Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Baojun Xu | -- | 1268 | 2022-04-15 03:47:14 | | | |
| 2 | Catherine Yang | -1 word(s) | 1267 | 2022-04-15 04:07:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, B.; Farooqi, A.A.; , .; Attar, R.; Taverna, S. Berbamine. Encyclopedia. Available online: https://encyclopedia.pub/entry/21805 (accessed on 07 February 2026).
Xu B, Farooqi AA, , Attar R, Taverna S. Berbamine. Encyclopedia. Available at: https://encyclopedia.pub/entry/21805. Accessed February 07, 2026.
Xu, Baojun, Ammad Ahmad Farooqi, , Rukset Attar, Simona Taverna. "Berbamine" Encyclopedia, https://encyclopedia.pub/entry/21805 (accessed February 07, 2026).
Xu, B., Farooqi, A.A., , ., Attar, R., & Taverna, S. (2022, April 15). Berbamine. In Encyclopedia. https://encyclopedia.pub/entry/21805
Xu, Baojun, et al. "Berbamine." Encyclopedia. Web. 15 April, 2022.
Copy Citation
Berbamine is a natural, potent, pharmacologically active biomolecule isolated from Berberis amurensis. Berbamine has been shown to modulate different oncogenic cell-signaling pathways in different cancers.
cancers
berbamine
1. Targeting of Ca2+/Calmodulin-Dependent Protein Kinase II (CAMKII) by Berbamine in Different Cancers
Targeting c-Myc with small-molecule inhibitors remains challenging. c-Myc protein stability can be controlled by phosphorylation at two different sites with opposing functions. Phosphorylation at 62nd serine residue stabilized c-Myc, whereas phosphorylation at 58th threonine residue promoted the degradation of c-Myc. Ca2+/calmodulin-dependent protein kinase II (CAMKII), a multifunctional serine/threonine kinase, has been shown to stabilize the oncogenic c-Myc level [1]. Expectedly, levels of phosphorylated-c-Myc (62nd serine residue) and total c-Myc were noted to be reduced in CAMKIIγ knockdown cells, whereas levels of phosphorylated-c-Myc (62nd serine residue) and total c-Myc were found to be significantly enhanced in CAMKIIγ overexpressing T cell lymphoma cells [1].
An orally administered, bioactive small molecule analog of berbamine, tosyl chloride-berbamine (TCB), considerably reduced phosphorylated levels of CaMKIIγ [2]. TCB induced a regression of leukemia growth in an orthotopic B-ALL model using NSG (NOD/SCID/IL2Rγ-/-) mice injected with CaMKIIγ/Myc-expressing leukemia cells [2].
Berbamine had the ability to bind to the ATP-binding pocket of CaMKIIγ, inhibiting its phosphorylation and inducing apoptosis in leukemia stem cells [3]. 4-Chlorobenzoyl berbamine (CBBM), a Berbamine derivative, effectively enhanced the proteasome-dependent degradation of c-Myc in OCI-Ly3 cells [4].
Berbamine has been reported to block VEGF- and BDNF-regulated angiogenesis, mainly through the inactivation of VEGFR2- and TrkB-mediated transduction cascades [5]. Berbamine considerably reduced VEGF-dependent phosphorylation of VEGFR2, as well as that of TrkB by BDNF in HUVECs, resulting in the deactivation of downstream effectors, such as PKB/AKT, NF-κB and ERK1/2. Berbamine efficiently reduced BDNF and VEGF-mediated CaMKIIγ phosphorylation. Berbamine significantly suppressed tumor growth in chorioallantoic membrane tumor models implanted with U87MG cells [5].
Berbamine and one of its derivatives, BBD24, strongly inhibited CAMKII phosphorylation in Huh7 cells [6]. Overall, these studies helped us develop a sharper understanding of the instrumental role of the CAMKII/c-Myc-signaling axis in carcinogenesis.
2. Regulation of Autophagy by Berbamine
Berbamine is a natural molecule from traditional Chinese medicine that is useful for the treatment of patients with inflammation and cancers such as leukaemia, lung, liver and breast cancer. Berbamine is administered to patients with leukopenia caused by conventional chemotherapy and/or radiotherapy. Several reports indicate that berbamine has a role in the modulation of deregulated pathways in cancers. Berbamine causes caspase-3-dependent apoptosis in leukaemia cells through surviving pathway activation [7]. Moreover, berbamine inhibits the cell growth and motility of highly metastatic breast cancer and lung cancer cells [8][9].
Recently, berbamine has been considered a novel autophagy inhibitor in breast and colon cancer cells. Autophagy is an essential catabolic process involved in many pathological conditions, including cancer, that can protect cells and organisms from stressors. The role of autophagy in cancer remains uncertain and has been reported as a pro- and anti-tumorigenic system [10]. In pre-malignant lesions, autophagy activation might prevent cancer development [11]. Conversely, in advanced cancers, autophagy induction can stimulate carcinogenesis, such as in melanoma, colorectal, pancreas and renal cancers, or suppress it, as in breast cancer [12][13][14][15][16][17]. In 1988, Ohsumi described, for the first time, autophagy mechanisms as a lysosomal degradation and cellular recycling pathway, evolutionarily conserved, that allows protein aggregates and damaged organelles to be eliminated through lysosomal degradation, thus maintaining cellular homeostasis [18]. Autophagy can be divided into three groups: macroautophagy, microautophagy and chaperon-mediated autophagy. Macroautophagy (refer to macroautophagy as autophagy) is the best known of the three pathways. Autophagy mediates the sequestration and delivery of cytoplasmic material to lysosome for degradation. This process induces the phagophore formation caused by the extension of an isolated membrane, which fuses to convey cytoplasmic components into an autophagic double membrane vacuole, the autophagosomes; the organelles fused with lysosomes become autolysosomes, which degrade the materials contained within it. The autophagy process is modulated at transcriptional and post-translational levels, and the genes involved in autophagy are regulated by ATF4 at the transcriptional level. Cellular stresses induce MIT/TFE transcription factors and other inhibitors of mTOR, a negative regulator of MIT/TFE. In the autophagy process, the fusion of membranes is usually realized by soluble SNARE complexes (N-ethylmaleimide-sensitive factor attachment protein receptor). Recently, it has been described that the SNARE syntaxin 17 (STX17) contained in autophagosomes interacts with the cytoplasmatic SNARE SNAP29 and SNARE VAMP8 of the lysosomes, and these proteins cooperate in autophagosome-lysosome fusion.
Fu et al. reported that berbamine causes the upregulation of BNIP3, inhibiting SNARE-mediated autophagy-lysosome fusion in breast cancer cells. Under hypoxia, BNIP3, a protein with homology to BCL2 in the BH3 domain, drives mitophagy in many different cell types. BNIP3 may play a role in autophagosome-lysosome fusion regulation. Berbamine induces the upregulation of BNIP3, which binds SNAP29 and inhibits the interaction between SNAP29 and VAMP8, which, in turn, causes a blockade of autophagosome-lysosome fusion and autophagosome increase (Figure 1) [19]. This study suggests that berbamine could be considered a potential autophagy inhibitor, which could be used in combination with chemotherapy in cancer management. Autophagic cell death can also be induced by RAS/RAF/MEK/ERK pathway activation [20]. Mou et al. reported that berbamine can exert anticancer effects on human colon cancer cells, inducing autophagy and apoptosis and inhibiting cell migration via the MEK/ERK pathway. Berbamine induces autophagic vesicles in colon cancer cells and an increase in Beclin-1, LC3B-II, ATG-5 and 12 expression [21].
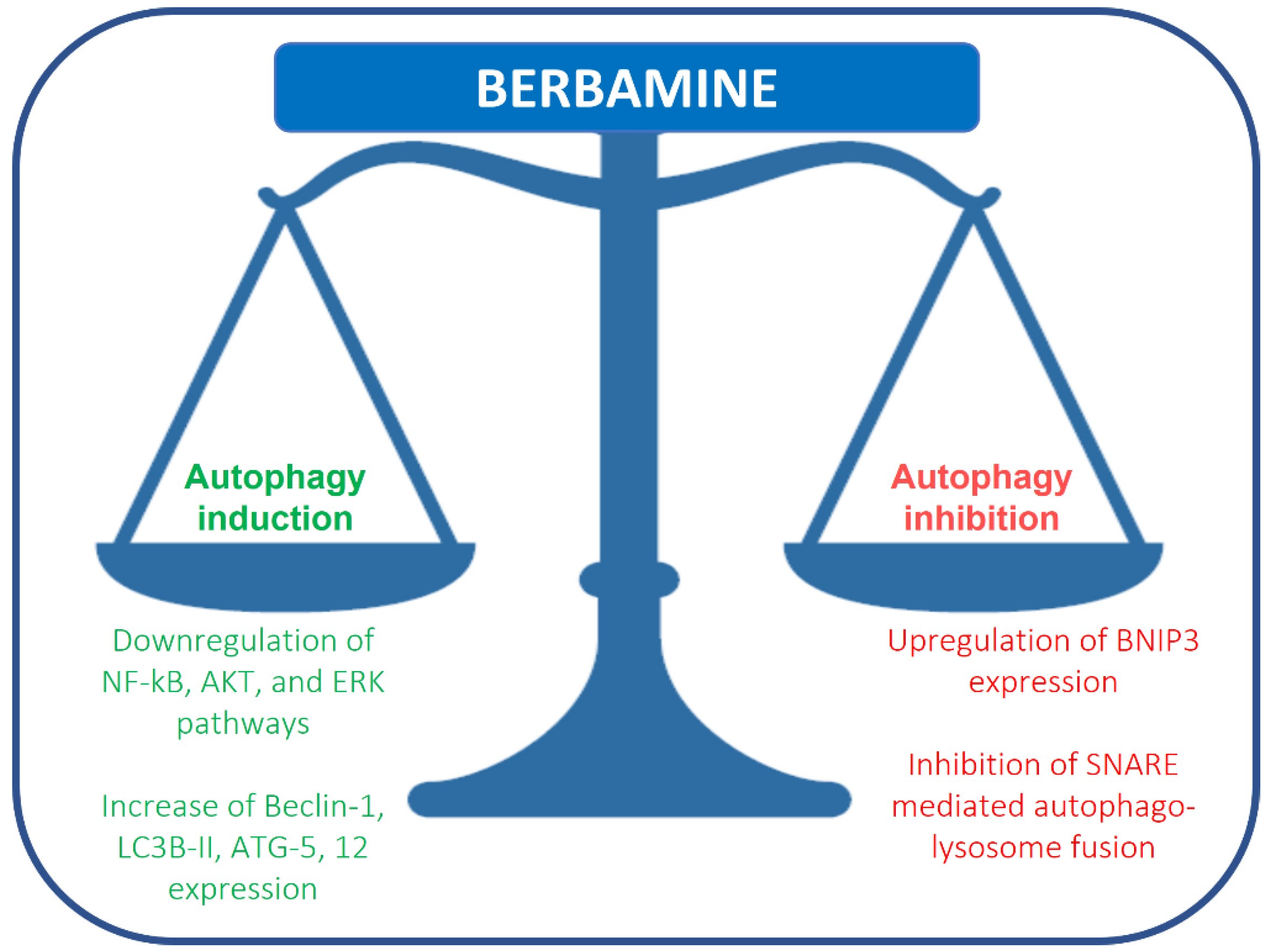
Figure 1. Regulation of autophagy by berbamine.
Preliminary studies on berbamine indicate its role in cancer treatment. It is efficient because other natural compounds could be limited by reduced water solubility, low absorption rate in the bowels and rapid metabolism. The role of berbamine in autophagy is still unclear. Further studies are needed to elucidate the effects of this natural compound on autophagy regulation.
3. Targeting of Protein Networks by Berbamine for Cancer Chemoprevention
Berbamine efficiently suppressed the migratory and invasive capacity of highly metastatic breast MDA-MB-231 cancer cells via the inhibition of pro-MMP2/MMP9 activation. It also reduced the phosphorylated levels of AKT and c-Met in MDA-MB-231 cancer cells [9].
Aspirin dose-dependently induced the phosphorylation of CREB and ATF1 [22]. Importantly, the treatment of HepG2 cells with AICAR (an AMPK agonist) also resulted in the phosphorylation of CREB and ATF1. However, knockdown of AMPKα1 abolished the phosphorylation of CREB/ATF1 by aspirin in Hep3B and HepG2 cells. PKA (Protein kinase A) is present downstream to AMPK and mediates the phosphorylation of CREB/ATF1 by aspirin. Accordingly, PKA inhibitors impaired the phosphorylation of CREB/ATF1 by aspirin in HepG2 and Hep3B cells. Soluble adenylyl cyclase (sAC) has an important role in cAMP synthesis. Aspirin did not induce phosphorylation of CREB/ATF1 in sAC knockdown cells. Aspirin caused marked reduction in the levels of CPS1 (carbamoyl phosphate synthetase I) in HepG2 and Hep3B cells. Similarly, AICAR (an AMPK agonist) inhibited CPS1 expression in HepG2 and Hep3B cells. AMPK knockdown abrogated the downregulation of CPS1 and upregulation of sAC by aspirin. CREB/ATF1 knockdown sensitized Hep3B and HepG2 cells to aspirin. CREB/ATF1 activation antagonized the anti-cancer effects of aspirin, and pharmacological targeting of CREB/ATF1 significantly enhanced the efficacy of aspirin against HCC cancer cells. Berbamine inhibited CREB/ATF1 phosphorylation and sensitized HCC cells to aspirin. Protein phosphatase-2A (PP2A) induced dephosphorylation of its substrates. However, CIP2A (a cellular inhibitor Of PP2A) negatively regulated PP2A. Berbamine reduced CIP2A levels in HCC cells and promoted PP2A-mediated abrogation of aspirin-induced phosphorylation of CREB/ATF1 [22].
Berbamine concentration-dependently inhibited the migratory and invasive potential of SMMC-7721 cells and increased expression of Cx32 in SMMC-7721 cells. However, after silencing Cx32, berbamine failed to inhibit cell invasion and metastasis [23].
References
- Gu, Y.; Zhang, J.; Ma, X.; Kim, B.-W.; Wang, H.; Li, J.; Pan, Y.; Xu, Y.; Ding, L.; Yang, L.; et al. Stabilization of the c-Myc Protein by CAMKIIγ Promotes T Cell Lymphoma. Cancer Cell 2017, 32, 115–128.e7.
- Yu, Q.; Wang, P.; Yang, L.; Wu, Z.; Li, S.; Xu, Y.; Wu, B.; Ma, A.; Gan, X.; Xu, R. Novel synthetic tosyl chloride-berbamine regresses lethal MYC-positive leukemia by targeting CaMKIIγ/Myc axis. Biomed. Pharmacother. 2019, 117, 109134.
- Gu, Y.; Chen, T.; Meng, Z.; Gan, Y.; Xu, X.; Lou, G.; Li, H.; Gan, X.; Zhou, H.; Tang, J.; et al. CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood 2012, 120, 4829–4839.
- Zhang, L.; Tong, J.; He, X.; Liang, Y.; Zhu, L.; Xu, R.; Zhao, X. Novel synthetic 4-chlorobenzoyl berbamine inhibits c-Myc expression and induces apoptosis of diffuse large B cell lymphoma cells. Ann. Hematol. 2018, 97, 2353–2362.
- Kim, Y.J.; Han, J.M.; Jung, H.J. Antiangiogenic and antitumor potential of berbamine, a natural CaMKIIγ inhibitor, against glioblastoma. Biochem. Biophys. Res. Commun. 2021, 566, 129–134.
- Meng, Z.; Li, T.; Ma, X.; Wang, X.; Van Ness, C.; Gan, Y.; Zhou, H.; Tang, J.; Lou, G.; Wang, Y.; et al. Berbamine Inhibits the Growth of Liver Cancer Cells and Cancer-Initiating Cells by Targeting Ca2+/Calmodulin-Dependent Protein Kinase II. Mol. Cancer Ther. 2013, 12, 2067–2077.
- Zhao, X.; He, Z.; Wu, D.; Xu, R. Berbamine selectively induces apoptosis of human acute promyelocytic leukemia cells via sur-vivin-mediated pathway. Chin. Med. J. 2007, 120, 802–806.
- Duan, H.; Luan, J.; Liu, Q.; Yagasaki, K.; Zhang, G. Suppression of human lung cancer cell growth and migration by berbamine. Cytotechnology 2009, 62, 341–348.
- Wang, S.; Liu, Q.; Zhang, Y.; Liu, K.; Yu, P.; Liu, K.; Luan, J.; Duan, H.; Lu, Z.; Wang, F.; et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol. Cancer 2009, 8, 81.
- White, E.; DiPaola, R.S. The Double-Edged Sword of Autophagy Modulation in Cancer. Clin. Cancer Res. 2009, 15, 5308–5316.
- Baehrecke, E.H.; Gewirtz, D.A.; Amaravadi, R.K.; Piacentini, M.; Levine, B.; Ryan, K.M.; Penninger, J.; Thorburn, A.M.; Martin, S.J.; Rubinsztein, D.C.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880.
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016, 30, 1913–1930.
- Gozuacik, D.; Akkoc, Y.; Öztürk, D.G.; Kocak, M. Autophagy-Regulating microRNAs and Cancer. Front. Oncol. 2017, 7, 65.
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741.
- Mathiassen, S.G.; De Zio, D.; Cecconi, F. Autophagy and the Cell Cycle: A Complex Landscape. Front. Oncol. 2017, 7, 51.
- Perera, R.M.; Stoykova, S.; Nicolay, B.N.; Ross, K.N.; Fitamant, J.; Boukhali, M.; Lengrand, J.; Deshpande, V.; Selig, M.K.; Ferrone, C.R.; et al. Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature 2015, 524, 361–365.
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The Hairpin-type Tail-Anchored SNARE Syntaxin 17 Targets to Autophagosomes for Fusion with Endosomes/Lysosomes. Cell 2012, 151, 1256–1269.
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2013, 24, 9–23.
- Fu, R.; Deng, Q.; Zhang, H.; Hu, X.; Li, Y.; Liu, Y.; Hu, J.; Luo, J.; Zhang, Y.; Jiang, X.; et al. A novel autophagy inhibitor berbamine blocks SNARE-mediated autopha-gosome-lysosome fusion through upregulation of BNIP3. Cell Death Dis. 2018, 9, 243.
- Sooro, M.A.; Zhang, N.; Zhang, P. Targeting EGFR-mediated autophagy as a potential strategy for cancer therapy. Int. J. Cancer 2018, 143, 2116–2125.
- Mou, L.; Liang, B.; Liu, G.; Jiang, J.; Liu, J.; Zhou, B.; Huang, J.; Zang, N.; Liao, Y.; Ye, L.; et al. Berbamine exerts anticancer effects on human colon cancer cells via induction of autophagy and apoptosis, inhibition of cell migration and MEK/ERK signalling pathway. J. BUON. 2019, 24, 1870–1875.
- Zhang, H.; Yang, S.; Wang, J.; Jiang, Y. Blockade of AMPK-Mediated cAMP–PKA–CREB/ATF1 Signaling Synergizes with Aspirin to Inhibit Hepatocellular Carcinoma. Cancers 2021, 13, 1738.
- Yu, B.-B.; Liu, L.-L.; Yan, J.-D.; Cao, J.-B.; Cao, Y. Effect of berbamine on invasion and metastasis of human liver cancer SMMC-7721 cells and its possible mechanism. Anti-Cancer Drugs 2021, 33, e178–e185.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
15 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




