Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Meng-Che Lu | -- | 2063 | 2022-04-14 06:43:03 | | | |
| 2 | Catherine Yang | Meta information modification | 2063 | 2022-04-14 07:38:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lu, M.; , .; Hsu, Y.; Chen, S. Norovirus. Encyclopedia. Available online: https://encyclopedia.pub/entry/21748 (accessed on 07 February 2026).
Lu M, , Hsu Y, Chen S. Norovirus. Encyclopedia. Available at: https://encyclopedia.pub/entry/21748. Accessed February 07, 2026.
Lu, Meng-Che, , Yi-Hsiang Hsu, Shih-Yen Chen. "Norovirus" Encyclopedia, https://encyclopedia.pub/entry/21748 (accessed February 07, 2026).
Lu, M., , ., Hsu, Y., & Chen, S. (2022, April 14). Norovirus. In Encyclopedia. https://encyclopedia.pub/entry/21748
Lu, Meng-Che, et al. "Norovirus." Encyclopedia. Web. 14 April, 2022.
Copy Citation
Noroviruses (NoVs) are one of the emerging and rapidly spreading groups of pathogens threatening human health. A reduction in sporadic NoV infections was noted following the start of the COVID-19 pandemic, but the return of NoV gastroenteritis during the COVID-19 pandemic has been noted recently. Research in recent years has shown that different virus strains are associated with different clinical characteristics; moreover, there is a paucity of research into extraintestinal or unusual complications that may be associated with NoV.
norovirus
epidemiology
gastroenteritis
complications
1. Introduction
Norovirus (NoV) is a nonenveloped virus with a single-stranded, positive-sense, polyadenylated RNA genome that encodes three major open reading frames (ORFs) [1]. ORF1 encodes a large polyprotein that is cleaved by the viral proteinase into six nonstructural proteins, which are involved in NoV replication in host cells. ORF2 encodes the major viral capsid protein (VP) 1 [2], whereas ORF3 encodes VP2, a minor capsid protein involved in viral packaging [3]. The capsid protein consists of a shell (S) domain encoded by conserved genetic lineages and two protruding (P) subdomains: the relatively conserved P1 and highly variable P2 subdomains. The P1 subdomain plays a role in viral-particle stability, whereas the outermost P2 subdomain contains epitopes recognized by neutralizing antibodies and the clefts required for binding to human histo-blood group antigen (HBGA) receptors [4][5][6]. Recombination at the junction of ORF1 and ORF 2 can result in the emergence of a novel viral strain, similar to other RNA viruses [7].
NoVs are highly diverse viruses that can be genetically grouped into 10 genogroups from GI to GX. Only the GI, GII, GIV, GVIII, and GIX genogroups can infect humans, and the GII genogroup is the most prevalent [8][9]. Variants (subgenotypes) are determined by sequence analysis of highly variable regions of the ORF2 and named according to the location and year they were first described [9]. NoV is an emerging pathogen that causes enteric infections in humans and has diverse clinical presentations. Genetic diversity of NoV indicates its rapid evolution with genotype polymorphism. NoV variants related to severe infection and complications have been reported sporadically, and the rapid genetic evolution of NoV is believed to drive changes in its clinical manifestations [10][11][12]. In addition to gastrointestinal (GI) symptoms, NoV infection is associated with several complications, including benign infantile convulsion [13], encephalopathy [14][15][16], necrotizing enterocolitis [17][18], dysregulation of the enteric nervous and immune system [19][20], exacerbation of inflammatory bowel disease [21], and chronic, serious outcomes in immunocompromised patients [22][23].
2. Associated Complications Related to Norovirus Infection
According to the volunteer challenge studies, the asymptomatic infections were estimated at 32.1% [24]. In Taiwan, from winter 2004 to winter 2005, the most common complications were electrolyte imbalance (14.3%) and hypoglycemia (11.4%). After the rotavirus vaccination introduction (from late 2006), the most common complications were convulsive disorder (28%) and hypoglycemia (20%) from winter 2006 to winter 2007. In the 2008/09/10 winters, major complications included GI hemorrhage (22.2%) and prominent hyperthermia (13.8%). From winter 2011 to winter 2012, hyperthermia (34.9%) and GI hemorrhage (23.3%) were the most prominent complications caused by NoV infection [25]. Figure 1 demonstrates the overall complications of NoV infection in different periods in northern Taiwan.
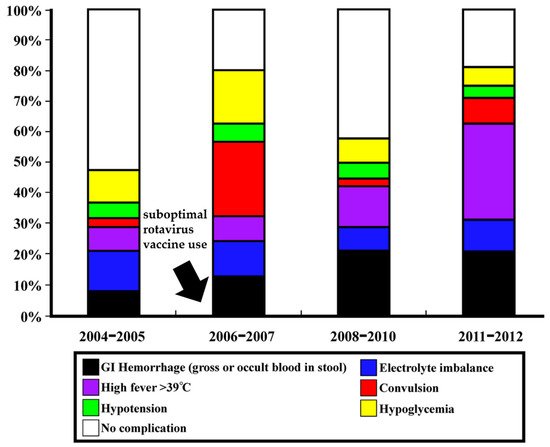
Figure 1. Complications in different periods of NoV outbreaks in northern Taiwan before and after suboptimal rotavirus vaccine introduction from late 2006 (highlight by arrow). The figure has been modified and originated from the research by Wang et al. [25].
Although the specific NoV subgenotypes related to severe infection and complications were only sporadically reported, the rapid genetic evolution of NoV is believed to be the main factor driving the changing clinical manifestations in infected patients. In the 2006/2007 winters, the NoV subgenotype or variant GII.4 Den_Haag_2006b (40%) caused the most complications of convulsion (67.9%) in infected children. The major subgenotype in the 2008/09/10 winters, GII.4 2010 (New Orleans) (48.5%), caused GI hemorrhage (37.5%). Furthermore, GII.4 2012 subgenotypes (56.8%), the predominant NoV strains in the 2011/2012 winter, caused more high fever (57.1%) and GI hemorrhage (33.3%) [25]. Research in northern Taiwan for the clinical relevance and genotypes of circulating NoVs found that patients infected by NoV GII.4 2006b had a higher frequency of diarrhea, longer duration of diarrhea, and more frequent hypoglycemia and electrolyte imbalance compared with those with gastroenteritis caused by NoV GII.4 2010 [26].
Studies in the last 15 years have reported that NoV infection is also associated with a range of sequelae and complications other than gastroenteritis. A recently developed model for NoV infection contributed critical knowledge for extraintestinal or unusual complications. There is still a paucity of research into associated complications in Taiwan. The details of possible associated sequelae or complications with NoV are given below.
2.1. Chronic Gastroenteritis
Chronic NoV gastroenteritis is the major sequela of NoV infection in primary immune deficient and oncologic patients, transplant recipients, and those infected with the human immunodeficiency virus [22][23][27]. Chronic NoV gastroenteritis can present specific clinical challenges in immunocompromised patients. Immunosuppressed patients experience prolonged fecal NoV shedding [28]. NoV diarrhea in cancer patients can contribute to decreased quality of life, interruption of cancer care, malnutrition, and altered mucosal barrier function [29]; in primary immune deficient groups, it is associated with protracted diarrhea, weight loss, and the requirement of parenteral nutrition [30]. Transplant recipients have a similar risk for developing recurrent or chronic infection with NoV as other groups [31]. Immunocompromised patients with chronic NoV infection only have limited options beyond supportive care. For cancer or transplant patients, though only few data support this strategy, immunosuppression should be decreased [32]. There is currently no effective antiviral regimen for chronic NoV infections. Several treatments have been attempted, including serum-derived human immunoglobulin, Nitazoxanide, antiviral agents such as Favipiravir, adoptive T-cell therapy, fecal microbial transplantation, probiotics, and complex microbial communities with glycans with affinity to NoV [33][34][35][36][37]. Further studies are needed to determine if those strategies could be suitable therapies to treat chronic NoV.
2.2. Necrotizing Enterocolitis
The gut microbiota composition during infancy or early childhood is variable, dynamic, and influenced by both prenatal and postnatal factors [38]. Many studies have shown that intestinal viral infections may be associated with severe illness such as hemorrhagic enteritis and even necrotizing enterocolitis (NEC) [17][18][39][40]. Although studies mentioned previously indicated an episodic association of enteric viruses in NEC, one study by Skeath et al. [41] in 2016 for 17 NEC infants without detection of any related viruses may suggest that viral etiology is unlikely to be causative for most sporadic forms of NEC. Understanding of the mechanisms in microbiota-immunity-infectious agent axis is necessary to define potential preventive or therapeutic tools against significant infections in children [38].
2.3. Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a prolonged and disabling functional GI syndrome that affects 9–23% of the population across the world [42]. Acute gastroenteritis is a risk factor for postinfectious irritable bowel syndrome. The pathophysiology of IBS includes several possible mechanisms, such as visceral hypersensitivity, irregular gut motility, abnormal brain–gut relations, and the role of infectious agents; a systematic review showed similar risks for bacterial pathogens and indicated that studies are still limited for viral and parasitic pathogens [43]. There are already many studies showing that a number of pathogens correspond with the IBS disease, such as Clostridium difficile, Escherichia coli, Campylobacter jejuni, Chlamydia trachomatis, Helicobacter pylori, Pseudomonas aeruginosa, Salmonella spp., and Shigella spp.; viruses, particularly NoV; and even parasites [44]. Innate and adaptive immune mechanisms are involved in control of human NoV infection. NoVs may serve as viral triggers for IBS in some environmental and genetic contexts and also indicate that the microbiota may be important for NoV pathogenesis [45]. This still needs further work for understanding interactions between NoVs and bacteria in the gut and understanding the phenotypic outcomes from NoV mutation and evolution.
2.4. Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) includes ulcerative colitis and Crohn’s disease. The burden of IBD is rising globally, with substantial variation in levels and trends of the disease in different countries and regions. There are an estimated 6.8 million IBD cases worldwide, and the prevalence rates range from 79.5 to 84.3 per 100,000 individuals [46]. Although there is some evidence that NoV is involved in the dysregulation of enteric nervous and immune system [19][20] or exacerbation of IBD [21], IBD is influenced by gut microbiota, complex inflammatory pathways, and cell–virus interactions. IBD is also affected by confounding variables such as diet, age, smoking, or psychological stress, as demonstrated in monozygotic twins [47]. While the effect of cytomegalovirus infection on IBD has been confirmed, the role of other viruses in IBD pathogenesis or exacerbation of disease symptoms is still controversial [48][49]. A recent study by Tarris et al. [50] found a strong expression of sialylated Lewis a and Lewis x antigens and human NoV viral-like particles (VLPs) binding in the absence of ABO antigen expression in IBD regenerative mucosa. Further studies are required to explore the implications of NoV in the impairment of epithelial repair and dysregulation of inflammatory pathways [50]. Recent findings regarding human NoV replication in intestinal enteroids and organoids are promising [51][52]. The use of organoids derived from IBD patients might be useful to investigate the virus–host interactions and genetic responses related to NoV [53].
2.5. Convulsions and Encephalopathy
Some patients with acute gastroenteritis develop convulsions that may be febrile or afebrile convulsions [54]. Specific enteric pathogens have been linked to convulsions in children, such as rotavirus, NoV, Campylobacter, and Shigella [13][14][15][55][56][57][58]. The change in the prevalence of convulsions related to gastroenteritis might be associated with rotavirus vaccination [59]. Several reports have shown that NoV related to mild gastroenteritis causes convulsions without fever, severe dehydration, electrolyte imbalance, and hypoglycemia [13][60][61][62]. Severe central nervous system complications such as meningitis, encephalitis, and encephalopathy have been also described [14][15][16][63][64][65]. A study in the era of post-rotavirus vaccination in Taiwan for molecular epidemiology of NoV gastroenteritis showed that seizures occurred in 20.9% of children with NoV infection. GII.4 Den_Haag_2006b (42.3%) and GII.4 Sydney 2012 (19.2%) were major variants correlated with convulsions. Compared with GII.4 Den_Haag_2006b, the GII.4 Sydney 2012-associated convulsions had similar manifestations except without significant winter preponderance. The NoV infection with convulsions had less febrile course, specific genotype (GII.4) infections, and shorter instances of vomiting [66]. More studies and continuous surveillance are essential for uncommon convulsions associated with emerging NoV strain infections.
2.5.1. Benign Convulsions with Mild Gastroenteritis
Benign convulsions with mild gastroenteritis (CwG) were first reported by Morooka in 1982 [67]. CwG is characterized by afebrile convulsions within 5 days of acute viral gastroenteritis in previously healthy infants and children between the ages of 6 months and 3 years without electrolyte balance or abnormal blood sugar level or abnormal result of cerebrospinal fluid analysis. Several reports have broadened the range of definitions for children aged <6 years with CwG [61][62][68][69][70][71][72][73]. Convulsions in CwG are mostly generalized, usually clustered, and last less than 5 min. Although clustering seizures frequently occur in the acute phase of CwG, the prognosis is good and long-term treatment is usually not required [68].
After rotavirus vaccination, some changes in epidemiology and clinical characteristics occurred in NoV-associated CwG [61][62][71][72][74]. The ratio of NoV-associated CwG to NoV gastroenteritis and that of rotavirus-associated CwG to rotavirus gastroenteritis were different among studies [72][75]. One nationwide dataset from health insurance reviews and associated services in South Korea showed that the prevalence of rotavirus-associated CwG did not change (0.013–0.024%) but the annual prevalence of NoV-associated CwG is increasing by 1.790 times each year from 0.00001% [71][72]. However, these studies have limitations as they use diagnostic codes without compiling data directly from patient charts. Therefore, further studies are necessary to determine the actual prevalence after rotavirus vaccination.
There are many reports on the pathophysiological mechanism of CwG but most of them are for rotavirus [76][77][78][79][80][81]. Several studies have attempted to identify the mechanisms of NoV-associated CwG. NoV penetration into the gastrointestinal tract and RNA in the serum and cerebrospinal fluid have been observed [64][82]. There is still a paucity of studies on the pathophysiology of NoV-associated CwG.
2.5.2. Encephalopathy/Encephalitis
Encephalitis is associated with significant mortality despite intensive care [83]. There were few reports or case series, mainly from Japan, related to NoV-associated encephalopathy or encephalitis [16][64][65][84]. A nationwide survey of NoV-associated encephalitis/encephalopathy in Japan showed that the outcome of children with NoV-associated encephalitis was poor. Poor prognosis included early onset of neurological symptoms, an elevated serum creatinine level, and an abnormal blood glucose level [16]. Elevated concentrations of cerebrospinal fluid interleukin-6, interleukin-10, interferon-γ, and tumor necrosis factor-α indicated that the encephalopathy may be related to hypercytokinemia rather than direct viral invasion [84]. Future studies are required since there is no effective treatment and the definite pathophysiology of NoV-associated encephalitis/encephalopathy is still unknown.
2.6. Other Possible Associated Extraintestinal Complications
There have been reports that NoV infection is associated with Guillain–Barre syndrome and its variant Miller Fisher syndrome [85][86]. Although it is still unclear if NoV is the one of the antecedent infections in Guillain–Barre syndrome, it might be linked to the molecular mimicry [87]. As NoV serology is usually unavailable in routine clinical practice, further studies related to this response are needed.
References
- Xi, J.N.; Graham, D.Y.; Wang, K.N.; Estes, M.K. Norwalk Virus Genome Cloning and Characterization. Science 1990, 250, 1580–1583.
- Prasad, B.V.; Hardy, M.E.; Dokland, T.; Bella, J.; Rossmann, M.G.; Estes, M.K. X-Ray Crystallographic Structure of the Norwalk Virus Capsid. Science 1999, 286, 287–290.
- Glass, P.J.; White, L.J.; Ball, J.M.; Leparc-Goffart, I.; Hardy, M.E.; Estes, M.K. Norwalk Virus Open Reading Frame 3 Encodes a Minor Structural Protein. J. Virol. 2000, 74, 6581–6591.
- Bertolotti-Ciarlet, A.; White, L.J.; Chen, R.; Prasad, B.V.V.; Estes, M.K. Structural Requirements for the Assembly of Norwalk Virus-like Particles. J. Virol. 2002, 76, 4044–4055.
- Lochridge, V.P.; Hardy, M.E. A Single-Amino-Acid Substitution in the P2 Domain of VP1 of Murine Norovirus Is Sufficient for Escape from Antibody Neutralization. J. Virol. 2007, 81, 12316–12322.
- Tan, M.; Huang, P.; Meller, J.; Zhong, W.; Farkas, T.; Jiang, X. Mutations within the P2 Domain of Norovirus Capsid Affect Binding to Human Histo-Blood Group Antigens: Evidence for a Binding Pocket. J. Virol. 2003, 77, 12562–12571.
- Martella, V.; Medici, M.C.; De Grazia, S.; Tummolo, F.; Calderaro, A.; Bonura, F.; Saporito, L.; Terio, V.; Catella, C.; Lanave, G.; et al. Evidence for Recombination between Pandemic GII.4 Norovirus Strains New Orleans 2009 and Sydney 2012. J. Clin. Microbiol. 2013, 51, 3855–3857.
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373–381.
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated Classification of Norovirus Genogroups and Genotypes. J. Gen. Virol. 2019, 100, 1393–1406.
- Buesa, J.; Montava, R.; Abu-Mallouh, R.; Fos, M.; Ribes, J.M.; Bartolomé, R.; Vanaclocha, H.; Torner, N.; Domínguez, A. Sequential Evolution of Genotype GII.4 Norovirus Variants Causing Gastroenteritis Outbreaks from 2001 to 2006 in Eastern Spain. J. Med. Virol. 2008, 80, 1288–1295.
- Eden, J.-S.; Hewitt, J.; Lim, K.L.; Boni, M.F.; Merif, J.; Greening, G.; Ratcliff, R.M.; Holmes, E.C.; Tanaka, M.M.; Rawlinson, W.D.; et al. The Emergence and Evolution of the Novel Epidemic Norovirus GII.4 Variant Sydney 2012. Virology 2014, 450–451, 106–113.
- Mahar, J.E.; Bok, K.; Green, K.Y.; Kirkwood, C.D. The Importance of Intergenic Recombination in Norovirus GII.3 Evolution. J. Virol. 2013, 87, 3687–3698.
- Chen, S.-Y.; Tsai, C.-N.; Lai, M.-W.; Chen, C.-Y.; Lin, K.-L.; Lin, T.-Y.; Chiu, C.-H. Norovirus Infection as a Cause of Diarrhea-Associated Benign Infantile Seizures. Clin. Infect. Dis. 2009, 48, 849–855.
- Hu, M.-H.; Lin, K.-L.; Wu, C.-T.; Chen, S.-Y.; Huang, G.-S. Clinical Characteristics and Risk Factors for Seizures Associated with Norovirus Gastroenteritis in Childhood. J. Child Neurol. 2017, 32, 810–814.
- Ueda, H.; Tajiri, H.; Kimura, S.; Etani, Y.; Hosoi, G.; Maruyama, T.; Noma, H.; Kusumoto, Y.; Takano, T.; Baba, Y.; et al. Clinical Characteristics of Seizures Associated with Viral Gastroenteritis in Children. Epilepsy Res. 2015, 109, 146–154.
- Shima, T.; Okumura, A.; Kurahashi, H.; Numoto, S.; Abe, S.; Ikeno, M.; Shimizu, T. Norovirus-associated Encephalitis/Encephalopathy Collaborative Study investigators A Nationwide Survey of Norovirus-Associated Encephalitis/Encephalopathy in Japan. Brain Dev. 2019, 41, 263–270.
- Turcios-Ruiz, R.M.; Axelrod, P.; St John, K.; Bullitt, E.; Donahue, J.; Robinson, N.; Friss, H.E. Outbreak of Necrotizing Enterocolitis Caused by Norovirus in a Neonatal Intensive Care Unit. J. Pediatr. 2008, 153, 339–344.
- Stuart, R.L.; Tan, K.; Mahar, J.E.; Kirkwood, C.D.; Andrew Ramsden, C.; Andrianopoulos, N.; Jolley, D.; Bawden, K.; Doherty, R.; Kotsanas, D.; et al. An Outbreak of Necrotizing Enterocolitis Associated with Norovirus Genotype GII.3. Pediatr. Infect. Dis. J. 2010, 29, 644–647.
- Porter, C.K.; Faix, D.J.; Shiau, D.; Espiritu, J.; Espinosa, B.J.; Riddle, M.S. Postinfectious Gastrointestinal Disorders Following Norovirus Outbreaks. Clin. Infect. Dis. 2012, 55, 915–922.
- Zanini, B.; Ricci, C.; Bandera, F.; Caselani, F.; Magni, A.; Laronga, A.M.; Lanzini, A. San Felice del Benaco Study Investigators Incidence of Post-Infectious Irritable Bowel Syndrome and Functional Intestinal Disorders Following a Water-Borne Viral Gastroenteritis Outbreak. Am. J. Gastroenterol. 2012, 107, 891–899.
- Khan, R.R.; Lawson, A.D.; Minnich, L.L.; Martin, K.; Nasir, A.; Emmett, M.K.; Welch, C.A.; Udall, J.N. Gastrointestinal Norovirus Infection Associated with Exacerbation of Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 328–333.
- Haessler, S.; Granowitz, E.V. Norovirus Gastroenteritis in Immunocompromised Patients. N. Engl. J. Med. 2013, 368, 971.
- Woodward, J.; Gkrania-Klotsas, E.; Kumararatne, D. Chronic Norovirus Infection and Common Variable Immunodeficiency. Clin. Exp. Immunol. 2017, 188, 363–370.
- Miura, F.; Matsuyama, R.; Nishiura, H. Estimating the Asymptomatic Ratio of Norovirus Infection During Foodborne Outbreaks With Laboratory Testing in Japan. J. Epidemiol. 2018, 28, 382–387.
- Wang, P.-L.; Chen, S.-Y.; Tsai, C.-N.; Chao, H.-C.; Lai, M.-W.; Chang, Y.-J.; Chen, C.-L.; Chiu, C.-H. Complicated Norovirus Infection and Assessment of Severity by a Modified Vesikari Disease Score System in Hospitalized Children. BMC Pediatr. 2016, 16, 162.
- Vega, E.; Barclay, L.; Gregoricus, N.; Williams, K.; Lee, D.; Vinjé, J. Novel Surveillance Network for Norovirus Gastroenteritis Outbreaks, United States. Emerg. Infect. Dis. 2011, 17, 1389–1395.
- Fishman, J.A. Infections in Immunocompromised Hosts and Organ Transplant Recipients: Essentials. Liver Transplant. 2011, 17 (Suppl. 3), S34–S37.
- Henke-Gendo, C.; Harste, G.; Juergens-Saathoff, B.; Mattner, F.; Deppe, H.; Heim, A. New Real-Time PCR Detects Prolonged Norovirus Excretion in Highly Immunosuppressed Patients and Children. J. Clin. Microbiol. 2009, 47, 2855–2862.
- Kondapi, D.S.; Ramani, S.; Estes, M.K.; Atmar, R.L.; Okhuysen, P.C. Norovirus in Cancer Patients: A Review. Open Forum Infect. Dis. 2021, 8, ofab126.
- Brown, L.-A.K.; Clark, I.; Brown, J.R.; Breuer, J.; Lowe, D.M. Norovirus Infection in Primary Immune Deficiency. Rev. Med. Virol. 2017, 27, e1926.
- Abbas, A.; Zimmer, A.J.; Florescu, D. Viral Enteritis in Solid-Organ Transplantation. Viruses 2021, 13, 2019.
- Boillat Blanco, N.; Kuonen, R.; Bellini, C.; Manuel, O.; Estrade, C.; Mazza-Stalder, J.; Aubert, J.D.; Sahli, R.; Meylan, P. Chronic Norovirus Gastroenteritis in a Double Hematopoietic Stem Cell and Lung Transplant Recipient. Transpl. Infect. Dis. 2011, 13, 213–215.
- Gairard-Dory, A.-C.; Dégot, T.; Hirschi, S.; Schuller, A.; Leclercq, A.; Renaud-Picard, B.; Gourieux, B.; Kessler, R. Clinical Usefulness of Oral Immunoglobulins in Lung Transplant Recipients with Norovirus Gastroenteritis: A Case Series. Transplant. Proc. 2014, 46, 3603–3605.
- Keeffe, E.B.; Rossignol, J.-F. Treatment of Chronic Viral Hepatitis with Nitazoxanide and Second Generation Thiazolides. World J. Gastroenterol. 2009, 15, 1805–1808.
- Ruis, C.; Brown, L.-A.K.; Roy, S.; Atkinson, C.; Williams, R.; Burns, S.O.; Yara-Romero, E.; Jacobs, M.; Goldstein, R.; Breuer, J.; et al. Mutagenesis in Norovirus in Response to Favipiravir Treatment. N. Engl. J. Med. 2018, 379, 2173–2176.
- Muftuoglu, M.; Olson, A.; Marin, D.; Ahmed, S.; Mulanovich, V.; Tummala, S.; Chi, T.L.; Ferrajoli, A.; Kaur, I.; Li, L.; et al. Allogeneic BK Virus-Specific T Cells for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2018, 379, 1443–1451.
- Barberio, B.; Massimi, D.; Bonfante, L.; Facchin, S.; Calò, L.; Trevenzoli, M.; Savarino, E.V.; Cattelan, A.M. Fecal Microbiota Transplantation for Norovirus Infection: A Clinical and Microbiological Success. Ther. Adv. Gastroenterol. 2020, 13, 1756284820934589.
- George, S.; Aguilera, X.; Gallardo, P.; Farfán, M.; Lucero, Y.; Torres, J.P.; Vidal, R.; O’Ryan, M. Bacterial Gut Microbiota and Infections During Early Childhood. Front. Microbiol. 2021, 12, 793050.
- Bagci, S.; Eis-Hübinger, A.M.; Yassin, A.F.; Simon, A.; Bartmann, P.; Franz, A.R.; Mueller, A. Clinical Characteristics of Viral Intestinal Infection in Preterm and Term Neonates. Eur. J. Clin. Microbiol. 2010, 29, 1079–1084.
- Pelizzo, G.; Nakib, G.; Goruppi, I.; Fusillo, M.; Scorletti, F.; Mencherini, S.; Parigi, G.B.; Stronati, M.; Calcaterra, V. Isolated Colon Ischemia with Norovirus Infection in Preterm Babies: A Case Series. J. Med. Case Rep. 2013, 7, 108.
- Skeath, T.; Stewart, C.; Waugh, S.; Embleton, N.; Cummings, S.; Berrington, J. Cytomegalovirus and Other Common Enteric Viruses Are Not Commonly Associated with NEC. Acta Paediatr. 2016, 105, 50–52.
- Saha, L. Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence-Based Medicine. World J. Gastroenterol. 2014, 20, 6759–6773.
- Svendsen, A.T.; Bytzer, P.; Engsbro, A.L. Systematic Review with Meta-Analyses: Does the Pathogen Matter in Post-Infectious Irritable Bowel Syndrome? Scand. J. Gastroenterol. 2019, 54, 546–562.
- Shariati, A.; Fallah, F.; Pormohammad, A.; Taghipour, A.; Safari, H.; Chirani, A.S.; Sabour, S.; Alizadeh-Sani, M.; Azimi, T. The Possible Role of Bacteria, Viruses, and Parasites in Initiation and Exacerbation of Irritable Bowel Syndrome. J. Cell. Physiol. 2019, 234, 8550–8569.
- Hassan, E.; Baldridge, M.T. Norovirus Encounters in the Gut: Multifaceted Interactions and Disease Outcomes. Mucosal Immunol. 2019, 12, 1259–1267.
- GBD 2017 Inflammatory Bowel Disease Collaborators The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30.
- Tarris, G.; de Rougemont, A.; Charkaoui, M.; Michiels, C.; Martin, L.; Belliot, G. Enteric Viruses and Inflammatory Bowel Disease. Viruses 2021, 13, 104.
- Al-Zafiri, R.; Gologan, A.; Galiatsatos, P.; Szilagyi, A. Cytomegalovirus Complicating Inflammatory Bowel Disease: A 10-Year Experience in a Community-Based, University-Affiliated Hospital. Gastroenterol. Hepatol. 2012, 8, 230–239.
- Römkens, T.E.H.; Bulte, G.J.; Nissen, L.H.C.; Drenth, J.P.H. Cytomegalovirus in Inflammatory Bowel Disease: A Systematic Review. World J. Gastroenterol. 2016, 22, 1321–1330.
- Tarris, G.; de Rougemont, A.; Estienney, M.; Charkaoui, M.; Mouillot, T.; Bonnotte, B.; Michiels, C.; Martin, L.; Belliot, G. Specific Norovirus Interaction with Lewis x and Lewis a on Human Intestinal Inflammatory Mucosa during Refractory Inflammatory Bowel Disease. mSphere 2021, 6, e01185-20.
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.-L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393.
- Zhang, D.; Tan, M.; Zhong, W.; Xia, M.; Huang, P.; Jiang, X. Human Intestinal Organoids Express Histo-Blood Group Antigens, Bind Norovirus VLPs, and Support Limited Norovirus Replication. Sci. Rep. 2017, 7, 12621.
- Dotti, I.; Mora-Buch, R.; Ferrer-Picón, E.; Planell, N.; Jung, P.; Masamunt, M.C.; Leal, R.F.; Martín de Carpi, J.; Llach, J.; Ordás, I.; et al. Alterations in the Epithelial Stem Cell Compartment Could Contribute to Permanent Changes in the Mucosa of Patients with Ulcerative Colitis. Gut 2017, 66, 2069–2079.
- Iflah, M.; Kassem, E.; Rubinstein, U.; Goren, S.; Ephros, M.; Cohen, D.; Muhsen, K. Convulsions in Children Hospitalized for Acute Gastroenteritis. Sci. Rep. 2021, 11, 15874.
- Ma, X.; Luan, S.; Zhao, Y.; Lv, X.; Zhang, R. Clini.ical Characteristics and Follow-up of Benign Convulsions with Mild Gastroenteritis among Children. Medicine 2019, 98, e14082.
- Kang, B.; Kwon, Y.S. Benign Convulsion with Mild Gastroenteritis. Korean J. Pediatr. 2014, 57, 304–309.
- Hiranrattana, A.; Mekmullica, J.; Chatsuwan, T.; Pancharoen, C.; Thisyakorn, U. Childhood Shigellosis at King Chulalongkorn Memorial Hospital, Bangkok, Thailand: A 5-Year Review (1996–2000). Southeast Asian J. Trop. Med. Public Health 2005, 36, 683–685.
- Solomon, N.H.; Lavie, S.; Tenney, B.L.; Blaser, M.J. Campylobacter Enteritis Presenting with Convulsions. Clin. Pediatr. 1982, 21, 118–119.
- Lu, M.-C.; Shia, B.-C.; Kao, Y.-W.; Lin, S.-C.; Wang, C.-Y.; Lin, W.-C.; Chen, S.-Y. The Impact of Rotavirus Vaccination in the Prevalence of Gastroenteritis and Comorbidities among Children after Suboptimal Rotavirus Vaccines Implementation in Taiwan: A Population-Based Study. Medicine 2021, 100, e25925.
- Komori, H.; Wada, M.; Eto, M.; Oki, H.; Aida, K.; Fujimoto, T. Benign Convulsions with Mild Gastroenteritis: A Report of 10 Recent Cases Detailing Clinical Varieties. Brain Dev. 1995, 17, 334–337.
- Kim, G.-H.; Byeon, J.H.; Lee, D.-Y.; Jeong, H.J.; Eun, B.-L. Norovirus in Benign Convulsions with Mild Gastroenteritis. Ital. J. Pediatr. 2016, 42, 94.
- Kim, B.R.; Choi, G.E.; Kim, Y.O.; Kim, M.J.; Song, E.S.; Woo, Y.J. Incidence and Characteristics of Norovirus-Associated Benign Convulsions with Mild Gastroenteritis, in Comparison with Rotavirus Ones. Brain Dev. 2018, 40, 699–706.
- Bartolini, L.; Mardari, R.; Toldo, I.; Calderone, M.; Battistella, P.A.; Laverda, A.M.; Sartori, S. Norovirus Gastroenteritis and Seizures: An Atypical Case with Neuroradiological Abnormalities. Neuropediatrics 2011, 42, 167–169.
- Ito, S.; Takeshita, S.; Nezu, A.; Aihara, Y.; Usuku, S.; Noguchi, Y.; Yokota, S. Norovirus-Associated Encephalopathy. Pediatr. Infect. Dis. J. 2006, 25, 651–652.
- Sánchez-Fauquier, A.; González-Galán, V.; Arroyo, S.; Rodà, D.; Pons, M.; García, J.-J. Norovirus-Associated Encephalitis in a Previously Healthy 2-Year-Old Girl. Pediatr. Infect. Dis. J. 2015, 34, 222–223.
- Tsai, C.-N.; Lin, C.-Y.; Lin, C.-W.; Shih, K.-C.; Chiu, C.-H.; Chen, S.-Y. Clinical Relevance and Genotypes of Circulating Noroviruses in Northern Taiwan, 2006–2011. J. Med Virol. 2014, 86, 335–346.
- Morooka, K. Convulsions and mild diarrhea. Shonika 1982, 23, 131–137.
- Lee, Y.S.; Lee, G.H.; Kwon, Y.S. Update on Benign Convulsions with Mild Gastroenteritis. Clin. Exp. Pediatr. 2021. published online ahead of print.
- Hung, J.-J.; Wen, H.-Y.; Yen, M.-H.; Chen, H.-W.; Yan, D.-C.; Lin, K.-L.; Lin, S.-J.; Lin, T.-Y.; Hsu, C.-Y. Rotavirus Gastroenteritis Associated with Afebrile Convulsion in Children: Clinical Analysis of 40 Cases. Chang. Gung Med. J. 2003, 26, 654–659.
- Uemura, N.; Okumura, A.; Negoro, T.; Watanabe, K. Clinical Features of Benign Convulsions with Mild Gastroenteritis. Brain Dev. 2002, 24, 745–749.
- Kim, D.H.; Lee, Y.S.; Ha, D.J.; Chun, M.J.; Kwon, Y.S. Epidemiology of Rotavirus Gastroenteritis and Rotavirus-Associated Benign Convulsions with Mild Gastroenteritis after the Introduction of Rotavirus Vaccines in South Korea: Nationwide Data from the Health Insurance Review and Assessment Service. Int. J. Environ. Res. Public Health 2020, 17, E8374.
- Kim, D.H.; Ha, D.J.; Lee, Y.S.; Chun, M.J.; Kwon, Y.S. Benign Convulsions with Mild Rotavirus and Norovirus Gastroenteritis: Nationwide Data from the Health Insurance Review and Assessment Service in South Korea. Children 2021, 8, 263.
- Verrotti, A.; Nanni, G.; Agostinelli, S.; Parisi, P.; Capovilla, G.; Beccaria, F.; Iannetti, P.; Spalice, A.; Coppola, G.; Franzoni, E.; et al. Benign Convulsions Associated with Mild Gastroenteritis: A Multicenter Clinical Study. Epilepsy Res. 2011, 93, 107–114.
- Lloyd, M.B.; Lloyd, J.C.; Gesteland, P.H.; Bale, J.F. Rotavirus Gastroenteritis and Seizures in Young Children. Pediatr. Neurol. 2010, 42, 404–408.
- Chan, C.V.; Chan, C.D.; Ma, C.; Chan, H. Norovirus as Cause of Benign Convulsion Associated with Gastro-Enteritis. J. Paediatr. Child Health 2011, 47, 373–377.
- Liu, B.; Fujita, Y.; Arakawa, C.; Kohira, R.; Fuchigami, T.; Mugishima, H.; Kuzuya, M. Detection of Rotavirus RNA and Antigens in Serum and Cerebrospinal Fluid Samples from Diarrheic Children with Seizures. Jpn. J. Infect. Dis. 2009, 62, 279–283.
- Nishimura, S.; Ushijima, H.; Nishimura, S.; Shiraishi, H.; Kanazawa, C.; Abe, T.; Kaneko, K.; Fukuyama, Y. Detection of Rotavirus in Cerebrospinal Fluid and Blood of Patients with Convulsions and Gastroenteritis by Means of the Reverse Transcription Polymerase Chain Reaction. Brain Dev. 1993, 15, 457–459.
- Blutt, S.E.; Kirkwood, C.D.; Parreño, V.; Warfield, K.L.; Ciarlet, M.; Estes, M.K.; Bok, K.; Bishop, R.F.; Conner, M.E. Rotavirus Antigenaemia and Viraemia: A Common Event? Lancet 2003, 362, 1445–1449.
- Weclewicz, K.; Svensson, L.; Kristensson, K. Targeting of Endoplasmic Reticulum-Associated Proteins to Axons and Dendrites in Rotavirus-Infected Neurons. Brain Res. Bull. 1998, 46, 353–360.
- Díaz, Y.; Chemello, M.E.; Peña, F.; Aristimuño, O.C.; Zambrano, J.L.; Rojas, H.; Bartoli, F.; Salazar, L.; Chwetzoff, S.; Sapin, C.; et al. Expression of Nonstructural Rotavirus Protein NSP4 Mimics Ca2+ Homeostasis Changes Induced by Rotavirus Infection in Cultured Cells. J. Virol. 2008, 82, 11331–11343.
- Yeom, J.S.; Kim, Y.-S.; Jun, J.-S.; Do, H.J.; Park, J.S.; Seo, J.-H.; Park, E.S.; Lim, J.-Y.; Woo, H.-O.; Park, C.-H.; et al. NSP4 Antibody Levels in Rotavirus Gastroenteritis Patients with Seizures. Eur. J. Paediatr. Neurol. 2017, 21, 367–373.
- Medici, M.C.; Abelli, L.A.; Dodi, I.; Dettori, G.; Chezzi, C. Norovirus RNA in the Blood of a Child with Gastroenteritis and Convulsions--A Case Report. J. Clin. Virol. 2010, 48, 147–149.
- Hon, K.-L.E.; Tsang, Y.C.K.; Chan, L.C.N.; Tsang, H.W.; Wong, K.Y.K.; Wu, Y.H.G.; Chan, P.K.S.; Cheung, K.L.; Ng, E.Y.K.; Totapally, B.R. Outcome of Encephalitis in Pediatric Intensive Care Unit. Indian J. Pediatr. 2016, 83, 1098–1103.
- Obinata, K.; Okumura, A.; Nakazawa, T.; Kamata, A.; Niizuma, T.; Kinoshita, K.; Shimizu, T. Norovirus Encephalopathy in a Previously Healthy Child. Pediatr. Infect. Dis. J. 2010, 29, 1057–1059.
- Eltayeb, K.G.; Crowley, P. Guillain-Barre Syndrome Associated with Norovirus Infection. BMJ Case Rep. 2012, 2012, bcr0220125865.
- Shimizu, T.; Tokuda, Y. Miller Fisher Syndrome Linked to Norovirus Infection. BMJ Case Rep. 2012, 2012, bcr2012007776.
- Ang, C.W.; Jacobs, B.C.; Laman, J.D. The Guillain-Barré Syndrome: A True Case of Molecular Mimicry. Trends Immunol. 2004, 25, 61–66.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
14 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




