [35], 51.5% [6], 63% [19] |
| Anxiety | 287, 402, 134 | 38.0% [38], 42% [39], 47.8% [6] |
| Sleep disturbance | 1733, 96, 134, 35, 138 | 21.1% [35], 26.0% [19], 26.0% [28], 35.1% [6], 40.0% [39], 46% [23], 49% [27] |
| Joint pain | 277, 143, 287 | 19.6% [25], 27.3% [36], 31.4% [38] |
| Headache | 242, 270, 3065, 287 | 19.0% [35], 19.8% [25], 23.4% [30], 28.6% [38] |
| Chest pain | 596, 242, 538, 143, 287, 35, 5440 | 0.8% [34], 10.7% [35], 12.3% [7], 21.7% [36], 28.9% [38], 34.8% [23], up to 89% [16] |
3. Post-COVID-19 Condition Risk Factors
4. Post-COVID-19 Condition Pathophysiology
Different lines of research are trying to explain these protracted symptoms. A persistent immune activation and/or inflammation may contribute to post-COVID-19 condition, which could explain why many patients with mild COVID-19 disease experience chronic persistent symptoms, involving the cardiovascular, nervous, and respiratory systems [57]. In fact, the persistently elevated inflammatory markers observed in long-COVID patients point towards chronic persistence of inflammation [18][58].
Seessle et al. [28] observed several neurocognitive symptoms that were associated with antinuclear antibody titer elevation, pointing to autoimmunity as a cofactor in the etiology of post-COVID-19 neurologic conditions [28]. The autoimmune hypothesis could explain the greater incidence of this condition in women [14][57]. Since thyroid is closely linked to T-cell-mediated autoimmunity, thyroid dysfunction may be important in the pathophysiology of post-COVID-19 condition, as discussed in more detail below [3].
Post-COVID-19 condition has been related to additional characteristics of the innate and adaptive response, involving a weaker initial inflammatory response, with lower baseline levels of C-reactive protein and ferritin [45]. The participation of the immune system in post-COVID-19 condition has been reported in other studies [8][21][57][59][60]. Symptoms such as cognitive dysfunction, persistent fatigue, muscle aches, depression, and other mental health issues are highly associated with an initial immune challenge and/or with a constant dysregulation of the immune system [29][60].
The involvement of inflammatory cytokines in the etiology of the neuropsychiatric symptoms, reported in current large-scale population-based epidemiological and genetic studies, indicates that these cytokines may have a role in the etiology of the neuropsychiatric symptoms usually observed in patients with post-COVID-19 condition [3][29][60]. This cytokine storm must also be considered as a possible driving factor for the expansion of neuropathies after severe COVID-19 infection, contributing to the chronic pain that appears after acute infection recovery [61].
Studies have shown that patients with severe symptoms may have more severe autonomic dysfunction when compared with patients presenting mild symptoms, as indicated by the heart rate variability (HRV) analysis [2], which is a reliable non-invasive tool used to evaluate autonomic modulation [2][62]. Patients with severe symptoms presenting amelioration in autonomic parameters also show enhancements in immune and coagulation functions, as well as in cardiac injury biomarkers [2].
5. Thyroid Involvement in COVID-19
Due to the reported high expression of ACE2, the thyroid may become a target of coronavirus infection, and thyroid involvement in COVID-19 patients has been demonstrated [63]. In fact, SARS-CoV-2 uses ACE2, combined with the transmembrane protease serine 2 (TMPRSS2), as the main molecular complex for the host cell infection [64]. Interestingly, ACE2 and TMPRSS2 expression levels are higher in the thyroid gland than in the lungs [64]. Scappaticcio et al. [64], in their literature review on thyroid dysfunction in COVID-19 patients, presented strong evidence that the thyroid gland and the entire hypothalamic–pituitary–thyroid (HPT) axis may be important targets for SARS-CoV-2 damage.
Campi et al. [63] found a temporary situation of low TSH with normal T4 and low T3 levels in patients hospitalized for SARS-CoV-2 infection, which was inversely associated with C-reactive protein, cortisol, and IL-6, and positively associated with normal Tg levels. These authors stated that this temporary change was probably due to the cytokine storm induced by the virus, with a direct or mediated impact on TSH secretion and deiodinase activity, and probably not to a destructive thyroiditis. The THYRCOV study offers early evidence that patients with acute SARS-CoV-2 infection with thyrotoxicosis have statistically significantly higher levels of IL-6 [65]. In a short-term follow-up, Pizzocaro et al. [66] showed a spontaneous normalization of thyroid function in most infected patients with SARS-CoV-2-related thyrotoxicosis. Nevertheless, these authors stated that long-lasting studies are needed, since they found a frequent thyroid hypoecogenicity pattern in the ultrasonographic evaluation of these patients, which may predispose them to late-onset thyroid dysfunction development [66].
Even though clear evidence is missing, infection of the thyrocyte, thyrotroph, and corticotroph may lead to a decrease in T3, T4, TSH, ACTH, and cortisol levels [67]. HPT dysregulation has been considered, at least in part, responsible for hypothyroidism in COVID-19 [67][68]. Low FT3 levels are independently associated with increased mortality [67][69][70] and disease severity [68][71][72][73] and may be used as a surrogate prognostic biomarker [67][69][70].
Researchers' knowledge of the thyroid patterns of COVID-19 is still incomplete, as is the etiologic view of COVID-19 and thyroid insults [67][74]. To find direct evidence concerning the nature and cause of thyroid SARS-CoV-2 injury, and the full immune response in those patients with thyroid dysfunction, researchers need a histologic and cytological examination of the thyroid gland in a wide number of patients [67][68].
6. Post-COVID-19 Condition Health Burden and Patient Management
Post-COVID-19 condition (or long COVID) first gained extensive credit among social support groups, and then in scientific and medical communities [3][5][75][76]. It is probably the first illness to be cooperatively identified by patients discovering one another using Twitter and other social media [75].
Patients with post-COVID-19 condition are a heterogeneous group, which makes it difficult to advise treatment [77][78]. It is crucial for each patient to find the correct equilibrium between mild activity to avoid deconditioning and not triggering post-exercise malaise [77]. Strategies tackling levels of stress and/or the stress response, comprising psychosocial intervention, physical exercise, or possibly dietary interventions of people could be a good approach to counteract some of the negative effects of chronic inflammation [29].
Management of post-acute COVID-19 syndrome requires a comprehensive team, including physicians of various specialties (primary care, pulmonology, cardiology, and infectious disease), physiatrists, behavioural health experts, physical and occupational therapists, and social workers, which will address the clinical and psychological aspects of the disease [79].
7. Conclusions
It is urgent to better understand this emerging, complex, and puzzling medical condition [16][80]. Post-COVID-19 condition can become a crisis for health systems, which are already facing the challenge of the pandemic [81]. The primary care services, which represent the first approach for patient diagnosis, still have little information or resources to deal with these patients [82].
There is an urgent need to identify affected individuals early so the most appropriate and efficient treatments may be provided [79][80], helping them to thrive through the uncertainty of their condition [15][83].
References
- Sancak, B.; Kilic, C. A Psychiatrist’s Own Experience of Long COVID: Looking beyond the Psychosomatic Perspective. Psychiatr. Danub. 2021, 33, 250.
- Pan, Y.; Yu, Z.; Yuan, Y.; Han, J.; Wang, Z.; Chen, H.; Wang, S.; Wang, Z.; Hu, H.; Zhou, L. Alteration of Autonomic Nervous System Is Associated with Severity and Outcomes in Patients with COVID-19. Front. Physiol. 2021, 12, 630038.
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754.
- Shuwa, H.A.; Shaw, T.N.; Knight, S.B.; Wemyss, K.; McClure, F.A.; Pearmain, L.; Prise, I.; Jagger, C.; Morgan, D.J.; Khan, S.; et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med 2021, 2, 720–735.e4.
- Marshall, M. The four most urgent questions about long COVID. Nature 2021, 594, 168–170.
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119.
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95.
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67.
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202.
- WHO. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 3 March 2022).
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394.
- Jacobs, J.J. Persistent SARS-2 infections contribute to long COVID-19. Med. Hypotheses 2021, 149, 110538.
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33.
- Ortona, E.; Buonsenso, D.; Carfi, A.; Malorni, W. Long Covid Kids study g. Long COVID: An estrogen-associated autoimmune disease? Cell Death Discov. 2021, 7, 77.
- Amenta, E.M.; Spallone, A.; Rodriguez-Barradas, M.C.; El Sahly, H.M.; Atmar, R.L.; Kulkarni, P.A. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect. Dis. 2020, 7, ofaa509.
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, A.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021, 75, e14357.
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580.
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875.
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232.
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830.
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front. Immunol. 2021, 12, 686029.
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880.
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Pediatr. 2021, 48, 2823–2833.
- Naeije, R.; Caravita, S. Phenotyping long COVID. Eur. Respir. J. 2021, 58, 2101763.
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.-M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383.
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003.
- Gaber, T.A.-Z.K.; Ashish, A.; Unsworth, A. Persistent post-covid symptoms in healthcare workers. Occup. Med. 2021, 71, 144–146.
- Seessle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent symptoms in adult patients one year after COVID-19: A prospective cohort study. Clin. Infect. Dis. 2021, ciab611.
- Mondelli, V.; Pariante, C.M. What can neuroimmunology teach us about the symptoms of long-COVID? Oxf. Open Immunol. 2021, 2, iqab004.
- Stephenson, T.; Pereira, S.M.P.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc. Health 2022, 6, 230–239.
- Becker, R.C. Autonomic dysfunction in SARS-CoV-2 infection acute and long-term implications COVID-19 editor’s page series. J. Thromb. Thrombolysis 2021, 52, 692–707.
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337.
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J. Clin. Transl. Endocrinol. 2022, 27, 100284.
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513.
- Kashif, A.; Chaudhry, M.; Fayyaz, T.; Abdullah, M.; Malik, A.; Anwer, J.M.A.; Inam, S.H.A.; Fatima, T.; Iqbal, N.; Shoaib, K. Follow-up of COVID-19 recovered patients with mild disease. Sci. Rep. 2021, 11, 13414.
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605.
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398.
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746.
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600.
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427.
- Wildwing, T.; Holt, N. The neurological symptoms of COVID-19: A systematic overview of systematic reviews, comparison with other neurological conditions and implications for healthcare services. Ther. Adv. Chronic Dis. 2021, 12.
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019.
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022.
- Duggal, P.; Penson, T.; Manley, H.N.; Vergara, C.; Munday, R.M.; Duchen, D.; Linton, E.A.; Zurn, A.; Kerulz, J.C.; Mehta, S.H.; et al. Post-sequelae symptoms and comorbidities after COVID-19. J. Med. Virol. 2022, 94, 2060–2066.
- Garcia-Abellan, J.; Padilla, S.; Fernandez-Gonzalez, M.; Garcia, J.A.; Agullo, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutiérrez, F.; Masiá, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021, 41, 1490–1501.
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. eClinicalMedicine 2021, 36, 100899.
- Cortinovis, M.; Perico, N.; Remuzzi, G. Long-term follow-up of recovered patients with COVID-19. Lancet 2021, 397, 173–175.
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631.
- Fernandez-de-Las-Penas, C.; Pellicer-Valero, O.J.; Navarro-Pardo, E.; Palacios-Cena, D.; Florencio, L.L.; Guijarro, C.; Martín-Guerrero, J.D. Symptoms Experienced at the Acute Phase of SARS-CoV-2 Infection as Risk Factor of Long-term Post-COVID Symptoms: The LONG-COVID-EXP-CM Multicenter Study. Int. J. Infect. Dis. 2022, 116, 241–244.
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.; Florencio, L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621.
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67.
- Iyengar, K.P.; Jain, V.K.; Vaishya, R.; Ish, P. Long COVID-19: An emerging pandemic in itself. Adv. Respir. Med. 2021, 89, 234–236.
- Khunti, K.; Davies, M.J.; Kosiborod, M.N.; Nauck, M.A. Long COVID—metabolic risk factors and novel therapeutic management. Nat. Rev. Endocrinol. 2021, 17, 379–380.
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285.
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 1–11.
- Yelin, D.; Margalit, I.; Yahav, D.; Runold, M.; Bruchfeld, J. Long COVID-19-it’s not over until? Clin. Microbiol. Infect. 2021, 27, 506–508.
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 5, eabe8063.
- Doykov, I.; Hallqvist, J.; Gilmour, K.C.; Grandjean, L.; Mills, K.; Heywood, W.E. ‘The long tail of COVID-19’—The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research 2020, 9, 1349.
- Pasrija, R.; Naime, M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int. Immunopharmacol. 2021, 90, 107225.
- Kappelmann, N.; Dantzer, R.; Khandaker, G.M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology 2021, 131, 105295.
- McFarland, A.J.; Yousuf, M.S.; Shiers, S.; Price, T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: Implications for COVID-19 and pain. Pain Rep. 2021, 6, e885.
- Barizien, N.; Le Guen, M.; Russel, S.; Touche, P.; Huang, F.; Vallée, A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021, 11, 14042.
- Campi, I.; Bulgarelli, I.; Dubini, A.; Perego, G.B.; Tortorici, E.; Torlasco, C.; Torresani, E.; Rocco, L.; Persani, L.; Fugazzola, L. The spectrum of thyroid function tests during hospitalization for SARS-CoV-2 infection. Eur. J. Endocrinol. 2021, 184, 699–709.
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2020, 22, 803–815.
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in Patients with COVID-19: The Thyrcov Study. Eur. J. Endocrinol. 2020, 183, 381–387.
- Pizzocaro, A.; Colombo, P.; Vena, W.; Ariano, S.; Magnoni, P.; Reggiani, F.; Favacchio, G.; Mirani, M.; Lavezzi, E.; Voza, A.; et al. Outcome of SARS-CoV-2-related thyrotoxicosis in survivors of COVID-19: A prospective study. Endocrine 2021, 73, 255–260.
- Inaba, H.; Aizawa, T. Coronavirus Disease 2019 and the Thyroid—Progress and Perspectives. Front. Endocrinol. 2021, 12, 708333.
- Chen, W.; Tian, Y.; Li, Z.; Zhu, J.; Wei, T.; Lei, J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology 2021, 162, bqab004.
- Ruggeri, R.M.; Campennì, A.; Deandreis, D.; Siracusa, M.; Tozzoli, R.; Ovčariček, P.P.; Giovanella, L. SARS-CoV-2-related immune-inflammatory thyroid disorders: Facts and perspectives. Expert Rev. Clin. Immunol. 2021, 17, 737–759.
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935.
- Schwarz, Y.; Percik, R.; Oberman, B.; Yaffe, D.; Zimlichman, E.; Tirosh, A. Sick Euthyroid Syndrome on Presentation of Patients With COVID-19: A Potential Marker for Disease Severity. Endocr. Pr. 2021, 27, 101–109.
- Güven, M.; Gültekin, H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: Results of single-centre pandemic hospital. Int. J. Clin. Pr. 2021, 75, e14129.
- Beltrão, F.E.d.L.; Beltrão, D.C.d.A.; Carvalhal, G.; Beltrão, M.F.E.d.L.; Brito, M.A.d.S.; Capistrano, M.K.H.R.; Bastos, I.H.D.A.; Hecht, F.; Daltro, C.H.D.C.; Bianco, A.C.; et al. Thyroid Hormone Levels During Hospital Admission Inform Disease Severity and Mortality in COVID-19 Patients. Thyroid 2021, 31, 1639–1649.
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031.
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426.
- Roth, P.H.; Gadebusch-Bondio, M. The contested meaning of “long COVID”—Patients, doctors, and the politics of subjective evidence. Soc. Sci. Med. 2022, 292, 114619.
- Newman, M. Chronic fatigue syndrome and long covid: Moving beyond the controversy. BMJ 2021, 373, n1559.
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Physiol. 2022, 322, C1–C11.
- Chippa, V.; Aleem, A.; Anjum, F. Post Acute Coronavirus (COVID-19) Syndrome; StatPearls: Treasure Island, FL, USA, 2022.
- Moghimi, N.; Di Napoli, M.; Biller, J.; Siegler, J.E.; Shekhar, R.; McCullough, L.D.; Harkins, M.S.; Hong, E.; Alaouieh, D.A.; Mansueto, G.; et al. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021, 21, 44.
- Murray, T. Unpacking “long COVID”. CMAJ 2021, 193, E318–E319.
- Hussain, F.A. Facilitating care: A biopsychosocial perspective on long COVID. Br. J. Gen. Pr. 2021, 72, 30–31.
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310.




