
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bhajan Lal | -- | 5935 | 2022-04-14 15:32:38 | | | |
| 2 | Beatrix Zheng | Meta information modification | 5935 | 2022-04-15 04:50:09 | | |
Video Upload Options
Innovating methods for treating industrial wastewater containing heavy metals frequently incorporate toxicity-reduction technologies to keep up with regulatory requirements. This research reviews the latest advances, benefits, opportunities and drawbacks of several heavy metal removal treatment systems for industrial wastewater in detail. The conventional physicochemical techniques used in heavy metal removal processes with their advantages and limitations are evaluated. A particular focus is given to innovative gas hydrate-based separation of heavy metals from industrial effluent with their comparison, advantages and limitations in the direction of commercialization as well as prospective remedies. Clathrate hydrate-based removal is a potential technology for the treatment of metal-contaminated wastewater. In this research, a complete assessment of the literature is addressed based on removal efficiency, enrichment factor and water recovery, utilizing the gas hydrate approach. It is shown that gas hydrate-based treatment technology may be the way of the future for water management purposes, as the industrial treated water may be utilized for process industries, watering, irrigation and be safe to drink.
1. Introduction
2. Overview of Gas Hydrate Technology
2.1. Gas Hydrate-Based Desalination
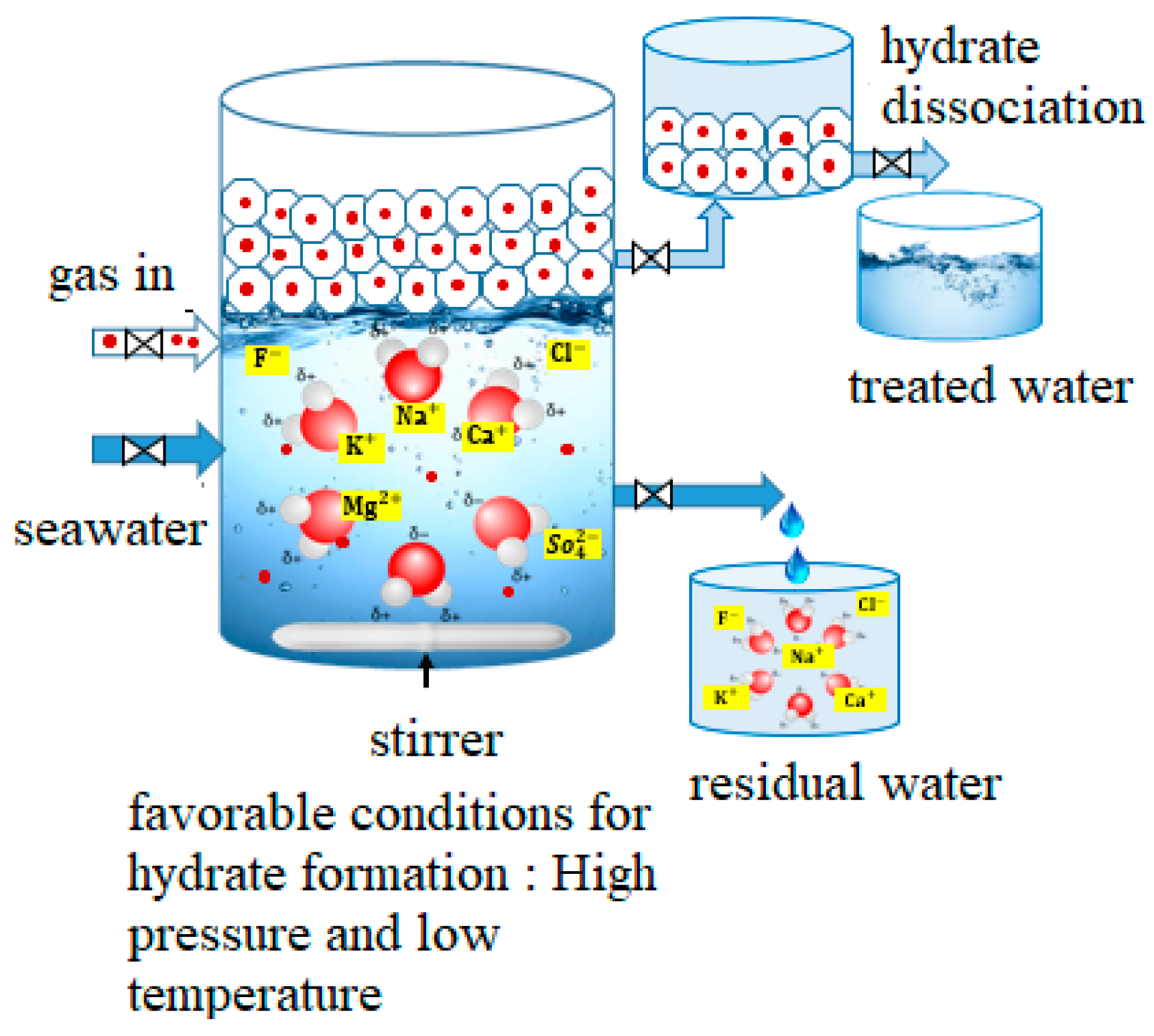
2.2. Gas Hydrate Desalination Reactor Design Innovations
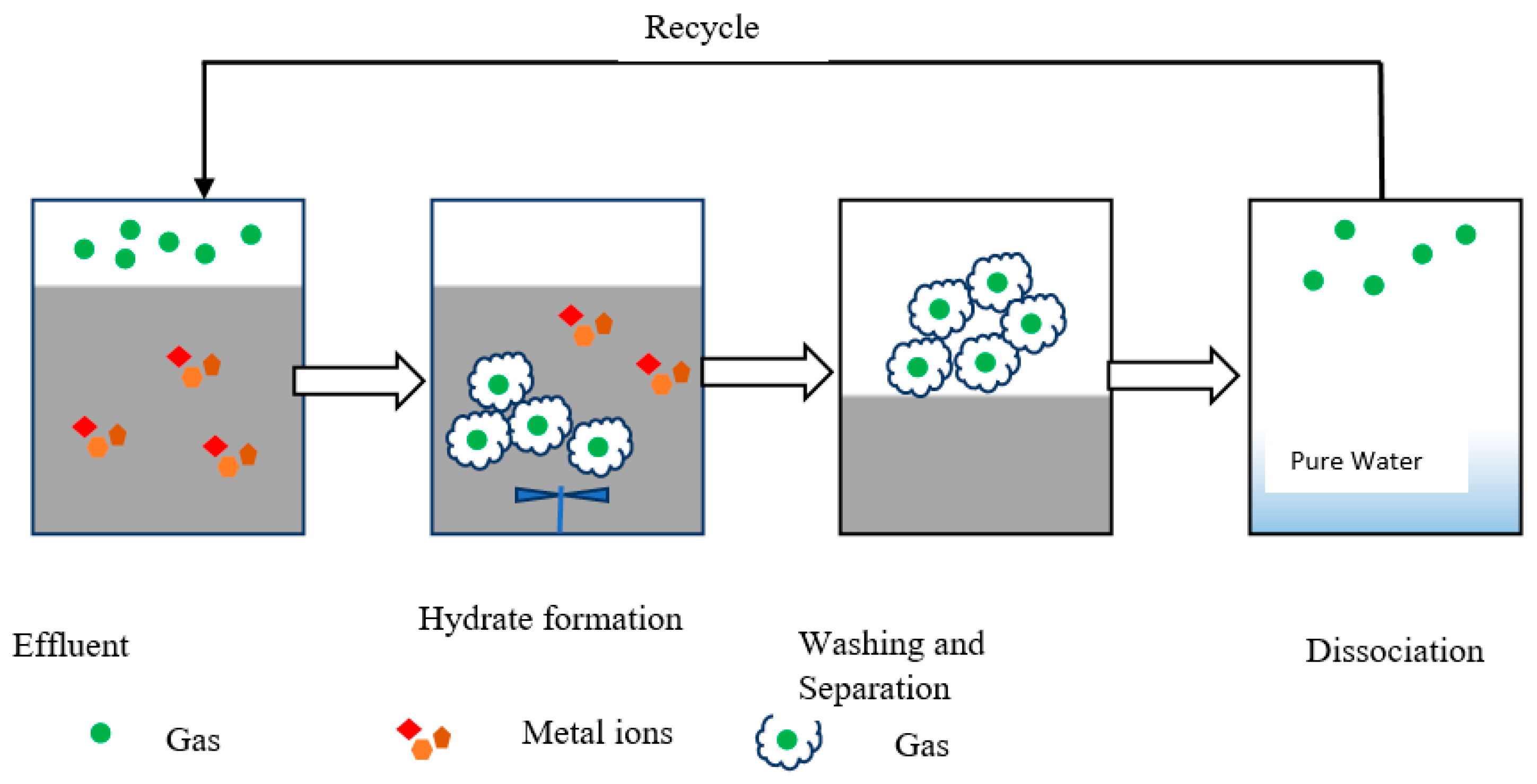
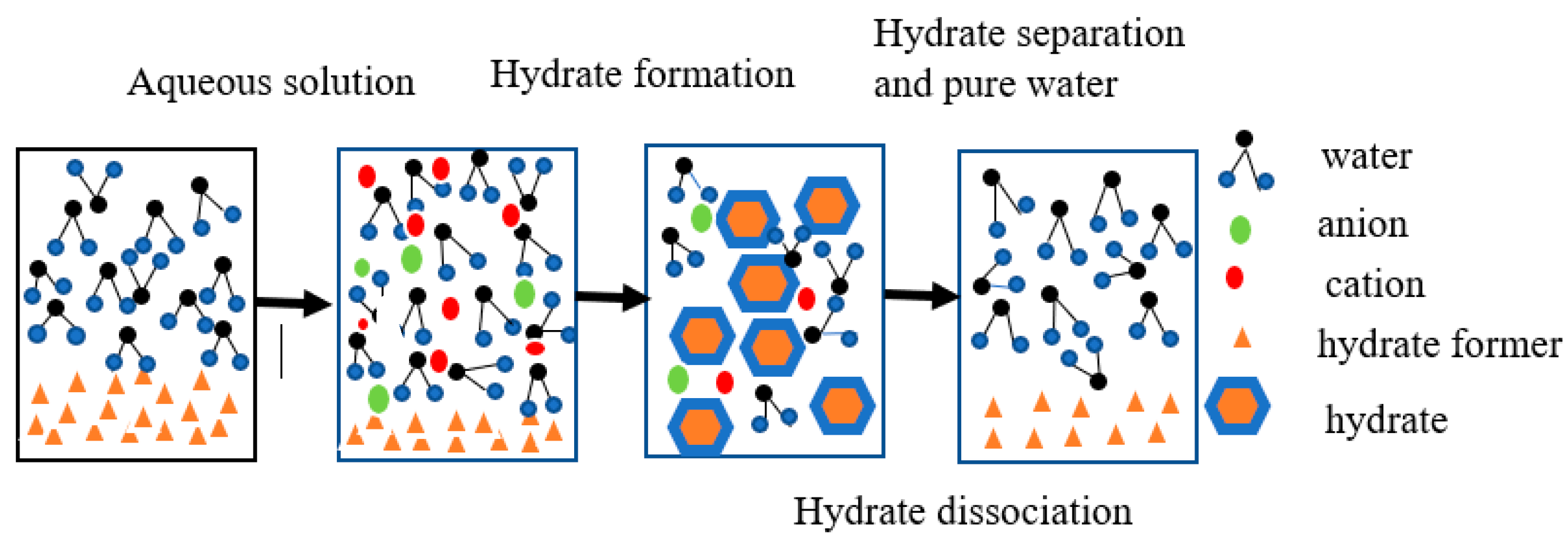
2.3. Heavy Metal Separation Mechanism Based on Gas Hydrates
2.4. Water Recovery
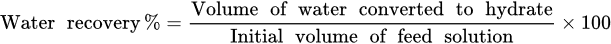
2.5. Removal Efficiency
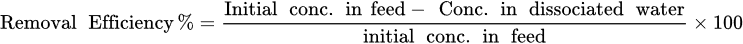
2.6. Enrichment Factor (Ef)
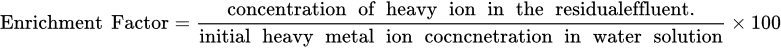
2.7. Gas Hydrate and Hybrid Technologies
References
- Rosado, D.; Usero, J.; Morillo, J. Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 2016, 153, 10–17.
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Badjah, A.Y. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem. Photobiol. 2018, 94, 935–941.
- Ali, I.; Alharbi, O.M.; ALOthman, Z.A.; Alwarthan, A.; Al-Mohaimeed, A.M. Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int. J. Biol. Macromol. 2019, 132, 244–253.
- Khan, N.A.; Ahmed, S.; Farooqi, I.H.; Ali, I.; Vambol, V.; Changani, F.; Khan, A.H. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: A critical review. TrAC Trends Anal. Chem. 2020, 129, 115921.
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177.
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 116–117.
- Rehman Ur, A.; Zaini, D.B.; Lal, B. Application of Gas Hydrate Based Technique in Wastewater Treatment—A Mini Review. In Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020), Kelantan, Malaysia, 15 August 2020; pp. 249–254.
- Nallakukkala, S.; Kassim, Z.; Othman, N.A.; Lal, B. Advancement in Gas Hydrate Water Based Produced Water Desalination: An Overview. In Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020), Kelantan, Malaysia, 15 August 2020.
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates, 1st ed.; Springer Publication: Cham, Switzerland, 2020; pp. 7–14.
- Leopercio, B.C. Kinetics of Cyclopentane Hydrate Formation—An Interfacial Rheology Study. Master’s Thesis, Pontifical Catholic University of Rio de Janeiro, PUC-Rio, Brazil, 2016.
- Sangwai, J.S.; Patel, R.S.; Mekala, P.; Mech, D.; Busch, M. Desalination of seawater using gas hydrate technology-current status and future direction. In Proceedings of the 18th International Conference on Hydraulics, Water Resources, Coastal and Environmental Engineering, HYDRO, Madras, India, 4–6 December 2013.
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400.
- Kezirian, M.T.; Phoenix, S.L. Natural gas hydrate as a storage mechanism for safe, sustainable and economical production from offshore petroleum reserves. Energies 2017, 10, 828.
- Zhou, H. Hydrate Slurry as Cold Energy Storage and Distribution Medium: Enhancing the Performance of Refrigeration Systems. Master’s Thesis, Guangzhou Institute of Energy Conversion, Guangzhou, China, 2017.
- Aregbe, A.G. Gas hydrate—Properties, formation and benefits. Open J. Yangtze Oil Gas 2017, 2, 27–44.
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 64–187.
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207.
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A review of clathrate hydrate based desalination to strengthen energy—Water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107.
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053.
- Lv, Y.N.; Wang, S.S.; Sun, C.Y.; Gong, J.; Chen, G.J. Desalination by forming hydrate from brine in cyclopentane dispersion system. Desalination 2017, 413, 217–222.
- Xu, C.; Li, X.; Yan, K.; Ruan, X.; Chen, Z.; Xia, Z. Research progress in hydrate-based technologies and processes in China: A review. Chin. J. Chem. Eng. 2019, 27, 1998–2013.
- Sahu, P.; Krishnaswamy, S.; Ponnani, K.; Pande, N.K. A thermodynamic approach to selection of suitable hydrate formers for seawater desalination. Desalination 2018, 436, 144–151.
- Knox, W.G.; Hess, M.; Jones, G.E.; Smith, H.B. The clathrate process. Chem. Eng. Prog. 1961, 57, 66–71.
- Ngan, Y.T.; Englezos, P. Concentration of mechanical pulp mill effluents and NaCl solutions through propane hydrate formation. Ind. Eng. Chem. Res. 1996, 35, 1894–1900.
- Nakajima, M.; Ohmura, R.; Mori, Y.H. Clathrate hydrate formation from cyclopentane-in-water emulsions. Ind. Eng. Chem. Res. 2008, 47, 8933–8939.
- Corak, D.; Barth, T.; Høiland, S.; Skodvin, T.; Larsen, R.; Skjetne, T. Effect of subcooling and amount of hydrate former on formation of cyclopentane hydrates in brine. Desalination 2011, 278, 268–274.
- Cai, L.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation kinetics of cyclopentane–methane binary clathrate hydrate. Chem. Eng. Sci. 2014, 119, 147–157.
- Cai, L.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation of cyclopentane methane binary clathrate hydrate in brine solutions. Chem. Eng. Sci. 2016, 141, 125–132.
- Misyura, S.Y.; Manakov, A.Y.; Morozov, V.S.; Nyashina, G.S.; Gaidukova, O.S.; Skiba, S.S.; Volkov, R.S.; Voytkov, I.S. The influence of key parameters on combustion of double gas hydrate. J. Nat. Gas Sci. Eng. 2020, 80, 103396.
- Yang, M.; Song, Y.; Jiang, L.; Liu, W.; Dou, B.; Jing, W. Effects of operating mode and pressure on hydrate-based desalination and CO2 capture in porous media. Appl. Energy 2014, 135, 504–511.
- Kang, K.C.; Linga, P.; Park, K.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90.
- McCormack, R.A.; Niblock, G.A. Investigation of High Freezing Temperature, Zero Ozone, and Zero Global Warming Potential, Clathrate Formers for Desalination; US Department of the Interior, Bureau of Reclamation, Technical Service: Denver, CO, USA, 2000.
- Simmons, B.A.; Bradshaw, R.W.; Dedrick, D.E.; Cygan, R.T.; Greathouse, J.A.; Majzoub, E.H. Desalination Utilizing Clathrate Hydrates (LDRD Final Report); National Technical Information Service: Livermore, CA, USA, 2008.
- Karamoddin, M.; Varaminian, F. Water desalination using R141b gas hydrate formation. Desalin. Water Treat. 2014, 52, 2450–2456.
- Ngema, P.T.; Petticrew, C.; Naidoo, P.; Mohammadi, A.H.; Ramjugernath, D. Experimental measurements and thermodynamic modeling of the dissociation conditions of clathrate hydrates for (refrigerant + NaCl + water) systems. J. Chem. Eng. Data. 2014, 59, 466–475.
- Seo, Y.; Moon, D.; Lee, C.; Park, J.W.; Kim, B.S.; Lee, G.W.; Yoon, J.H. Equilibrium, kinetics, and spectroscopic studies of SF6 hydrate in NaCl electrolyte solution. Environ. Sci. Technol. 2015, 49, 6045–6050.
- Wang, L.J.; Zhang, X.M.; Li, H.H.; Shao, L.; Zhang, D.; Jiao, L. Theory research on desalination of brackish water using gas hydrate method. Adv. Mater. Res. 2013, 616, 1202–1207.
- Lee, D.; Lee, Y.; Lee, S.; Seo, Y. Accurate measurement of phase equilibria and dissociation enthalpies of HFC-134a hydrates in the presence of NaCl for potential application in desalination. Korean J. Chem. Eng. 2016, 33, 1425–1430.
- Khan, M.N. Phase Equilibria Modeling of Inhibited Gas Hydrate Systems Including Salts: Applications in Flow Assurance, Seawater Desalination and Gas Separation. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2016.
- Zhang, Y.; Sheng, S.M.; Shen, X.D.; Zhou, X.B.; Wu, W.Z.; Wu, X.P.; Liang, D.Q. Phase equilibrium of cyclopentane + carbon dioxide binary hydrates in aqueous sodium chloride solutions. J. Environ. Chem. Eng. 2017, 62, 2461–2465.
- Ho-Van, S.; Bouillot, B.; Douzet, J.; Babakhani, S.M.; Herri, J.M. Implementing cyclopentane hydrates phase equilibrium data and simulations in brine solutions. Ind. Eng. Chem. Res. 2018, 43, 14774–14783.
- Ho-Van, S.; Bouillot, B.; Douzet, J.; Babakhani, S.M.; Herri, J.M. Cyclopentane hydrates–A candidate for desalination? J. Environ. Chem. Eng. 2019, 7, 103359.
- Lv, Q.; Li, X.; Li, G. Seawater desalination by hydrate formation and pellet production process. Energy Procedia 2019, 158, 5144–5148.
- Choi, W.; Lee, Y.; Mok, J.; Lee, S.; Lee, J.D.; Seo, Y. Thermodynamic and kinetic influences of NaCl on HFC-125a hydrates and their significance in gas hydrate-based desalination. Chem. Eng. J. 2019, 358, 598–605.
- Seo, S.D.; Hong, S.Y.; Sum, A.K.; Lee, K.H.; Lee, J.D.; Lee, B.R. Thermodynamic and kinetic analysis of gas hydrates for desalination of saturated salinity water. Chem. Eng. J. 2019, 370, 980–987.
- Han, S.; Shin, J.Y.; Rhee, Y.W.; Kang, S.P. Enhanced efficiency of salt removal from brine for cyclopentane hydrates by washing, centrifuging, and sweating. Desalination 2014, 354, 17–22.
- Karamoddin, M.; Varaminian, F. Water purification by freezing and gas hydrate processes, and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+). J. Mol. Liq. 2016, 223, 1021–1031.
- Fakharian, H.; Ganji, H.; Naderifar, A.; Mofrad, H.R.; Kakavand, M. Effect of gas type and salinity on performance of produced water desalination using gas hydrates. J. Water Reuse Desalin. 2019, 9, 396–404.
- Kang, K.C.; Hong, S.Y.; Cho, S.J.; Kim, D.H.; Lee, J.D. Evaluation of desalination by nanostructured hydrate formation and pellet production process. J. Nanosci. Nanotechnol. 2017, 17, 4059–4062.
- Javanmardi, J.; Moshfeghian, M. Energy consumption and economic evaluation of water desalination by hydrate phenomenon. Appl. Therm. Eng. 2003, 23, 845–857.
- Ghalavand, Y.; Hatamipour, M.S.; Rahimi, A. A review on energy consumption of desalination processes. Desalin. Water Treat. 2015, 54, 1526–1541.
- Singh, J.; Lal, B. Prospectives on gas hydrates-based desalination. In Gas Hydrate in Water Treatment, 1st ed.; Lal, B., Nallakukkala, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 1, pp. 31–53.
- Chong, Z.R.; He, T.; Babu, P.; Zheng, J.; Linga, P. Economic evaluation of energy efficient hydrate-based desalination utilizing cold energy from liquefied natural gas (LNG). Desalination 2019, 463, 69–80.
- He, T.; Chong, Z.R.; Zheng, J.; Ju, Y.; Linga, P. LNG cold energy utilization: Prospects and challenges. Energy 2019, 170, 557–568.
- Zheng, J.; Yang, M. Experimental investigation on novel desalination system via gas hydrate. Desalination 2020, 478, 114284.
- Sarshar, M.; Sharafi, A.H. Simultaneous water desalination and CO2 capturing by hydrate formation. Desalin. Water Treat. 2011, 28, 59–64.
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351.
- Yang, M.; Zheng, J.; Liu, W.; Liu, Y.; Song, Y. Effects of C3H8 on hydrate formation and dissociation for integrated CO2 capture and desalination technology. Energy 2015, 93, 1971–1979.
- Donath, W.E. Method and Apparatus for Producing Purified Water from Aqueous Saline Solutions. U.S. Patent No. 2,904,511, 15 September 1959.
- Buchanan, B.B. Removing Salt from Sea Water. U.S. Patent 3,027,320, 27 March 1962.
- Walton, P.R. Continuous Saline Water Purification. U.S. Patent 3,132,096, 5 May 1964.
- Klass, D. Hydrate Forming in Water Desalination. U.S. Patent No 3,856,492, 24 December 1974.
- Guo, B.; Bretz, R.E.; Lee, R.L. Method and Apparatus for Generating, Transporting and Dissociating Gas Hydrates. U.S. Patent No. 5,473,904, 12 December 1995.
- McCormack, R.A. Clathrate Freeze Desalination Apparatus and Method. U.S. Patent No. 5,553,456, 10 September 1996.
- Max, M.D.; Pellenbarg, R.E. Desalination through Methane Hydrate. U.S. Patent No. 5,873,262, 23 February 1999.
- Heinemann, R.F.; Huang, D.D.T.; Long, J.; Saeger, R.B. Process for Making Gas Hydrates. U.S. Patent No. 6,028,234, 22 February 2000.
- Heinemann, R.F.; Huang, D.D.T.; Long, J.; Saeger, R.B. Method for Producing Gas Hydrates Utilizing a Fluidized Bed. U.S. Patent No. 6,180,843, 30 January 2001.
- Max, M.D. Hydrate Formation and Growth for Hydrate-Based Desalination by Means of Enriching Water to Be Treated. U.S. Patent No. 6,890,444, 10 May 2005.
- Max, M.D.; Korsgaard, J. Hydrate-Based Desalination with Hydrate-Elevating Density-Driven Circulation. U.S. Patent No. 6,969,467, 10 November 2005.
- Simmons, B.A.; Bradshaw, R.W.; Dedrick, D.E.; Anderson, D.W. Complex Admixtures of Clathrate Hydrates in a Water Desalination Method. U.S. Patent 7560028B1, 14 July 2009.
- Phelps, T.J.; Tsouris, C.; Palumbo, A.V.; Riestenberg, D.E.; McCallum, S.D. Method for Excluding Salt and Other Soluble Materials from Produced Water. U.S. Patent No. 7,569,737, 4 August 2009.
- Li, D.; Liang, D.; Tang, C. Test Device for Desalination of Sea Water by Hydrate Method. CN Patent CN101289231B, 6 June 2010.
- Osegovic, J.P.; Max, M.D.; Tatro, S.R. Seawater-Based Carbon Dioxide Disposal. U.S. Patent No. 8,048,309, 1 November 2011.
- Carstens, C.; Dickinson, W.; Dickinson, W.; Myers, J. Clathrate Hydrate Modular Storage, Applications and Utilization Processes. U.S. Patent No. 7914749, 29 March 2011.
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96.
- Katyal, A.A. System and Method for Hydrate-Based Desalination. U.S. Patent No. 9,643,860, 9 May 2017.
- McCormack, R.A.; Ripmeester, J.A. Clathrate Desalination Process Using an Ultrasonic Actuator. U.S. Patent 20140223958A1, 14 August 2014.
- Parker, A. Potable water from sea-water. Nature 1942, 149, 184–186.
- Hesse, R.; Harrison, W.E. Gas hydrates (clathrates) causing pore-water freshening and oxygen isotope fractionation in deep-water sedimentary sections of terrigenous continental margins. Earth Planet. Sci. Lett. 1981, 55, 453–462.
- Willson III, R.C.; Bulot, E.; Cooney, C. L Use of Hydrates for Aqueous Solution Treatment. U.S. Patent No. 4,678,583, 7 July 1987.
- Huang, C.P.; Fennema, O.; Powrie, W.D. Gas hydrates in aqueous-organic systems: II. Concentration by gas hydrate formation. Cryobiology 1966, 2, 240–245.
- Truong-Lam, H.S.; Kim, S.; Seo, S.D.; Jeon, C.; Lee, J.D. Water purifying by gas hydrate: Potential applications to desalination and wastewater treatments. Chem. Eng. Trans. 2020, 78, 67–72.
- Song, Y.; Dong, H.; Yang, L.; Yang, M.; Li, Y.; Ling, Z.; Zhao, J. Hydrate-based heavy metal separation from aqueous solution. Sci. Rep. 2016, 6, 21389.
- Yang, Y.; Zhou, H.; Li, F.; Shi, C.; Wang, S.; Ling, Z. Cyclopentane hydrate-based processes for treating heavy metal containing wastewater. E3S Web Conf. 2019, 118, 10–13.
- Li, X.S.; Yang, B.; Zhang, Y.; Li, G.; Duan, L.P.; Wang, Y.; Wu, H.J. Experimental investigation into gas production from methane hydrate in sediment by depressurization in a novel pilot-scale hydrate simulator. Appl. Energy 2012, 93, 722–732.
- Ohmura, R.; Ogawa, M.; Yasuoka, K.; Mori, Y.H. Statistical study of clathrate-hydrate nucleation in a water/hydrochlorofluorocarbon system: Search for the nature of the memory effect. J. Phys. Chem. B 2003, 107, 5289–5293.
- Murshed, M.M.; Faria, S.H.; Kuhs, W.F.; Kipfstuhl, S.; Wilhelms, F. The role of hydrochlorofluorocarbon densifiers in the formation of clathrate hydrates in deep boreholes and subglacial environments. Ann. Glaciol. 2007, 47, 109–114.
- Kato, M.; Iida, T.; Mori, Y.H. Drop formation behaviour of a hydrate-forming liquid in a water stream. J. Fluid Mech. 2000, 414, 367–378.
- Dong, H.; Zhang, L.; Ling, Z.; Zhao, J.; Song, Y. The Controlling Factors and Ion Exclusion Mechanism of Hydrate-Based Pollutant Removal. ACS Sustain. Chem. Eng. 2019, 7, 7932–7940.
- Gaikwad, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas Hydrate-Based Process for Desalination of Heavy Metal Ions from an Aqueous Solution: Kinetics and Rate of Recovery. ACS ES&T Water 2021, 1, 134–144.
- Nallakukkala, S.; Lal, B. Waste brine management. In Gas Hydrate in Water Treatment, 1st ed.; Lal, B., Nallakukkala, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 1, pp. 31–53.
- Babu, P.; Nambiar, A.; Chong, Z.R.; Daraboina, N.; Albeirutty, M.; Bamaga, O.A.; Linga, P. Hydrate-based desalination (HyDesal) process employing a novel prototype design. Chem. Eng. Sci. 2020, 218, 115563.
- Al-Hemeri, S.T.; Al-Mukhtar, R.S.; Hussine, M.N. Removal of heavy metals from industrial wastewater by use of Cyclopentane-Clathrate Hydrate formation technology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Istanbul, Turkey, 2020; Volume 737, p. 012178.
- Fakharian, H.; Ganji, H.; Naderifar, A. Desalination of high salinity produced water using natural gas hydrate. J. Taiwan Inst. Chem. Eng. 2017, 72, 157–162.
- Nallakukkala, S.; Lal, B.; Shariff, M.A. Influence of water volume on CO2 hydrate-based desalination of brine solution. Mater. Today Proc. 2021, 56, 2172–2177.
- Nambiar, A.; Babu, P.; Linga, P. Improved kinetics and water recovery with propane as co-guest gas on the hydrate-based desalination (hydesal) process. ChemEngineering 2019, 3, 31.
- Johannsen, P.; Karlapudi, R.; Reinhold, G. High pressure reverse osmosis for wastewater minimization and zero liquid discharge applications. Desalination 2006, 199, 84–85.
- Nallakukkala, S.; Abulkhair, H.; Alsaiari, A.; Ahmad, I.; Almatrafi, E.; Bamaga, O.; Mohd Shariff, A. Suitable Binary and Ternary Thermodynamic Conditions for Hydrate Mixtures of CH4, CO2, and C3H8 for Gas Hydrate-Based Applications. ACS Omega 2022, 7, 10877–10889.
- Falahieh, M.M.; Bonyadi, M.; Lashanizadegan, A. A new hybrid desalination method based on the CO2 gas hydrate and capacitive deionization processes. Desalination 2021, 502, 114932.
- Truong-Lam, H.S.; Seo, S.D.; Jeon, C.; Lee, G.P.; Lee, J.D. A gas hydrate process for high-salinity water and wastewater purification. Desalination 2022, 529, 115651.
- Dong, H.; Fan, Z.; Wang, B.; Xue, S.; Zhao, J.; Song, Y. Hydrate-based reduction of heavy metal ion from aqueous solution. Energy Procedia 2017, 105, 4706–4712.
- Youssef, P.; Al-Dadah, R.; Mahmoud, S. Comparative Analysis of Desalination Technologies. Energy Procedia 2014, 61, 2604–2607.
- Yun, S.H.; Woo, D.S. Analysis of seawater desalination energy consumption based on changes in raw water characteristics and operating condition. J. Korean Soc. Water Wastewater 2019, 33, 281–289.
- Lal, B.; Nallakukkala, S. Gas Hydrate in Water Treatment: Technological, Economic, and Industrial Aspects, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 14–301.




