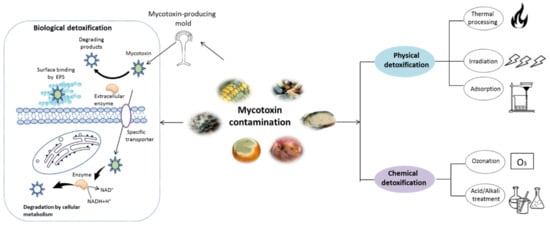
The continuous occurrence of food contaminants worldwide poses a critical threat to the health of human and livestock. One of the major contaminants in food and feed products are mycotoxins, the secondary metabolites synthesized by toxigenic fungi strains, mainly those belonging to Penicillium, Aspergillus, Alternaria and Fusarium genera. Both acute and chronic exposure to mycotoxin-contaminated food may cause deleterious health effects including retarded growth, suppression of the immune response, vomiting, infertility and gastrointestinal and carcinogenic diseases. These mycotoxins occur in various products, from raw agricultural products such as corn, barley, oats, fruits and herbs, to commercial commodities including aquafeeds, beverages, fruit and vegetable-derived products. The contamination of mycotoxins can occur during any part of the complex food chain, including harvest, industry processing, transportation and/or storage, imposing social burdens on the food industry due to the waste created by contaminated products. This creates an urgent demand for mycotoxin removal methods to minimize economic loss and hazards to consumers.
- mycotoxins
- biodegradation
- probiotics, recombinant enzyme, microbial consortia
1. Introduction
2. Chemical Structure and Toxic Effects of Mycotoxins
| Mycotoxins | Chemical Structure | Main Toxic Groups | Main Degradation Products | Organ/System Affected | Main Clinical Signs | Producing Fungi |
|---|---|---|---|---|---|---|
| Aflatoxins (B1, B2, G1, G2) |
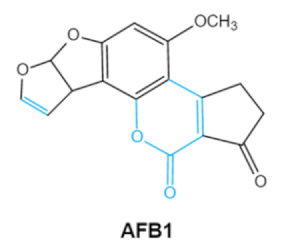 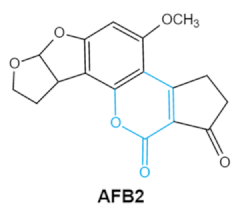 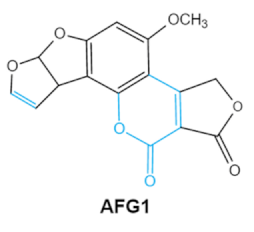 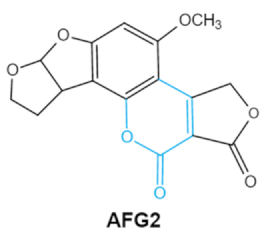 |
Lactone ring Double bond in difuran ring moiety |
AFB1-8,9dihydrodiol, AFB1-8,9-epoxide, dihydrohydroxyaflatoxin B1, | Liver, kidney, immune system | Hepatitis, carcinogenic, abdominal pain, vomiting, increased susceptibility to disease, immunosuppressive and carcinogenic effects |
Aspergillus. Flavus A. Parasiticus A. nomius |
| Zearalenones (ZEA) | 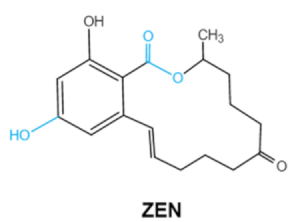 |
Lactone ring C-4 hydroxyl group |
α-/β-zearalenol, α-/β-zearalanol,zearalenone-4-sulfate, 1-(3,5-dihydroxyphenyl)-6′-hydroxy-l’-undecen-l0′-one, (5S)-5-({2,4-dihydroxy-6-[(1E)-5-hydroxypent-1-en-1-yl]benzoyl}oxy)hexanoic acid |
Reproductive tract, mainly female | Hyperestrogenism, Reproductive disorders | Fusarium graminearum(F. roseum) F. culmorum F. equiseti F. cerealis F. verticillioides F. incarnatum |
| Ochratoxins (A,B,C) (OTs) | 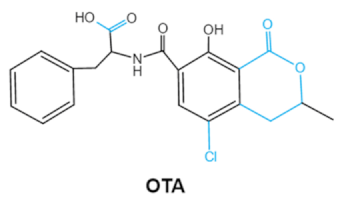 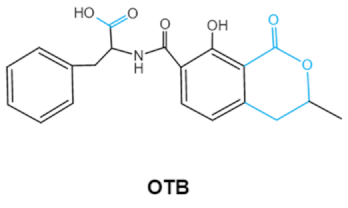 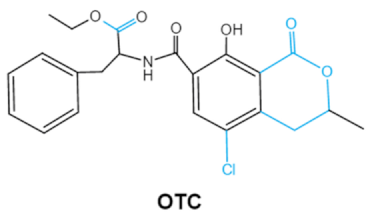 |
Isocoumarin moiety Carboxyl group of the phenylalanine moietyCl group |
L-βphenylalanine, OTα | Liver, kidney, immune system, inhibit RNA, DNA and protein synthesis in kidney | Nephritis, enlargement of kidney and hepatitis | A. ochraceus A. carbonarius A. niger P. verrucosum P. nordicum |
| Fumonisins FBs (B1, B2) | 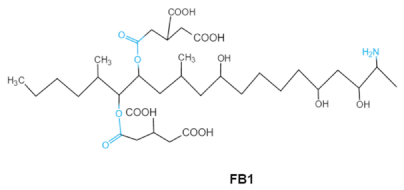 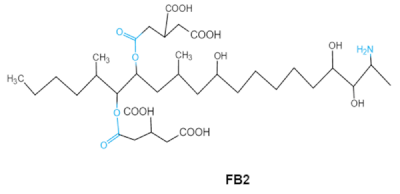 |
Two tricarballylic acid side chains Free amino group |
2-oxo-12,16-dimethyl-3,5,10,14,15-icosanepentol hemiketal, NacetylAP1, |
Lungs and heart (pig), central nervous system (horse), liver, immune system | Porcine pulmonary edema (PPE), equine leukoencephalomalacia | Fusarium section Liseola |
| Trichothecenes (DON, T-2, HT-2) TCNs | 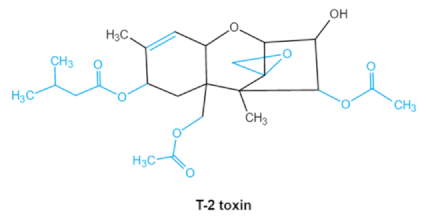 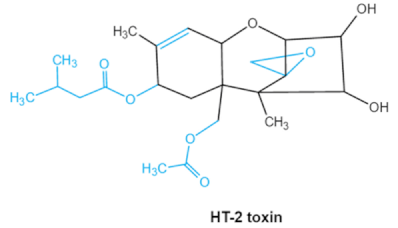 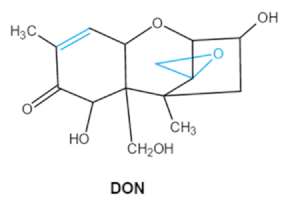 |
Epoxide group Acylated side groups C9-10 double bond |
HT-2 toxin, T-2 triol, T-2 tetraol, de-epoxy T-2 tetraol, 3α,7α,15α-triacetoxy-deoxynivalenol, de-epoxy deoxynivalenol, 3-acetyldeoxynivalenol, diaacetoxydeoxynivalenol. Epoxymonoacetoxyscirpenol, de-epoxyscirpentrio |
Central nervous system, gastrointestinal tract, liver, immune system | Anorexia, vomiting, abdominal pains, cardiovascular dysfunction | F. acuminatum F. sporotrichioides F. langsethiae Fusariumgraminearum, F. culmorum F. cerealis F. culmorum F. graminearum F. sporotrichioides F. poae |
| Patulin PAT |
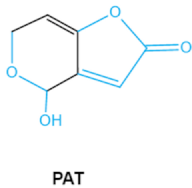 |
Furan, pyran or lactone ring Hemiacetal |
Ascladiol, hydroascladiol, desoxypatulinic acid, 3-keto-5-hydroxypentanal, glyoxylic acid |
Gut epithelium, liver, kidney, immune system | Oral and epithelial lesion, loss of appetite Nausea, vomitin, gastric ulcers |
Penicillim expansum Bysochlamis nívea Aspergillus clavatus P. griseofulvum |
2.1. Aflatoxins (AFs)
Aflatoxin (AFs) is the most widely studied mycotoxin encountered in agricultural commodities. They are mainly produced by2.1. Aflatoxins (AFs)
2.2. Trichothecenes
As one of the major classes of mycotoxins, trichothecenes (TCNs) cause a significant economic impact on the food chain each year. TCNs are formed in nature by some filamentous fungi such as2.2. Trichothecenes
2.3. Ochratoxins
Ochratoxins (OTs) belong to a family of mycotoxins commonly found in various food and feed mainly produced by2.3. Ochratoxins
2.4. Zearalenone
Zearalenone (ZEA) is a kind of nonsteroidal mycotoxin that contaminates a variety of cereals [42]. ZEA is a secondary metabolite that is synthesized by several species of mold fungi belonging to the2.4. Zearalenone
2.5. Fumonisins
Fumonisins (FBs), polyketide-derived mycotoxins, are mainly produced by2.5. Fumonisins
2.6. Patulin
Patulin (PAT) is a tetraketide mycotoxin most commonly found in apples and apple-derived products [51]. It is produced by a variety of filamentous fungi including2.6. Patulin
3. Mycotoxin Removal by Probiotics
3.1. Fast Glance to Probiotic Properties
Probiotics, defined as ‘‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’’ by the World Health Organization (WHO), have been incorporated into various kinds of products such as foods, drugs, and dietary supplements [55]. Beyond their basic nutritional value, probiotics provide numerous benefits such as the alleviation of lactose intolerance, maintaining balance of the digestive tract and modulation of inflammatory responses [56]. The most extensively studied and widely used probiotics include3.1. Fast Glance to Probiotic Properties
3.2. Mycotoxins Detoxification by Probiotics and the Related Mechanism
The biological detoxification of mycotoxins by probiotic bacteria was reviewed years ago [28][55][59]. Probiotics, particularly lactic acid bacteria (LAB) and yeasts such as3.2. Mycotoxins Detoxification by Probiotics and the Related Mechanism
3.2.1. Cell-Binding of Mycotoxins
In the last three decades, numerous studies have been developed regarding toxin binding by the cell-surface of probiotics such as3.2.1. Cell-Binding of Mycotoxins
3.2.2. Biodegradation
In contrast to cell-binding methods, research on the biodegradation of mycotoxins by probiotics is very limited. The available literatures about mycotoxins degradation by probiotics over the last decade was summarized in3.2.2. Biodegradation
| Mycotoxins | Microorganism | Reduction Rate (%) | Toxin Level | Degradation Condition | Reference | |
|---|---|---|---|---|---|---|
| AFB1 | Streptomyces cacaoi subsp. Asoensis K234 | 88.34 ± 15.62 | 1 μg mL | −1 | 5 days, 28 °C,170 rpm liquid LB medium |
Harkai et al. (2016) |
| Streptomyces luteogriseus K144 | 79.93 | 1 μg mL | −1 | 5 days, 28 °C,170 rpm liquid LB medium |
||
| Bacillus licheniformis CFR1 | 94.73 ± 1.09 | 500 ppb | liquid nutrient broth (NB) at 37 °C, 72 h | Rao et al. (2017) | ||
| T-2 | P. pentosaceus KTU05-10 | 78.0 | 12.8–19.5 μg kg | −1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Saccharomyces pastorianus A15 | 31.0 | 5000 µg L | −1 | 15 °C, 120 rpm, four days 11.5° Plato wort |
Nathanail et al. (2016) | |
| HT-2 | P. pentosaceus KTU05-10 | 79.0 | 258–819 μg L | −1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| ZEA | P. acidilactici | 38.0 | 19.5–873.7 μg L | −1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Streptomyces rimosus (K145, K189) | 100.0 | 1 μg mL | −1 | 5 days, 28 °C,170 rpm liquid LB medium |
Harkai et al. (2016) | |
| DON | P. pentosaceus KTU05-10 | 47.0 | 3370–6930 μg kg | −1 | malting wheat grains with bacterial suspension at 18 °C for 30 min |
Juodeikiene et al. (2018) |
| Saccharomyces pastorianus A15 | 15.0 | 400 µg L | −1 | 11.5° Plato wort, 15 °C, 120 rpm for 4 days | Nathanail et al. (2016) |
