
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lu Liu | -- | 2821 | 2022-04-18 07:05:12 | | | |
| 2 | Conner Chen | Meta information modification | 2821 | 2022-04-18 11:11:56 | | | | |
| 3 | Conner Chen | Meta information modification | 2821 | 2022-04-22 03:54:07 | | |
Video Upload Options
The continuous occurrence of food contaminants worldwide poses a critical threat to the health of human and livestock. One of the major contaminants in food and feed products are mycotoxins, the secondary metabolites synthesized by toxigenic fungi strains, mainly those belonging to Penicillium, Aspergillus, Alternaria and Fusarium genera. Both acute and chronic exposure to mycotoxin-contaminated food may cause deleterious health effects including retarded growth, suppression of the immune response, vomiting, infertility and gastrointestinal and carcinogenic diseases. These mycotoxins occur in various products, from raw agricultural products such as corn, barley, oats, fruits and herbs, to commercial commodities including aquafeeds, beverages, fruit and vegetable-derived products. The contamination of mycotoxins can occur during any part of the complex food chain, including harvest, industry processing, transportation and/or storage, imposing social burdens on the food industry due to the waste created by contaminated products. This creates an urgent demand for mycotoxin removal methods to minimize economic loss and hazards to consumers.
1. Introduction
2. Chemical Structure and Toxic Effects of Mycotoxins
| Mycotoxins | Chemical Structure | Main Toxic Groups | Main Degradation Products | Organ/System Affected | Main Clinical Signs | Producing Fungi |
|---|---|---|---|---|---|---|
| Aflatoxins (B1, B2, G1, G2) |
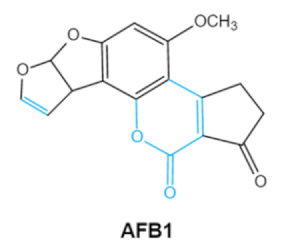 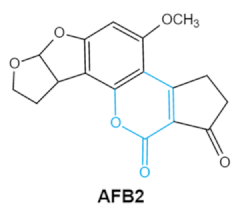 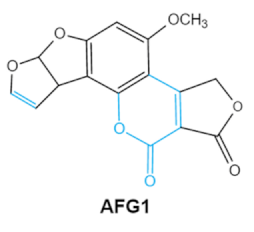  |
Lactone ring Double bond in difuran ring moiety |
AFB1-8,9dihydrodiol, AFB1-8,9-epoxide, dihydrohydroxyaflatoxin B1, | Liver, kidney, immune system | Hepatitis, carcinogenic, abdominal pain, vomiting, increased susceptibility to disease, immunosuppressive and carcinogenic effects |
Aspergillus. Flavus A. Parasiticus A. nomius |
| Zearalenones (ZEA) | 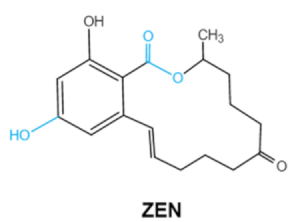 |
Lactone ring C-4 hydroxyl group |
α-/β-zearalenol, α-/β-zearalanol,zearalenone-4-sulfate, 1-(3,5-dihydroxyphenyl)-6′-hydroxy-l’-undecen-l0′-one, (5S)-5-({2,4-dihydroxy-6-[(1E)-5-hydroxypent-1-en-1-yl]benzoyl}oxy)hexanoic acid |
Reproductive tract, mainly female | Hyperestrogenism, Reproductive disorders | Fusarium graminearum(F. roseum) F. culmorum F. equiseti F. cerealis F. verticillioides F. incarnatum |
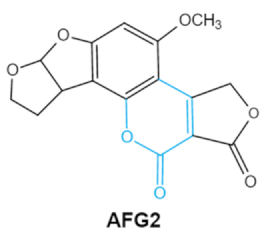
| Ochratoxins (A,B,C) (OTs) | 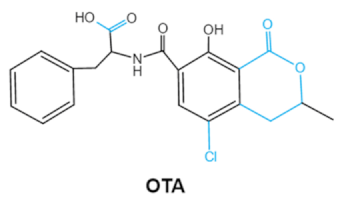 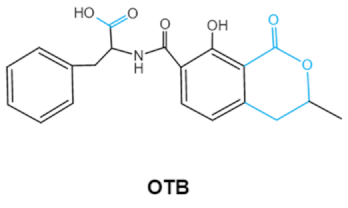 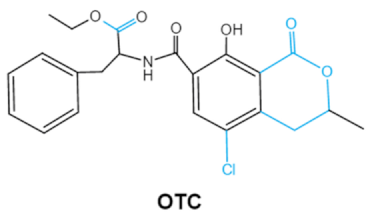 |
Isocoumarin moiety Carboxyl group of the phenylalanine moietyCl group |
L-βphenylalanine, OTα | Liver, kidney, immune system, inhibit RNA, DNA and protein synthesis in kidney | Nephritis, enlargement of kidney and hepatitis | A. ochraceus A. carbonarius A. niger P. verrucosum P. nordicum |
| Fumonisins FBs (B1, B2) | 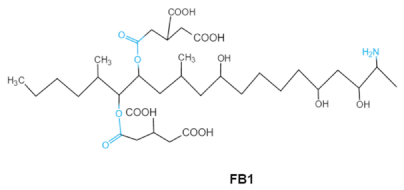 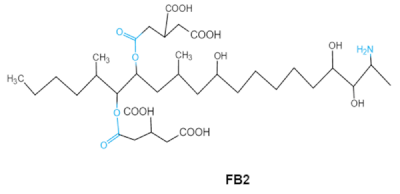 |
Two tricarballylic acid side chains Free amino group |
2-oxo-12,16-dimethyl-3,5,10,14,15-icosanepentol hemiketal, NacetylAP1, |
Lungs and heart (pig), central nervous system (horse), liver, immune system | Porcine pulmonary edema (PPE), equine leukoencephalomalacia | Fusarium section Liseola |
| Trichothecenes (DON, T-2, HT-2) TCNs | 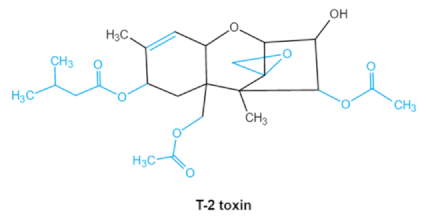 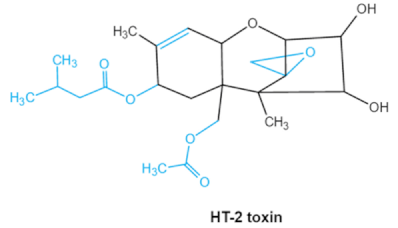  |
Epoxide group Acylated side groups C9-10 double bond |
HT-2 toxin, T-2 triol, T-2 tetraol, de-epoxy T-2 tetraol, 3α,7α,15α-triacetoxy-deoxynivalenol, de-epoxy deoxynivalenol, 3-acetyldeoxynivalenol, diaacetoxydeoxynivalenol. Epoxymonoacetoxyscirpenol, de-epoxyscirpentrio |
Central nervous system, gastrointestinal tract, liver, immune system | Anorexia, vomiting, abdominal pains, cardiovascular dysfunction | F. acuminatum F. sporotrichioides F. langsethiae Fusariumgraminearum, F. culmorum F. cerealis F. culmorum F. graminearum F. sporotrichioides F. poae |
| Patulin PAT |
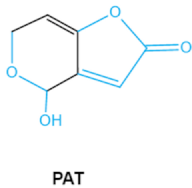 |
Furan, pyran or lactone ring Hemiacetal |
Ascladiol, hydroascladiol, desoxypatulinic acid, 3-keto-5-hydroxypentanal, glyoxylic acid |
Gut epithelium, liver, kidney, immune system | Oral and epithelial lesion, loss of appetite Nausea, vomitin, gastric ulcers |
Penicillim expansum Bysochlamis nívea Aspergillus clavatus P. griseofulvum |
2.1. Aflatoxins (AFs)
2.2. Trichothecenes
2.3. Ochratoxins
2.4. Zearalenone
2.5. Fumonisins
2.6. Patulin
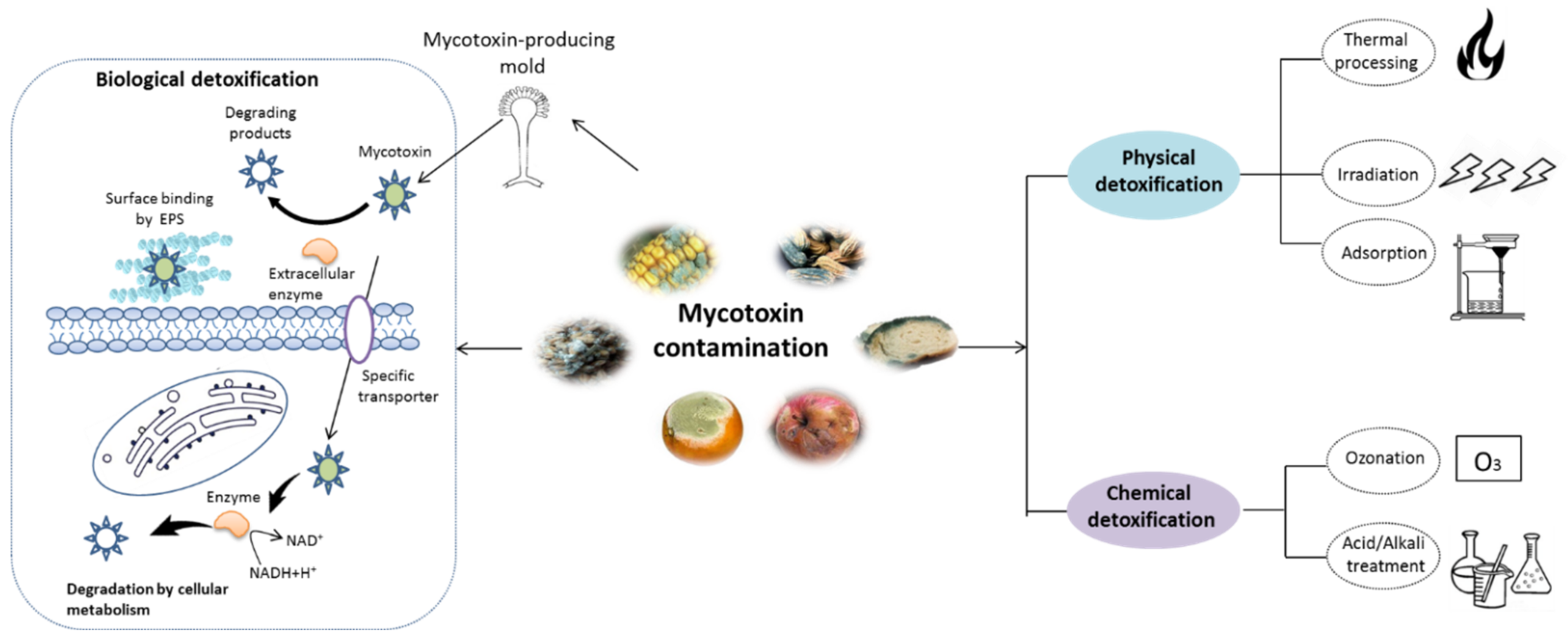
3. Mycotoxin Removal by Probiotics
3.1. Fast Glance to Probiotic Properties
3.2. Mycotoxins Detoxification by Probiotics and the Related Mechanism
3.2.1. Cell-Binding of Mycotoxins
3.2.2. Biodegradation
| Mycotoxins | Microorganism | Reduction Rate (%) | Toxin Level | Degradation Condition | Reference |
|---|---|---|---|---|---|
| AFB1 | Streptomyces cacaoi subsp. Asoensis K234 | 88.34 ± 15.62 | 1 μg mL−1 | 5 days, 28 °C,170 rpm liquid LB medium |
Harkai et al. (2016) |
| Streptomyces luteogriseus K144 | 79.93 | 1 μg mL−1 | 5 days, 28 °C,170 rpm liquid LB medium |
||
| Bacillus licheniformis CFR1 | 94.73 ± 1.09 | 500 ppb | liquid nutrient broth (NB) at 37 °C, 72 h | Rao et al. (2017) | |
| T-2 | P. pentosaceus KTU05-10 | 78.0 | 12.8–19.5 μg kg−1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Saccharomyces pastorianus A15 | 31.0 | 5000 µg L−1 | 15 °C, 120 rpm, four days 11.5° Plato wort |
Nathanail et al. (2016) | |
| HT-2 | P. pentosaceus KTU05-10 | 79.0 | 258–819 μg L−1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| ZEA | P. acidilactici | 38.0 | 19.5–873.7 μg L−1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Streptomyces rimosus (K145, K189) | 100.0 | 1 μg mL−1 | 5 days, 28 °C,170 rpm liquid LB medium |
Harkai et al. (2016) | |
| DON | P. pentosaceus KTU05-10 | 47.0 | 3370–6930 μg kg−1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Saccharomyces pastorianus A15 | 15.0 | 400 µg L−1 | 11.5° Plato wort, 15 °C, 120 rpm for 4 days | Nathanail et al. (2016) |
References
- Taheur, F.B.; Kouidhi, B.; al Qurashi, Y.M.A.; Salah-Abbes, J.B.; Chaieb, K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon 2019, 160, 12–22.
- Savi, G.D.; Piacentini, K.C.; Scussel, V.M. Ozone treatment efficiency in Aspergillus and Penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum L.). J. Food Process. Preserv. 2015, 39, 940–948.
- Temba, B.A.; Sultanbawa, Y.; Kriticos, D.J.; Fox, G.P.; Harvey, J.J.; Fletcher, M.T. Tools for defusing a major global food and feed safety risk: Nonbiological postharvest procedures to decontaminate mycotoxins in foods and feeds. J. Agric. Food Chem. 2016, 64, 8959–8972.
- Klaric, M.S.; Cvetnic, Z.; Pepeljnjak, S.; Kosalec, I. Co-occurrence of aflatoxins, ochratoxin A, fumonisins, and zearalenone in cereals and feed, determined by competitive direct enzyme-linked immunosorbent assay and thin-layer chromatography. Arh. Hig. Rada Toksikol. 2009, 60, 427–434.
- Schatzmayr, G.; Zehner, F.; Taubel, M.; Schatzmayr, D.; Klimitsch, A.; Loibner, A.P.; Binder, E.M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006, 50, 543–551.
- Bocarov-Stancic, A.; Adamovic, M.; Salma, N.; Bodroza-Solarov, M.; Vuckovic, J.; Pantic, V. In vitro efficacy of mycotoxins adsorption by natural mineral adsorbents. Biotechnol. Anim. Husb. 2011, 27, 1241–1251.
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010, 21, 67–76.
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121.
- Huang, W.; Chang, J.; Wan, P.; Liu, C.; Yin, Q.; Lu, F.; Gao, T. Effect of the combined compound probiotics with mycotoxin–degradation enzyme on detoxifying aflatoxin B1, and zearalenone. J. Toxicol. Sci. 2018, 43, 377–385.
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808.
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130.
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362.
- Sato, I.; Ito, M.; Ishizaka, M.; Ikunaga, Y.; Sato, Y.; Yoshida, S.; Koitabashi, M.; Tsushima, S. Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol. Lett. 2012, 327, 110–117.
- Liuzzi, V.C.; Fanelli, F.; Tristezza, M.; Haidukowski, M.; Picardi, E.; Manzari, C.; Lionetti, C.; Grieco, F.; Logrieco, A.F.; Thon, M.R.; et al. Transcriptional analysis of Acinetobacter sp. neg1, capable of degrading ochratoxin a. Front. Microbiol. 2016, 7, 2162.
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404.
- Rajasekharan, S.K.; Lee, J.H.; Zhao, Y.; Lee, J. The mycotoxin zearalenone hinders Candida albicans biofilm formation and hyphal morphogenesis. Indian J. Microbiol. 2018, 58, 19–27.
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125.
- Liu, B.; Peng, X.; Meng, X. Effective biodegradation of mycotoxin patulin by porcine pancreatic lipase. Front. Microbiol. 2018, 9, 615.
- Al-Nussairawi, M.; Risa, A.; Garai, E.; Varga, E.; Szabo, I.; Csenki-Bakos, Z.; Kriszt, B.; Cserhati, M. Mycotoxin biodegradation ability of the Cupriavidus Genus. Curr. Microbiol. 2020, 77, 2430–2440.
- Silva, L.J.G.; Pereira, A.; Pena, A.; Lino, C.M. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2020, 10, 14.
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237.
- Khan, G.I. Eco-friendly bacterial biodegradation of mycotoxins. Pure Appl. Biol. 2020, 9, 1708–1717.
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mule, G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins 2017, 9, 111.
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Mycotoxins in functional beverages: A Review. Beverages 2020, 6, 52.
- Vanhoutte, I.; Audenaert, K.; de Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561.
- Winter, G.; Pereg, L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019, 70, 882–897.
- Adebo, O.A.; Njobeh, P.B.; Sidu, S.; Adebiyi, J.A.; Mavumengwana, V. Aflatoxin B1, degradation by culture and lysate of a Pontibacter specie. Food Control 2017, 80, 99–103.
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58.
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644.
- Polak-Sliwinska, M.; Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454.
- Bezuidenhout, C.C.; Prinsloo, M.; van der Walt, A.M. Multiplex PCR-based detection of potential fumonisin-producing Fusarium in traditional African vegetables. Environ. Toxicol. 2006, 21, 360–366.
- Zhu, M.; Cen, Y.; Ye, W.; Li, S.; Zhang, W. Recent advances on macrocyclic trichothecenes, their bioactivities and biosynthetic pathway. Toxins 2020, 12, 417.
- Khaneghah, A.M.; Farhadi, A.; Nematollahi, A.; Vasseghian, Y.; Fakhri, Y. A systematic review and meta-analysis to investigate the concentration and prevalence of trichothecenes in the cereal-based food. Trends Food Sci. Technol. 2020, 102, 193–202.
- Van der Fels-Klerx, H.; Stratakou, I. T-2, toxin and HT-2, toxin in grain and grain-based commodities in Europe: Occurrence, factors affecting occurrence, co-occurrence and toxicological effects. World Mycotoxin J. 2010, 3, 349–367.
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814.
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378.
- Zhou, T.; He, J.; Gong, J. Microbial transformation of trichothecene mycotoxins. World Mycotoxin J. 2008, 1, 23–30.
- Stanciu, O.; Juan, C.; Miere, D.; Berrada, H.; Loghin, F.; Manes, J. First study on trichothecene and zearalenone exposure of the Romanian population through wheat-based products consumption. Food Chem. Toxicol. 2018, 121, 336–342.
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162.
- Bayman, P.; Baker, J.L. Ochratoxins: A global perspective. Mycopathologia 2006, 162, 215–223.
- Reddy, L.; Bhoola, K. Ochratoxins-food contaminants: Impact on human health. Toxins 2010, 2, 771–779.
- Rai, A.; Dixit, S.; Singh, S.P.; Gautam, N.K.; Das, M.; Tripathi, A. Presence of zearalenone in cereal grains and its exposure risk assessment in indian population. J. Food Sci. 2018, 83, 3126–3133.
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228.
- Kowalska, K.; Habrowska-Gorczynska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol 2016, 48, 141–149.
- Popiel, D.; Koczyk, G.; Dawidziuk, A.; Gromadzka, K.; Blaszczyk, L.; Chelkowski, J. Zearalenone lactonohydrolase activity in Hypocreales and its evolutionary relationships within the epoxide hydrolase subset of a/b-hydrolases. BMC Microbiol. 2014, 14, 82.
- Li, Y.-S.; Zhou, Y.; Lu, S.-Y.; Guo, D.-J.; Ren, H.-L.; Meng, X.-M.; Zhi, B.-H.; Lin, C.; Wang, Z.; Li, X.-B.; et al. Development of a one-step test strip for rapid screening of fumonisins B1, B2, and B3, in maize. Food Control 2012, 24, 72–77.
- Mirasoli, M.; Buragina, A.; Dolci, L.S.; Simoni, P.; Anfossi, L.; Giraudi, G.; Roda, A. Chemiluminescence-based biosensor for fumonisins quantitative detection in maize samples. Biosens. Bioelectron. 2012, 32, 283–287.
- Silva, L.J.; Pena, A.; Lino, C.M.; Fernandez, M.F.; Manes, J. Fumonisins determination in urine by LC-MS-MS. Anal. Bioanal. Chem. 2010, 396, 809–816.
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Sci. Technol. 2007, 137, 299–325.
- Morgavi, D.P.; Riley, R.T. Fusarium and their toxins: Mycology, occurrence, toxicity, control and economic impact. Animal Feed Sci. Technol. 2007, 137, 199–200.
- Diao, E.; Hou, H.; Hu, W.; Dong, H.; Li, X. Removing and detoxifying methods of patulin: A review. Trends Food Sci. Technol. 2018, 81, 139–145.
- Rodriguez, A.; Luque, M.I.; Andrade, M.J.; Rodriguez, M.; Asensio, M.A.; Cordoba, J.J. Development of real-time PCR methods to quantify patulin-producing molds in food products. Food Microbiol. 2011, 28, 1190–1199.
- Tannous, J.; el Khoury, R.; Snini, S.P.; Lippi, Y.; el Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60.
- Zhang, H.; Ahima, J.; Yang, Q.; Zhao, L.; Zhang, X.; Zheng, X. A review on citrinin: Its occurrence, risk implications, analytical techniques, biosynthesis, physiochemical properties and control. Food Res. Int. 2021, 141, 110075.
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333.
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185.
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380.
- Gaggia, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28.
- Kesarcodi-Watson, A.; Kaspar, H.; Lategan, M.J.; Gibson, L. Probiotics in aquaculture: The need, principles and mechanisms of action and screening processes. Aquaculture 2008, 274, 1–14.
- Zoghia, A.; Khosravi-Darani, K.; Sohrabvandib, S. Surface binding of toxins and heavy metals by probiotics. Mini Rev. Med. Chem. 2014, 14, 84–98.
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55.
- Chiocchetti, G.M.; Jadan-Piedra, C.; Monedero, V.; Zuniga, M.; Velez, D.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 1534–1545.
- Hatab, S.; Yue, T.; Mohamad, O. Removal of patulin from apple juice using inactivated lactic acid bacteria. J. Appl. Microbiol. 2012, 112, 892–899.
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1, binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156.
- Prete, V.D.; Rodriguez, H.; Carrascosa, A.V. In vitro removal of ochratoxin a by wine lactic acid bacteria. J. Food Prot. 2007, 70, 2155–2160.
- Sangsila, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Itsaranuwat, P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control 2016, 62, 187–192.
- Vinderola, G.; Ritieni, A. Role of probiotics against mycotoxins and their deleterious effects. J. Food Res. 2014, 4, 10–21.
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Factories 2014, 13, S9.
- Niderkorn, V.; Morgavi, D.P.; Aboab, B.; Lemaire, M.; Boudra, H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1, and B2, by lactic acid bacteria. J. Appl. Microbiol. 2009, 106, 977–985.
- Haskard, C.; Binnion, C.; Ahokas, J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 2000, 128, 39–49.
- Hernandez-Mendoza, A.; Guzman-de-Pena, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403.
- Jouany, J.P.; Yiannikouris, A.; Bertin, G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch. Zootech. 2005, 8, 26–50.
- Yiannikouris, A.; Poughon, L.; Cameleyre, X.; Dussap, C.G.; François, J.; Bertin, G.; Jouany, J.P. A novel technique to evaluate interactions between Saccharomyces cerevisiae cell wall and mycotoxins: Application to zearalenone. Biotechnol. Lett. 2003, 25, 783–789.
- Harkai, P.; Szabó, I.; Cserháti, M.; Krifaton, C.; Risa, A.; Radó, J.; Balázs, A.; Berta, K.; Kriszt, B. Biodegradation of aflatoxin-B1, and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegrad. 2016, 108, 48–56.
- Rao, K.R.; Vipin, A.V.; Hariprasad, P.; Appaiah, K.A.A.; Venkateswaran, G. Biological detoxification of Aflatoxin B1, by Bacillus licheniformis CFR1. Food Control 2017, 71, 234–241.
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT Food Sci. Technol. 2018, 89, 307–314.
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455.





