You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marina Carrasco-Llatas | -- | 1639 | 2022-04-14 09:53:46 | | | |
| 2 | Camila Xu | -1 word(s) | 1638 | 2022-04-14 11:02:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carrasco-Llatas, M.; , .; De Vito, A.; Chong, K.B. Drug-Induced Sleep Endoscopy. Encyclopedia. Available online: https://encyclopedia.pub/entry/21758 (accessed on 23 December 2025).
Carrasco-Llatas M, , De Vito A, Chong KB. Drug-Induced Sleep Endoscopy. Encyclopedia. Available at: https://encyclopedia.pub/entry/21758. Accessed December 23, 2025.
Carrasco-Llatas, Marina, , Andrea De Vito, Khai Beng Chong. "Drug-Induced Sleep Endoscopy" Encyclopedia, https://encyclopedia.pub/entry/21758 (accessed December 23, 2025).
Carrasco-Llatas, M., , ., De Vito, A., & Chong, K.B. (2022, April 14). Drug-Induced Sleep Endoscopy. In Encyclopedia. https://encyclopedia.pub/entry/21758
Carrasco-Llatas, Marina, et al. "Drug-Induced Sleep Endoscopy." Encyclopedia. Web. 14 April, 2022.
Copy Citation
Drug-induced sleep endoscopy (DISE) is a diagnostic tool to assess the upper airway of snorers and obstructive sleep apnea patients in conditions that mimic natural sleep.
drug-induced sleep endoscopy
DISE
sedation
obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA) is a common health problem that is associated with an increase in cardiovascular morbimortality, a decrease in quality of life, and a higher risk of traffic accidents due to hypersomnolence [1][2]. Standard treatment for OSA is positive upper airway pressure, usually delivered in a continuous mode, known as continuous positive airway pressure (CPAP) therapy. CPAP acts like a pneumatic splint that opens the upper airway (UA), regardless of the site of obstruction. Nevertheless, CPAP adherence can be poor, and therefore alternative therapies are necessary [3]. On the other hand, surgery has 100% compliance but has limited effect on unselected patients [4]. With the arrival of new technologies such as transoral robotic surgery (TORS) and coblation, new modalities for the treatment of tongue base obstruction is now available. The sleep surgeon needs to know the exact area or areas of upper airway collapse in order to select patients that may improve with surgery and determine the areas that need to be surgically addressed.
Routine UA assessment is performed when patients are awake—a state with no UA collapse or oxygen desaturation. In a small percentage of OSA patients, the anatomical causes of the obstruction are obvious (e.g., grade 4 palatine and/or lingual tonsils). However, for most patients, the cause is not so obvious, as they may have long soft palate, a relatively large tongue with grade 1 tonsils, or previous tonsillectomy. Therefore, it is of utmost importance to understand the UA behavior during sleep so that tailor-made treatment can be decided for every patient.
In the late 1970s, evaluation of the UA during natural sleep was proposed [5]. Unfortunately, observation of the UA during natural sleep is complicated and impractical in daily practice. On the other hand, with the aid of sedation, the UA can be observed dynamically to allow the sleep surgeon to have an accurate idea of what is happening during sleep and the possible treatment options. Other diagnostic tools such as MRI or CT scan under sedation are also possible. Although MRI and CT scans have the advantage of having complete visualization of the UA simultaneously, they are limited by the facilities available and the need to collaborate with the radiology department. Maneuvers or changes in the position during sleep MRI or CT scan are also almost impossible. For this reason, drug-induced sleep endoscopy (DISE) has spread all over the world and is the preferred diagnostic technique to assess the UA of patients who snore or have OSA in a state that simulates natural sleep [6].
2. Technique
Since the first publication on DISE [7], several drugs or drug combinations have been used for sedation. Some authors also use nasal decongestion, local anesthesia, and/or anti-secretory drugs. So far, there is also no uniformity regarding how to perform the sedation. Nevertheless, this point is critical to achieve a sedation level that resembles natural sleep in order to obtain reliable DISE findings.
In the European position paper on DISE, and its later update, Ear, Nose and throat (ENT) experts on DISE put in their collective opinion and recommended possible set-up and techniques for DISE to be reliably practiced in different centers [6][8].
2.1. Where to Perform DISE and Materials
DISE must be performed in a room where all the basic resuscitation equipment (e.g., oxygen supply) are available, because safety is of utmost importance. The room should also be quiet and dark.
In order to perform DISE, the patient should be monitored with electrocardiogram (ECG) and pulse oximeter. Monitoring of sedation depth such as Bi-spectral index (BIS) is highly recommended, as the BIS level of sedation should be between 70 and 50 when the UA is evaluated in adults [6]. Evaluation of the UA in lighter sedation may underestimate the obstruction, whereas deeper sedation may cause artificial obstruction due to over sedation [9][10][11]. Having said that, one must be aware that sedation levels observed with these devices are an estimate of actual sedation, as some people are not well sedated at those recommended levels, although the majority of adult people are. For children, these BIS levels might not be applicable.
The thinnest possible fiber endoscope, ideally with working channel to aspirate secretions, is recommended. The researchers also recommend a recording system for video and sound, if possible. The recording is useful, as the videos can be replayed to the patients to help them understand their problem better as well as the potential difficulty in addressing the obstruction. The videos may also be useful in the future if the results of the UA surgery do not achieve the desired outcomes.
2.2. Drugs for Sedation and Method
Based on the literature, many drugs (midazolam, propofol, dexmedetomidine, remifentanil, ketamine) are used, either alone or in combination, in order to achieve the ideal level of sedation [6]. All of them have their own advantages and disadvantages. Discussing this specific subject is beyond the scope of this entry; more details are explained elsewhere [12][13]. In this manuscript, only sedation with propofol using target-controlled infusion (TCI) pump will be described, as it is the sedation method that is evidence-proven to demonstrate UA collapses resembling natural sleep. Studies comparing natural sleep and TCI-propofol sleep have shown that the critical closing pressure, UA muscle responsiveness, apnea hypopnea index (AHI), levels of sedation measured by BIS and sites of obstruction are equivalent to those observed during N2 and N3 stages of natural sleep [14][15][16][17][18][19]. It is important to note that REM sleep cannot be reproduced with propofol sedation [15]. DISE usually takes 15–30 min. Within this short duration, only N2 sleep can be achieved [20]. Nevertheless, it is during this stage that most of the events occur (unless the patient has events only in REM sleep). Therefore, DISE should be considered a snapshot of what happens during the night, but it cannot provide information for the entire night. This is a limitation of the procedure, but as-long-as there is no tool that can show the behavior of every structure of the UA during natural sleep for the whole night, DISE will continue to be the best tool available.
It is recommended to titrate the TCI-propofol pump slowly to achieve the ideal level of sedation (BIS: 70–50, does not respond to verbal stimuli). According to Heiser et al. and the personal experience, this level of sedation is achieved when the brain concentration is approximately 3 µg/mL [9]. In order to reach this concentration as quickly as possible without causing central apneas, the pump can be programmed to 2 or 2.5 µg/mL, with an increase of 0.5 µg/mL every two minutes until the level of sedation is achieved. An addition of 1–2 mg of midazolam at the beginning of the sedation will shorten the time to the observation window, although it may also cause an increase in sneezing, making the assessment more difficult [21].
DISE is not a painful technique, therefore local anesthesia of the nose is not necessary. Neither is nasal decongestion [6]. Antisecretory drugs such as atropine are not recommended for routine use as atropine’s effect on the UA is unknown [6]. Nevertheless, the visualization of the UA dynamics can be extremely difficult in the presence of secretions, and therefore the physician performing DISE has to balance the pros and cons and act accordingly.
2.3. Maneuvers and Position
The patients are usually observed in supine decubitus position, because this is the position where most of the events occur. However, assessment of the UA in a lateral position does give additional information, especially for those patients that sleep mostly in a lateral decubitus position. If the patient’s history suggests that he or she should be assessed in a lateral decubitus position, it may be easier to start DISE in this position, including doing the necessary maneuvers, before changing to supine position. Although the initial study by the de Vries group noted that the UA findings were similar in lateral decubitus compared with supine decubitus with the head turned to one side [22], a recent study with a larger sample size proved that their initial findings were inaccurate, emphasizing the need to observe the UA in lateral decubitus [23].
It is very common for patients to open their mouths during DISE, as they usually do at home. If the mouth is open, the tongue may move backward, pushing the soft palate and further obstructing the UA. Changes to the areas and pattern of obstruction can be observed by just closing the mouth. This maneuver (often called chin-lift) is different from the Esmarch maneuver, which gently pulls the mandible forward to simulate the effect of a mandibular advancement device (MAD). As showed by the Antwerp group, maximum advancement of the mandible does not represent what a MAD can achieve [24]. Other than the advancement of the mandible, the thickness of a MAD needs to be simulated as well. Therefore, an interincisive distance of approximately 5 mm is necessary to mimic a MAD. The researchers pull the mandible by grabbing it with the fingers inside the mouth in order to mimic the thickness and the protrusion of the MAD in place. This maneuver is also believed to be less painful compared with pushing the mandible at its angle (Figure 1). Performing DISE with a custom-made MAD is also feasible. DISE can begin with the MAD in place. The device can be removed after observing the UA for potential collapse for at least two cycles (stable snoring, apneas/hypopneas and breathing), and the UA can be observed for another two or more cycles with different positions and maneuvers. This is because insertion of a MAD while the patient is sedated is extremely difficult, while removal is much easier with less arousal for the patient [24][25].
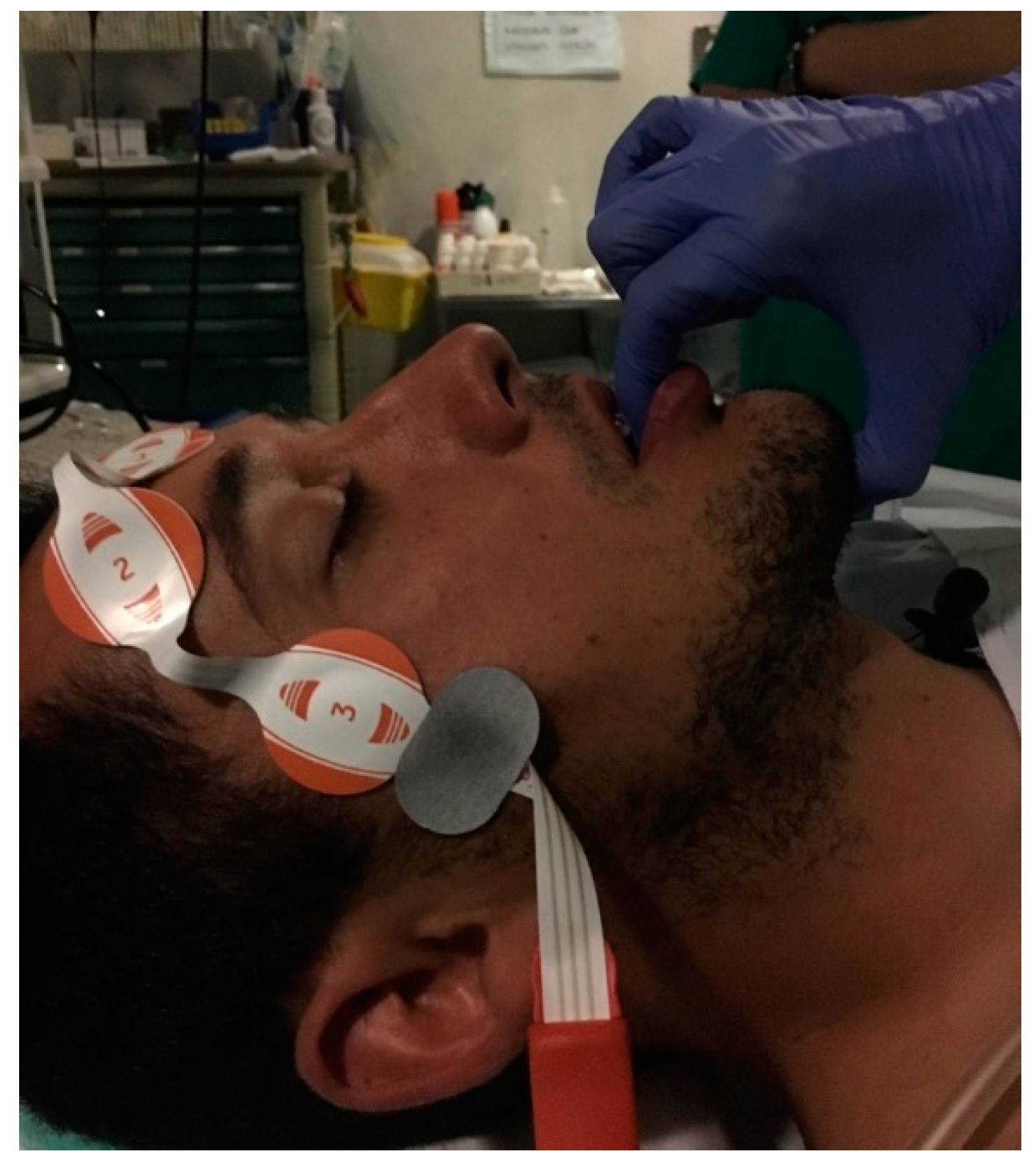
Figure 1. Modified Esmarch maneuverer grabbing the mandible of the patient to mimic the action of a mandibular advancement device (MAD).
References
- Molnar, M.Z.; Mucsi, I.; Novak, M.; Szabo, Z.; Freire, A.X.; Huch, K.M.; A Arah, O.; Ma, J.Z.; Lu, J.L.; Sim, J.J.; et al. Association of Incident Obstructive Sleep Apnea with Outcomes in a Large Cohort of US Veterans. Thorax 2015, 70, 888–895.
- Allen, A.J.M.H.; Bansback, N.; Ayas, N.T. The Effect of OSA on Work Disability and Work-Related Injuries. Chest 2015, 147, 1422–1428.
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head Neck Surg. 2016, 45, 1217.
- Caples, S.M.; Prinsell, J.R.; Pallanch, J.F.; Elamin, M.B.; Katz, S.G.; Harwick, J.D.; Rowley, J.A. Surgical Modifications of the Upper Airway for Obstructive Sleep Apnea in Adults: A Systematic Review and Meta-Analysis. Sleep 2010, 33, 1396–1407.
- Borowiecki, B.; Pollak, C.P.; Weitzman, E.D.; Rakoff, S.; Imperato, J.; Isaacson, S.R.; Snow, J.B. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope 1978, 88, 1310.
- De Vito, A.; Carrasco Llatas, M.; Ravesloot, M.J.; Kotecha, B.; De Vries, N.; Hamans, E.; Maurer, J.; Bosi, M.; Blumen, M.; Heiser, C.; et al. European position paper on drug-induced sleep endoscopy: 2017 Update. Clin. Otolaryngol. 2018, 43, 1541–1552.
- Croft, C.B.; Pringle, M. Sleep nasendoscopy: A technique of assessment in snoring and obstructive sleep apnoea. Clin. Otolaryngol. 1991, 16, 504–509.
- De Vito, A.; Carrasco Llatas, M.; Vanni, A.; Bosi, M.; Braghiroli, A.; Campanini, A.; de Vries, N.; Hamans, E.; Hohenhorst, W.; Kotecha, B.T.; et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 2014, 18, 453–465.
- Heiser, C.; Fthenakis, P.; Hofauer, B.; Hohenhorst, W.; Kochs, E.F.; Wagner, K.J.; Edenharter, G.M.; Hapfelmeier, A.; Berger, S. Drug-induced sleep endoscopy with target-controlled infusion using propofol and monitored depth of sedation to determine treatment strategies in obstructive sleep apnea. Sleep Breath 2017, 177, 1006–1744.
- Maddison, K.J.; Walsh, J.H.; Shepherd, K.L.; Bharat, C.; Lawther, B.K.; Platt, P.R.; Eastwood, P.R.; Hillman, D.R. Comparison of Collapsibility of the Human Upper Airway During Anesthesia and During Sleep. Anesth. Analg. 2019.
- Eastwood, P.R.; Platt, P.R.; Shepherd, K.; Maddison, K.; Hillman, D.R. Collapsibility of the Upper Airway at Different Concentrations of Propofol Anesthesia. Anesthesiology 2005, 103, 470–477.
- Ehsan, Z.; Mahmoud, M.; Shott, S.R.; Amin, R.S.; Ishman, S.L. The effects of Anesthesia and opioids on the upper airway: A systematic review. Laryngoscope 2016, 126, 270–284.
- Shteamer, J.W.; Dedhia, R.C. Sedative choice in drug-induced sleep endoscopy: A neuropharmacology-based review. Laryngoscope 2016, 1–7.
- Ordones, A.B. Sonoendoscopia Durante sono Natural Comparada com Sonoendoscopia Durante sono Induzido com Propofol. Ph.D. Thesis, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil, 2018.
- Rabelo, F.A.W.; Küpper, D.S.; Sander, H.H.; Fernandes, R.M.F.; Valera, F.C.P. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope 2013, 123, 2300–2305.
- Kasuya, Y.; Govinda, R.; Rauch, S.; Mascha, E.J.; Sessler, D.I.; Turan, A. The Correlation Between Bispectral Index and Observational Sedation Scale in Volunteers Sedated with Dexmedetomidine and Propofol. Anesth. Analg. 2009, 109, 1811–1815.
- Zhong, T.; Guo, Q.L.; Pang, Y.D.; Peng, L.F.; Li, C.L. Comparative evaluation of the cerebral state index and the bispectral index during target-controlled infusion of propofol. Br. J. Anaesth. 2005, 95, 798–802.
- Hoshino, Y.; Ayuse, T.; Kurata, S.; Ayuse, T.; Schneider, H.; Kirkness, J.P.; Patil, S.P.; Schwartz, A.R.; Oi, K. The compensatory responses to upper airway obstruction in normal subjects under propofol anesthesia. Respir. Physiol. Neurobiol. 2009, 166, 24–31.
- Hillman, D.R.; Walsh, J.H.; Maddison, K.J.; Platt, P.R.; Kirkness, J.P.; Noffsinger, W.J.; Eastwood, P.R. Evolution of Changes in Upper Airway Collapsibility during Slow Induction of Anesthesia with Propofol. Anesthesiology 2009, 111, 63–71.
- Carrasco Llatas, M.; Agostini Porras, G.; Cuesta González, M.T.; Rodrigo Sanbartolomé, A.; Giner Bayarri, P.; Gómez-Pajares, F.; Dalmau Galofre, J. Drug-induced sleep endoscopy: A two drug comparison and simultaneous polysomnography. Eur. Arch Otorhinolaryngol. 2014, 271, 181–187.
- Virk, J.S.; Kotecha, B. Sneezing during drug-induced sedation endoscopy. Sleep Breath 2014, 18, 451–452.
- Safiruddin, F.; Koutsourelakis, I.; De Vries, N. Analysis of the influence of head rotation during drug-induced sleep endoscopy in obstructive sleep apnea. Laryngoscope 2014, 124, 2195–2199.
- Vonk, P.E.; van de Beek, M.J.; Ravesloot, M.J.L.; de Vries, N. Drug-induced sleep endoscopy: New insights in lateral head rotation compared to lateral head and trunk rotation in (non)positional obstructive sleep apnea patients. Laryngoscope 2019.
- Vroegop, A.V.M.T.M.T.; Vanderveken, O.M.; Dieltjens, M.; Wouters, K.; Saldien, V.; Braem, M.J.; Van de Heyning, P.H. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J. Sleep. Res. 2013, 22, 348–355.
- Vroegop, A.V.; Vanderveken, O.M.; Van De Heyning, P.H.; Braem, M.J. Effects of vertical opening on pharyngeal dimensions in patients with obstructive sleep apnoea. Sleep Med. 2012, 13, 314–316.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
909
Revisions:
2 times
(View History)
Update Date:
14 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




