
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Corrado I. Angelini | + 1765 word(s) | 1765 | 2020-09-25 05:24:56 | | | |
| 2 | Catherine Yang | -15 word(s) | 1750 | 2020-10-09 11:17:23 | | |
Video Upload Options
Becker muscular dystrophy is a mild X-linked form of dystrophinopathy, with frequent cardiomyopathy.
1. Introduction
Becker muscular dystrophy (BMD) is an X-linked recessive disorder and has an incidence of 1 in 18,518 male births [1] and a prevalence of 0.01 in South Africa, 0.1 to 0.2 in Asia, 0.1 to 0.7 (per 10,000 males) in European countries [2]. It is caused by mutations of the DMD gene that encodes the muscle-specific dystrophin protein. The pivotal function of dystrophin is to link the cytoskeletal actin and dystrophin-associated protein complex to the extracellular matrix to stabilize the sarcolemma [3]. Some mutations of dystrophin compromise its binding at the sarcolemma and cause a specific sequence of muscle abnormalities such as muscle necrosis, altered mitochondrial metabolism, disruption of the sarcolemma, abnormality of calcium homeostasis, and reactive macrophagic inflammation [4]. The BMD typical clinical features consist of proximal muscle wasting and weakness, high creatine kinase (CK), cramps, myalgia, myoglobinuria, mild myopathy, and cardiomyopathy with fibrosis [5]. In young adults, the proximal muscle wasting and weakness are more evident at the thigh and pelvic girdle muscles and pronounced calf hypertrophy might be present [6]]. At the mild end of the BMD spectrum are patients characterized by muscle hypertrophy of the calves, cramps, and elevated CK levels, but virtually no muscle wasting or weakness [7][8]]. Becker muscular dystrophy differential diagnosis is important to distinguish it from other myopathies with muscle weakness; BMD patients remain ambulatory after the age of 16 years while Duchenne muscular dystrophy (DMD) patients lose ambulation and become wheelchair-bound by age of 12 [7][9]. BMD has a later onset and is clinically less severe compared to DMD. At the molecular level. DMD gene mutations disrupt the translational reading frame, which results in complete loss of dystrophin. In BMD, the mutation maintain the reading frame and result in truncated dystrophin protein with abnormal function [7][10]. In some instances, BMD could be confused with polymyositis, an idiopathic inflammatory myopathy characterized by bilateral proximal muscle weakness. BMD can be distinguished for the presence of calf pseudohypertrophy [7][11] and frequent cardiac involvement [12]].
Another muscle dystrophy group difficult to differentiate from BMD is limb-girdle muscular dystrophy, the hallmark of which is the absence of overt calf muscle pseudohypertrophy [13][14].
No definitive cure for BMD has been identified; however, steroid therapy appears useful to improve muscle function, to slow the decline of muscle strength, and to prolong in dystrophinopathy walking ability [5][15]]. In recent years, a potential strategy to detect and follow dystrophic symptoms could be the use of noninvasive biomarkers to monitor the disease.
MicroRNAs (miRNAs) are a class of small non-coding RNAs molecules with 22 nucleotides in length that regulate gene expression at the post-transcriptional level by destabilizing the mRNA and translation silencing [16]. MiRNAs are involved in several pathological and physiological processes such as development, proliferation, differentiation, and cell death. MiRNAs are expressed in muscle and stable in biofluids, such as plasma, serum, and urine [17]. Altered levels of circulating miRNAs have been linked to several neuromuscular diseases [18].
A specific group of circulating miRNAs is muscle tissue specific and they are called “myo-miRNAs” (miR-1, miR-206 and miR-133). MiR-1 and miR-133 are expressed in cardiac and skeletal muscle and are involved in the proliferation and differentiation processes [19]; miR-206 is a skeletal muscle-specific miRNA expressed in satellite cells and involved in muscle development and regeneration [20]. Several studies demonstrated that the serum levels of myo-miRs are increased in DMD and BMD patients [21]. Another common feature of muscular dystrophy is the inflammatory response that triggers a cascade of inflammatory cytokines and subsequently miRNAs induction. The activation of the inflammatory system is due to the muscle injury and the subsequent macrophagic reaction, which occurs to repair the muscle damage and to promote the regeneration process. Three inflammatory miRNAs (miR-146b, miR-221 and miR-155) were found to be dysregulated in several muscular dystrophies [22].
Myostatin (Mstn), also named “GDF-8,” is a member of the transforming growth factor β (TGFβ) superfamily and acts as a negative regulator of skeletal muscle growth [23]. It is known that the myostatin antagonist is follistatin (FST), a glycosylated secreted protein that controls muscle mass through different pathways [24].
2. Results
The clinical data of each patient are summarized in Table 1.
Table 1. Clinical features and dystrophin gene mutation in Becker muscular dystrophy (BMD) patients.
| Age at Study (Years) | Age at Onset (Years) | Deletion in the Dystrophin Gene | Dystrophin Quantity (%) and M.W. (kDa) * | Creatine Kinase Levels (U/L) | Muscle Involvement |
Left Ventricular Ejection Fraction ** | Treatment and Duration | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 42 | 5 | Exons 45–47 | 35%, 370 kDa |
1202 | HyperCKemia, waddling gait, calf hypertrophy, quadriceps weakness | 40–55% | Deflazacort ACE inhibitors 26 years |
| Patient 2 | 40 | 17 | Exons 45–49 | 60%, 380 kDa |
452 | HyperCKemia, pes cavus, difficulty rising from the floor, calf atrophy, weakness | 55% | Deflazacort 20 years |
| Patient 3 | 33 | 1 1/2 | Exons 48–51 | 90%, 370 kDa |
189 | Slight hyperCKemia, muscle cramps, quadriceps weakness | 65% | No |
| Patient 4 | 41 | 6 | Exons 31–44 | 35%, 320 kDa |
3086 | HyperCKemia, mild mitral valve insufficiency | 55% | No |
| Patient 5 | 17 | 6 | Exons 45–47 | 20%, 380 kDa |
1092 | HyperCKemia, calf hypertrophy | 64% | No |
| Patient 6 | 14 | 3 | Exons 45–51 | 50%, 370 kDa |
668 | HyperCKemia, mild winging scapulae | 65% | No |
| Patient 7 | 30 | 1 1/2 | Exons 48–49 | 50%, 380 kDa |
1305 | HyperCKemia, slight scoliosis, calf hypertrophy | 55% | No |
| Patient 8 | 45 | Childhood | Exons 47–49 | N.A. | 597–943 | HyperCKemia, slight scapular winging, calf hypertrophy | 55% | No |
2.1. Expression Levels of myo-miRNAs
We analyzed the expression levels of four “canonical” myo-miRNAs (miR-1, miR-133a, miR-133b and miR-206) in the serum of 8 BMD patients by real-time PCR, as shown in Figure 1.
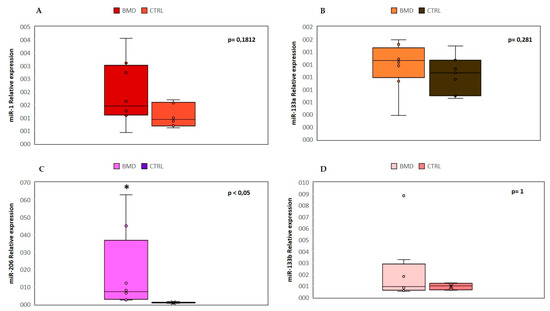
Figure 1. Comparison of the serum levels of myo-miRNAs in eight Becker muscular dystrophy (BMD) patients and six control subjects. Box plots show relative expression of miR-1 (A), miR-133a (B), miR-206 (C), and miR-133b (D) determined by quantitative real-time polymerase chain reaction (qRT-PCR). Single points show the individual myo-miRNAs levels, the box represents the quartiles (first and third) divided into two parts by the median (second quartile), and the maximum and minimum values are also indicated. The asterisk indicates significant p-values (p ≤ 0.05) between patients and controls using the Wilcoxon–Mann–Whitney test. BMD: Becker muscular dystrophy. CTRL: controls.
We found a significant (p < 0.05) upregulation of miR-206 in all patients, and in two cases (patient 1 and patient 8), there was an over 45-fold increase as compared to controls. No significant differences were observed in the mean value of miR-1, miR-133a, and miR-133b, but patient 8 showed a 9-fold higher value of miR-133b than control mean. The high variability detected in patient 8 could be attributed to a strenuous and eccentric muscle exercise done, few days before serum sample collection.
2.2. Expression Levels of Inflammatory MiRNAs
We then evaluated the expression levels of three selected inflammatory miRNAs (miR-146b, miR-155, and miR-221): miR-146b and miR-221 were found to be significantly overexpressed (p ≤ 0.05) in BMD patients in comparison to the control group, whereas miR-155 was not significantly different between patients and controls (Figure 2). In this group of inflammatory miRNAs, patient 8 showed over 10-fold increased levels as compared to control mean, in line with damage during the previous eccentric exercise and subsequent inflammatory macrophage reaction.
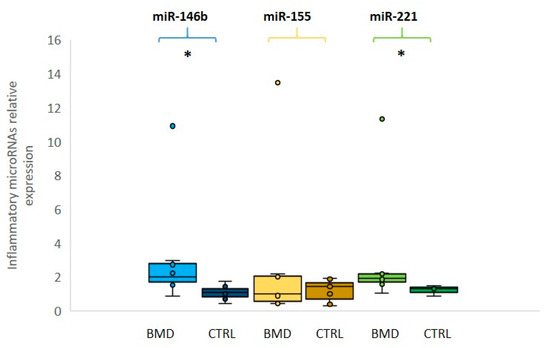
Figure 2. Relative expression of inflammatory microRNAs in BMD patients. Box plots showing the levels of miR-146b (light blue for patients and dark blue for controls), miR-155 (yellow for patients and dark yellow for controls) and miR-221 (green for patients and dark green for controls) (by qRT-PCR analyses in the serum of eight BMD patients and in six healthy controls). Data were significant, p-values (p ≤ 0.05), for miR-221 and miR-146b between patients and controls. Single points show the individual inflammatory miRNAs levels, the box represents the quartiles (first and third) divided into two parts by the median (second quartile), and the maximum and minimum values are also indicated BMD: Becker muscular dystrophy; CTRL: controls; * indicates significance (p ≤ 0.05).
2.3. Quantitative Expression of Myostatin/Follistatin
The analysis of the myostatin (GDF-8) and follistatin (FST) concentrations was an important aim of our study. The results showed that GDF8 and FST levels were not significantly different in BMD patients as compared to the control group. However, when we compared myostatin levels between the treated and the untreated patients’ groups, the steroid-treated BMD group showed lower values of GDF8 than untreated patients (Figure 3).
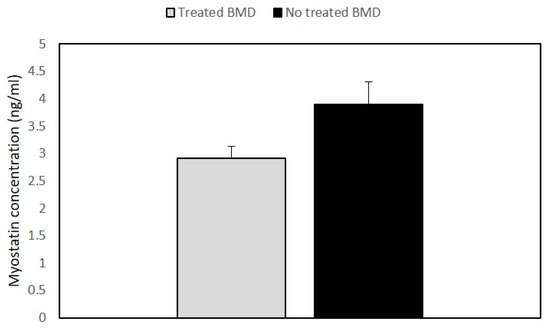
Figure 3. Histogram showing the concentration of myostatin in BMD patients. Quantitative myostatin expression was compared between treated (n = 2) and untreated BMD (n = 6) patients using the ELISA test. Data are expressed as mean + standard deviation.
2.4. Muscle MRI Changes
Thigh muscles showed significant fatty infiltration of muscles in the anterior and posterior compartment in patient 1 and patient 2 who were treated with steroids. Both quadriceps and posterior hamstrings muscles were equally affected; only gracilis and sartorius muscles were spared in these two patients (Figure 4).
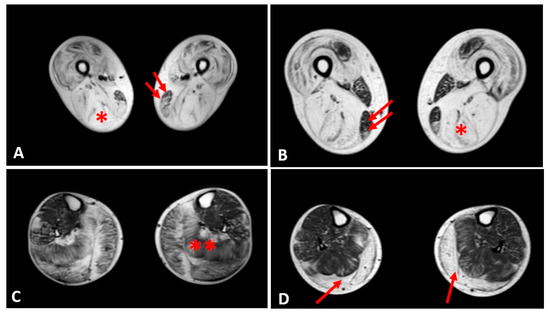
Figure 4. Evaluation of fibrofatty infiltration in lower limbs in two steroid-treated BMD patients. T1-sequences of thigh muscles shows marked atrophy of quadriceps and hamstrings (single asterisk) in both patients sparing of gracilis (double arrows) and sartorius muscles (A,B). In leg muscles, gastrocnemius and soleus muscles (double asterisk) presented advanced fatty and connective tissue infiltration in patient 1 (C), whereas marked atrophy and moderate fatty infiltration were observed in gastrocnemius muscles (single arrow) of patient 2 (D).
In the other patients, the thigh muscles were normal or slightly compromised, except for patients 7 and 8, in whom the fatty tissue infiltration in hamstring muscles reached Mercuri score 2a.
The posterior leg compartment was mostly affected in the two patients treated by steroids, showing severe fatty infiltration in gastrocnemius muscle. In the anterior leg, tibialis anterior was slightly involved; these 2 patients showed a Mercuri score 2a (Figure 4).
The other patients did not present a specific pattern of leg muscles involvement.
3. Conclusion
Concerning follistatin, the pivotal function of this protein is the inhibition of the myostatin pathway in order to regulate the muscle mass in muscular dystrophies. Several myostatin inhibitory drugs have been evaluated in neuromuscular diseases, but so far, the clinical results are limited [25]. In a gene therapy trial based on the use of adeno-associated virus (AAV) vector with preliminary prednisone treatment, follistatin was injected in six BMD ambulatory patients, and there was in four patients increase in the distance walked in six-minute test (6MWT) and reduced endomysial fibrosis, muscle hypertrophy especially at high dose [26][27]. It is possible that such follistatin gene therapy combined with long-term deflazacort regime could be used in the future to treat the symptoms and maintain muscle strength in BMD patients. In future studies, sonography could be also utilized as alternative to muscle MRI, to evaluate quality of muscle mass since this technique is a cost-effective tool recently developed for evaluation of metabolic syndrome and adults dynapenia [28][29]. We propose that circulating miRNAs and myostatin could be used as a noninvasive prognostic biomarkers to monitor the progression and the treatment of the disease. We suggest as a clinical prospective and possible applicability of this study the combined use of circulating biomarkers with muscle MRI and sonography in order to improve diagnosis and disease management in both young dystrophinopathy and chronic adult myopathy patients.
References
- Mah, J.K.; Korngut, L.; Dykeman, J.; Day, L.; Pringsheim, T.; Jette, N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 2014, 24, 482–491.
- Romitti, P.A.; Zhu, Y.; Puzhankara, S.; James, K.A.; Nabukera, S.K.; Zamba, G.K.D.; Ciafaloni, E.; Cunniff, C.; Druschel, C.M.; Mathews, K.D.; et al. HHS public access. Pediatrics 2016, 135, 513–521.
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928.
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 2016, 96, 253–305.
- Angelini, C.; Marozzo, R.; Pegoraro, V. Current and emerging therapies in Becker muscular dystrophy (BMD). Acta Myol. 2019, 38, 172–179.
- Barp, A.; Bello, L.; Caumo, L.; Campadello, P.; Semplicini, C.; Lazzarotto, A.; Sorarù, G.; Calore, C.; Rampado, A.; Motta, R.; et al. Muscle MRI and functional outcome measures in Becker muscular dystrophy. Sci. Rep. 2017, 7, 16060.
- Thangarajh, M. The dystrophinopathies. Contin. Lifelong Learn. Neurol. 2019, 25, 1619–1639.
- Angelini, C.; Fanin, M.; Freda, M.; Martinello, F.; Miorin, M.; Melacini, P.; Siciliano, G.; Pegoraro, E.; Rosa, M.; Danieli, G. Prognostic factors in mild dystrophinopathies. J. Neurol. Sci. 1996, 142, 70–78.
- Babbs, A.; Chatzopoulou, M.; Edwards, B.; Squire, S.E.; Wilkinson, I.V.; Wynne, G.M.; Russell, A.J.; Davies, K. From diagnosis to therapy in Duchenne muscular dystrophy. Biochem. Soc. Trans. 2020, 48, 813–821.
- Monaco, A.P.; Bertelson, C.J.; Liechti-Gallati, S.; Moser, H.; Kunkel, L.M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988, 2, 90–95.
- Haque, A.; Cox, M.; Sandler, R.D.; Hughes, M. A systematic review of internet-based information on dermatomyositis and polymyositis. Int. J. Rheum. Dis. 2020, 1–6.
- Rochitte, C.E.; Liberato, G.; Silva, M.C. Comprehensive assessment of cardiac involvement in muscular dystrophies by cardiac MR imaging. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 521–531.
- Palmieri, A.; Manara, R.; Bello, L.; Mento, G.; Lazzarini, L.; Borsato, C.; Bortolussi, L.; Angelini, C.; Pegoraro, E. Cognitive profile and MRI findings in limb-girdle muscular dystrophy 2I. J. Neurol. 2011, 258, 1312–1320.
- Angelini, C.; Pegoraro, V.; Cenacchi, G. The clinical and molecular spectrum of autosomal dominant limb-girdle muscular dystrophies focusing on transportinopathy. Expert Opin. Orphan Drugs 2019, 7, 223–232.
- Griggs, R.C.; Miller, J.P.; Rockman-Greenberg, C.; Fehlings, D.L.; Pestronk, A.; Mendell, J.R.; Moxley, R.T.; King, W.; Kissel, J.T.; Cwik, V.; et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology 2016, 87, 2123–2131.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 1–12.
- Zen, K.; Zhang, C.-Y. Circulating MicroRNAs: A novel class of biomarkers to diagnose and monitor human cancers. Med. Res. Rev. 2010, 32, 326–348.
- Matsuzaka, Y.; Kishi, S.; Aoki, Y.; Komaki, H.; Oya, Y.; Takeda, S.-I.; Hashido, K. Three novel serum biomarkers, miR-1, miR-133a, and miR-206 for Limb-girdle muscular dystrophy, Facioscapulohumeral muscular dystrophy, and Becker muscular dystrophy. Environ. Health Prev. Med. 2014, 19, 452–458.
- Haematology, T.L. Proliferation and differentiation. Lancet Haematol. 2020, 7, e1.
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352.
- Cacchiarelli, D.; Legnini, I.; Martone, J.; Cazzella, V.; D’Amico, A.; Bertini, E.; Bozzoni, I. MiRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol. Med. 2011, 3, 258–265.
- Eisenberg, I.; Eran, A.; Nishino, I.; Moggio, M.; Lamperti, C.; Amato, A.A.; Lidov, H.G.; Kang, P.B.; North, K.N.; Mitrani-Rosenbaum, S.; et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc. Natl. Acad. Sci. USA 2007, 104, 17016–17021.
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90.
- Lee, S.-J. Quadrupling muscle mass in mice by targeting TGF-ß signaling pathways. PLoS ONE 2007, 2, e789.
- Mariot, V.; Joubert, R.; Hourde, C.; Féasson, L.; Hanna, M.; Muntoni, F.; Maisonobe, T.; Servais, L.; Bogni, C.; Le Panse, R.; et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 2017, 8, 1859.
- Al-Zaidy, S.A.; Sahenk, Z.; Rodino-Klapac, L.R.; Kaspar, B.; Mendell, J.R. Follistatin gene therapy improves ambulation in Becker muscular dystrophy. J. Neuromuscul. Dis. 2015, 2, 185–192.
- Mendell, J.R.; Sahenk, Z.; Malik, V.; Gomez, A.M.; Flanigan, K.M.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Meadows, E.; Lewis, S.; et al. A phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol. Ther. 2015, 23, 192–201.
- Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Jan, W.H.; Han, D. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: An ultrasound imaging study. Exp. Gerontol. 2018, 108, 54–61.
- Chang, K.-V.; Yang, K.-C.; Wu, W.-T.; Huang, K.-C.; Han, D. Association between metabolic syndrome and limb muscle quantity and quality in older adults: A pilot ultrasound study. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1821–1830.




