| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Caixia Liu | + 1614 word(s) | 1614 | 2020-09-17 08:14:59 | | | |
| 2 | Nora Tang | Meta information modification | 1614 | 2020-11-09 08:38:44 | | |
Video Upload Options
The selective catalytic reduction (SCR) has been widely used in industrial denitrification owing to its high denitrification efficiency, low operating costs, and simple operating procedures. However, coal containing a large amount of sulfur will produce SO2 during combustion, which makes the catalyst easy to be deactivated, thus limiting the application of this technology. This review summarizes the latest NH3-SCR reaction mechanisms and the deactivation mechanism of catalyst in SO2-containing flue gas. Some strategies are summarized for enhancing the poison-resistance through modification, improvement of support, the preparation of complex oxide catalyst, optimizing the preparation methods, and acidification. The mechanism of improving sulfur resistance of catalysts at low temperatures is summarized, and the further development of the catalyst is also prospected. This paper could provide a reference and guidance for the development of SO2 resistance of the catalyst at low temperatures.
1. Introduction
Nitrogen oxide (NOx) is a general term composed of nitrogen, oxygen, and other compounds. It is one of the major pollutants from the exhaust gas of thermal power plants, industrial furnaces, motor vehicles, ship exhaust emissions, and includes N2O, NO, NO2, etc.—among which NO and NO2 account for the largest proportion[1]. A large amount of NOx emitted into the air will cause a series of environmental concerns. Therefore, exploring and developing efficient exhaust gas deNOx technology has been an area of intense investigation. Among all flue gas denitrification technologies, selective catalytic reduction (SCR) is an extensively applied technology due to its low reaction temperature and high denitrification efficiency[2][3]. Selective catalytic reduction (SCR) mainly refers to the reaction of NOx using NH3 as a reducing agent in the presence of O2 to produce pollution-free N2 and H2O, whose core is the catalyst.
2. SO2 Poisoning Mechanism of Low-Temperature Catalyst
At present, some power plants adopt wet desulfurization to remove SO2 with lower flue gas temperature, failing to meet the reaction requirements of V2O5/TiO2 catalyst. Therefore, there are many drawbacks such as low denitrification efficiency and catalyst waste. After desulfurization, tiny amounts of SO2 still exist in the exhaust gas, bringing about the deactivation of SCR catalyst. Therefore, developing a vanadium-free catalyst with great denitrification performance and sulfur and water resistance at low temperatures is extremely necessary[4]. To solve the sulfur poisoning of catalysts, many scholars have done a lot of research and elaborated on the poisoning mechanism in detail.
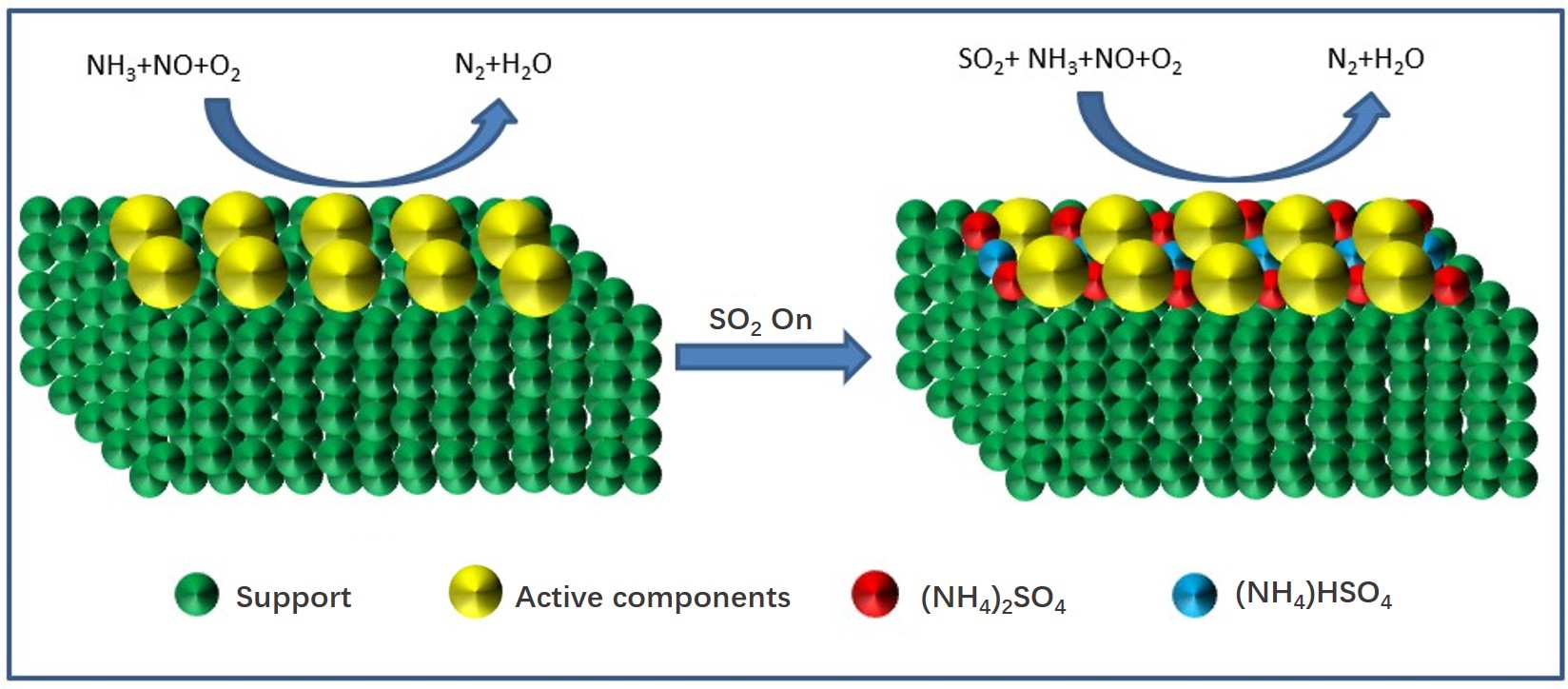
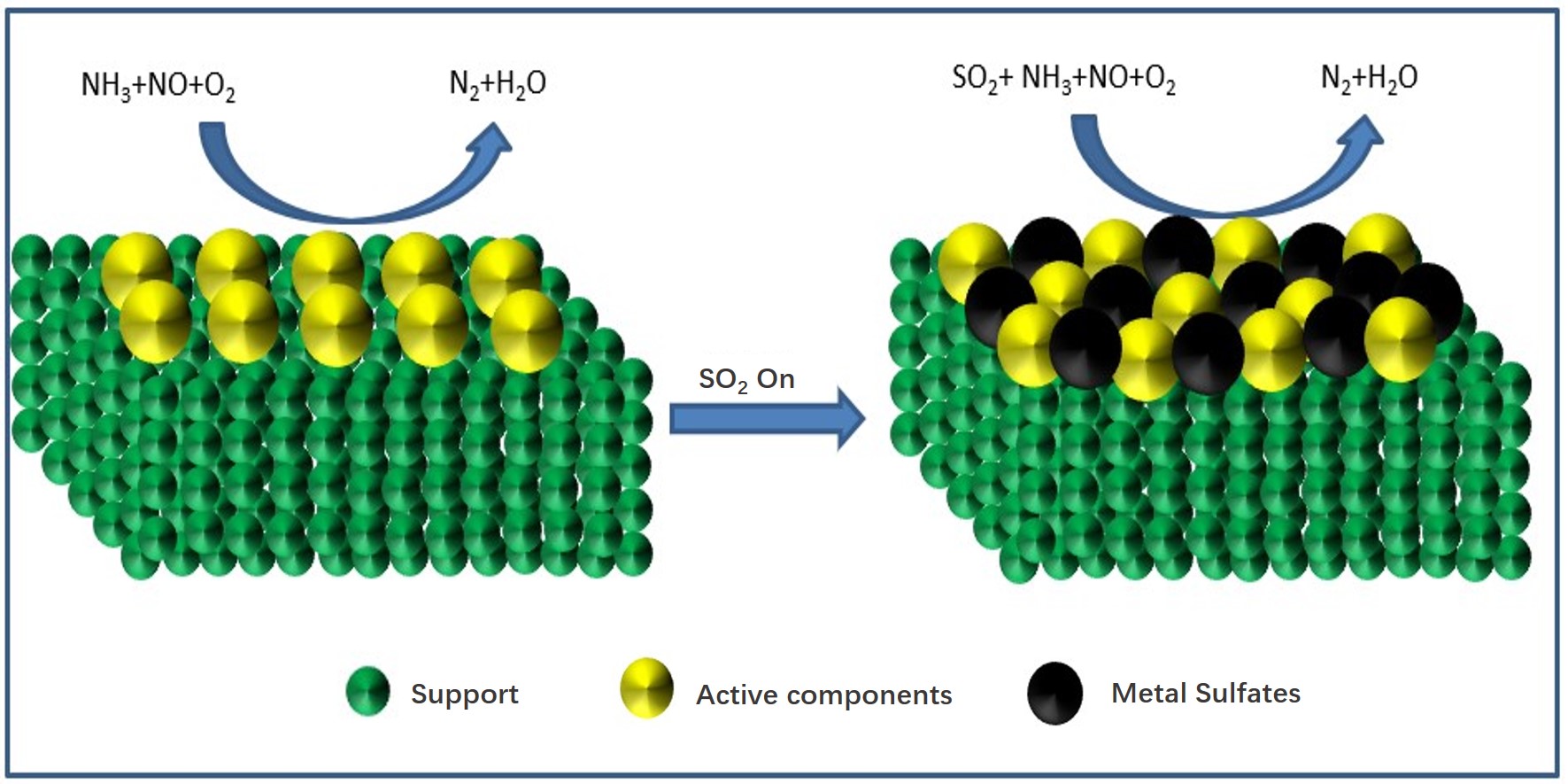
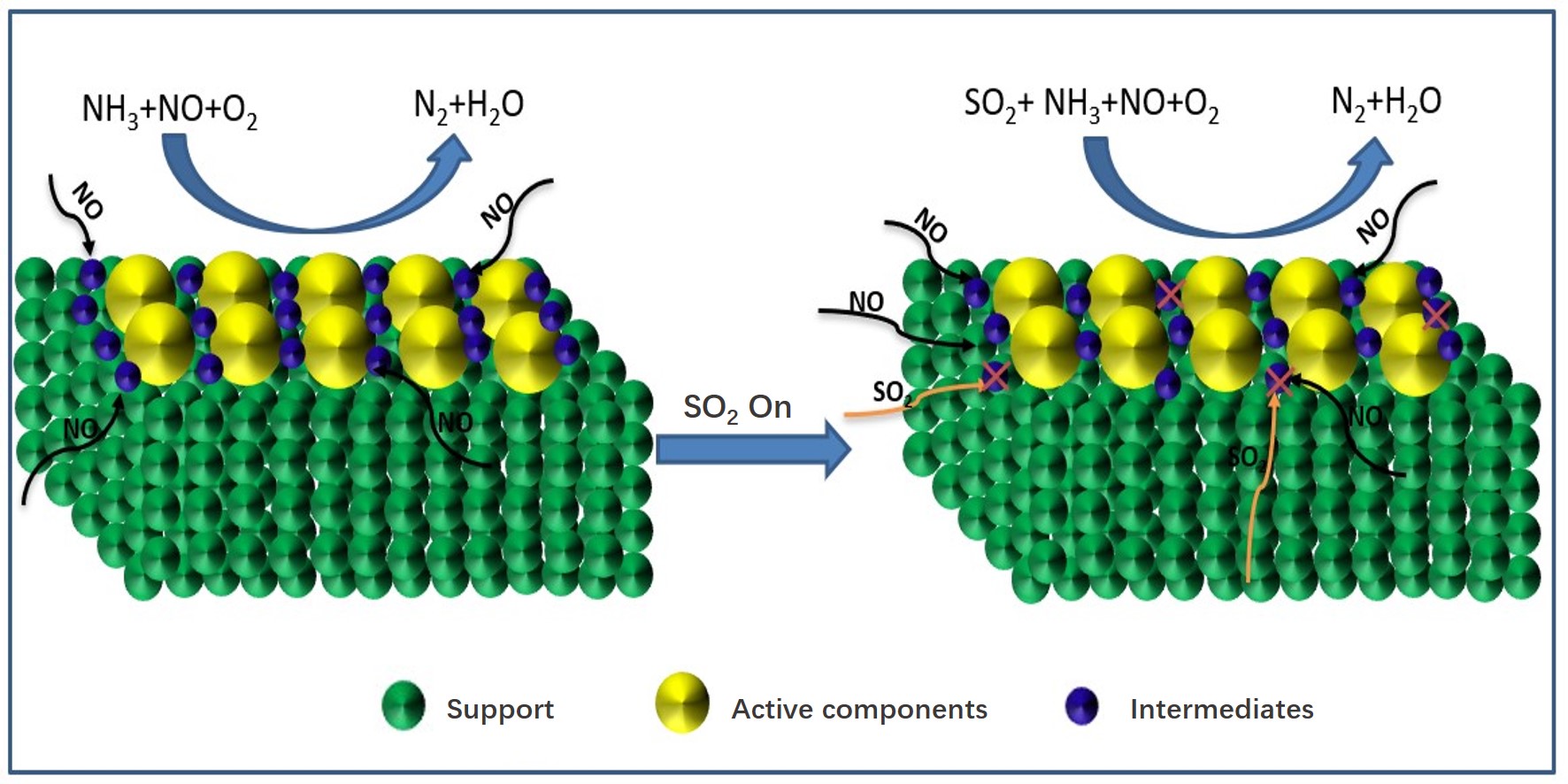
From the above studies, the SO2 deactivation mechanism on the catalyst at low temperatures can be observed mainly in the following three aspects. (1) The ammonium sulfate and ammonium bisulfate are formed by the reaction of SO2 and NH3 in the presence of O2 and attach to the catalyst surface, which can decrease the surface area, pore volume, and pore size of the catalyst, and then reduce the reaction rate. However, ammonium sulfate and ammonium bicarbonate will self-decompose when the NH3-SCR reaction is carried out above 280 °C and 350 °C, respectively, so the catalytic activity is able to be restored by the washing method at low temperatures[5]. (2) In the presence of O2, SO2 will react with the active component (mainly transition metal) on the catalyst surface to generate metal sulfate salt, which will cause irreversible deactivation of the catalyst. (3) SO2 will compete with NO at the adsorption sites on the catalyst surface when these acidic gases are present in the reaction system, which would reduce the formation of SCR intermediate products and the catalytic efficiency of catalyst. Figure 1-3 show the mechanism of catalyst sulfur poisoning.
Figure 1. The formation process of (NH4)2SO4 and NH4HSO4.
Figure 2. Sulfation of active components.
Figure 3. Competitive adsorption of NO and SO2.
3. Research Progress of SO2 Resistance Catalyst at Low Temperatures
Catalyst is usually composed of active component and support, and there are other forms of catalysts such as composite oxide catalysts. To enhance the low-temperature sulfur resistance of catalysts, many scholars have focused their attention on the improvement of active components and supports. In addition, some scholars have found that the preparation method—the handling catalyst by acidification and reaction conditions—can make a difference in the SO2 resistance of catalyst.
3.1. Effects of Active Components
The active component, which is composed of one or more substances, is the main unit of catalyst and affects the NH3-SCR reaction significantly. Using rare earth metals as well as transition metal oxides to improve active components is one of the most effective methods to improve sulfur resistance at low temperatures. We mainly summarized the SCR performance and/or SO2 resistance mechanism of catalysts modified with Ce, Fe, Cu, W and other metal elements, as shown in Figure 4-9.
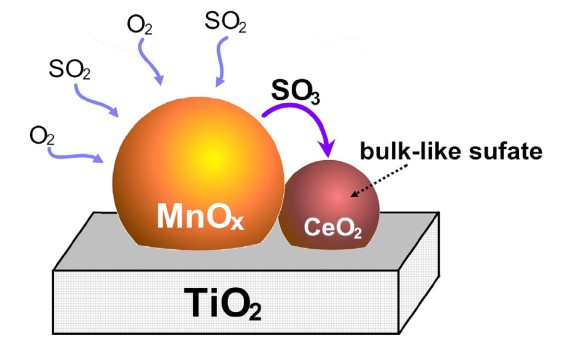
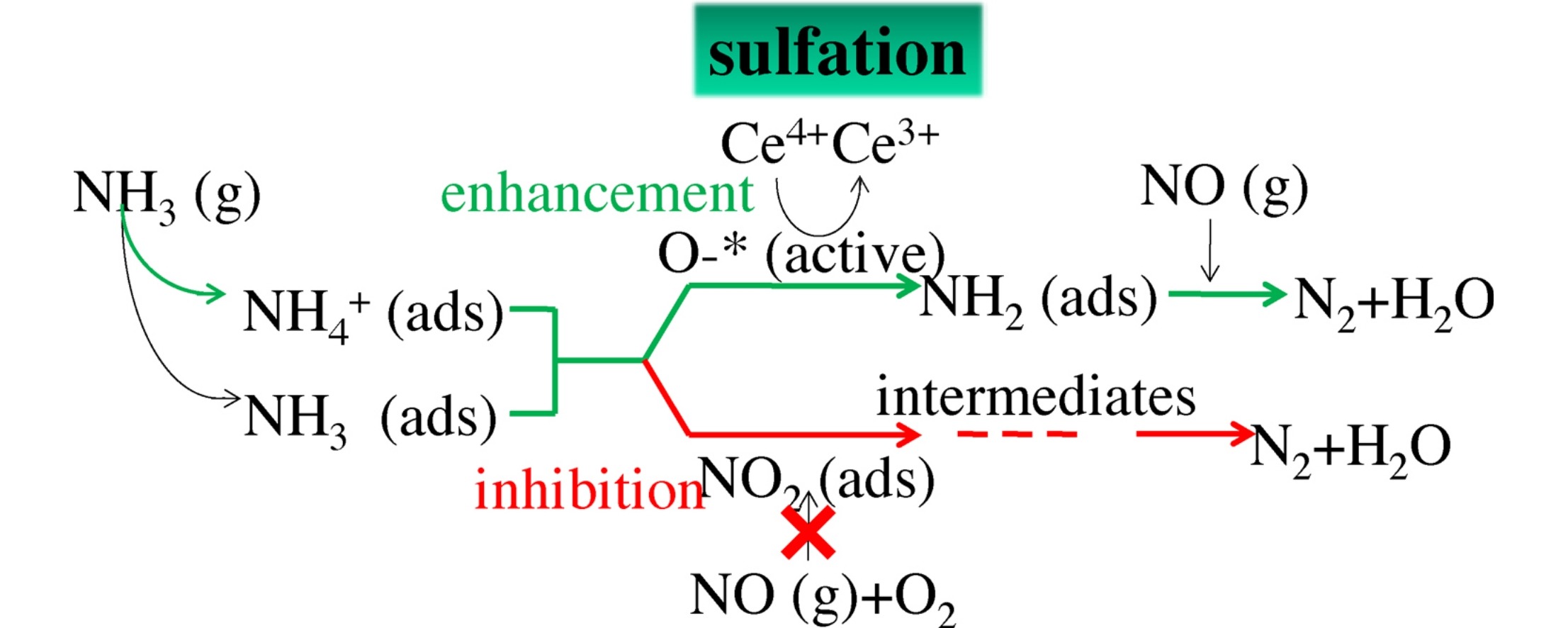
Figure 4. The sulfation mechanism of Ce-modified Mn–Ce/TiO2 catalyst[6].
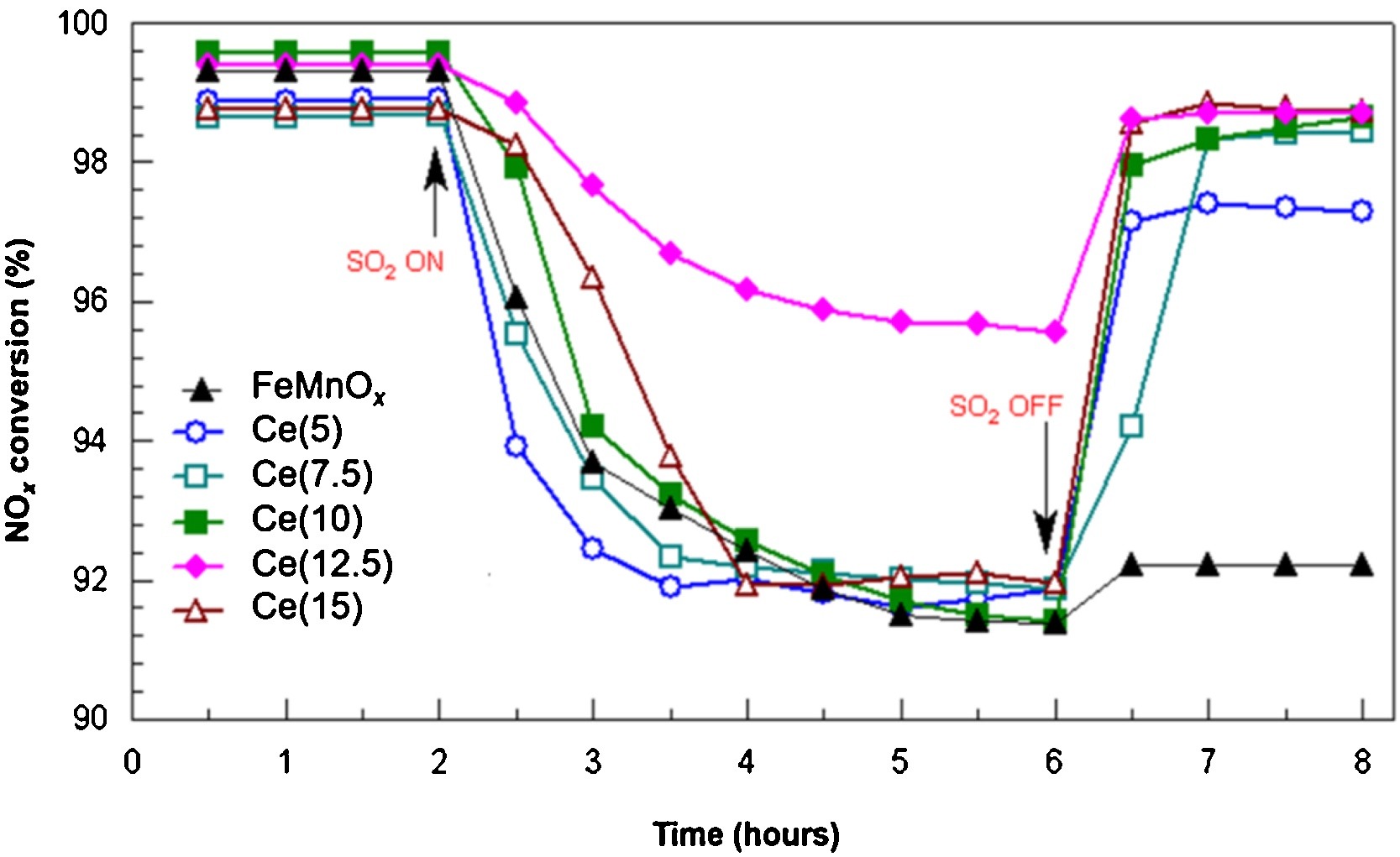
Figure 5. Influence of SO2 on NOx conversion of FeMnOx and Ce(y) catalysts. Reaction conditions: [NO] = [NH3] = 0.1%; [SO2] = 100 ppm; [O2] = 3%; N2 balance, GHSV = 30,000 h−1; reaction temperature = 120 °C[7].
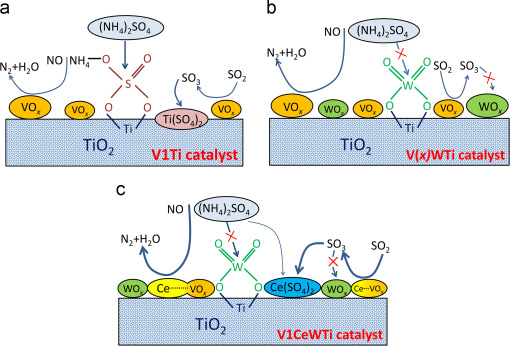
Figure 6. The mechanism of sulfur poisoning at low temperatures of catalysts (a) V1Ti; (b) V(x)WTi; (c) V1CeWTi[8].
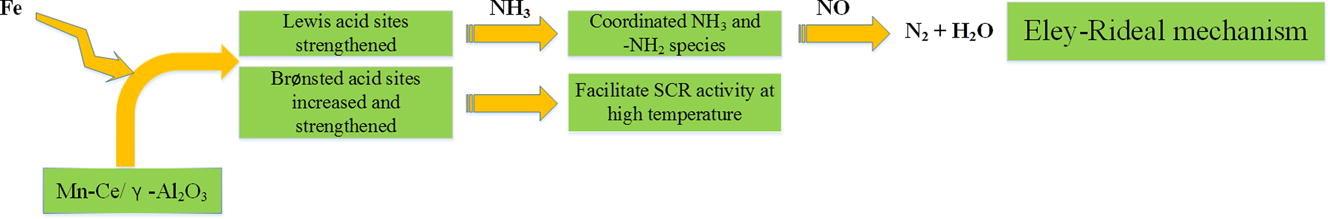
Figure 7. The reaction flow chart of Fe–Mn–Ce/γ-Al2O3 catalyst[9].
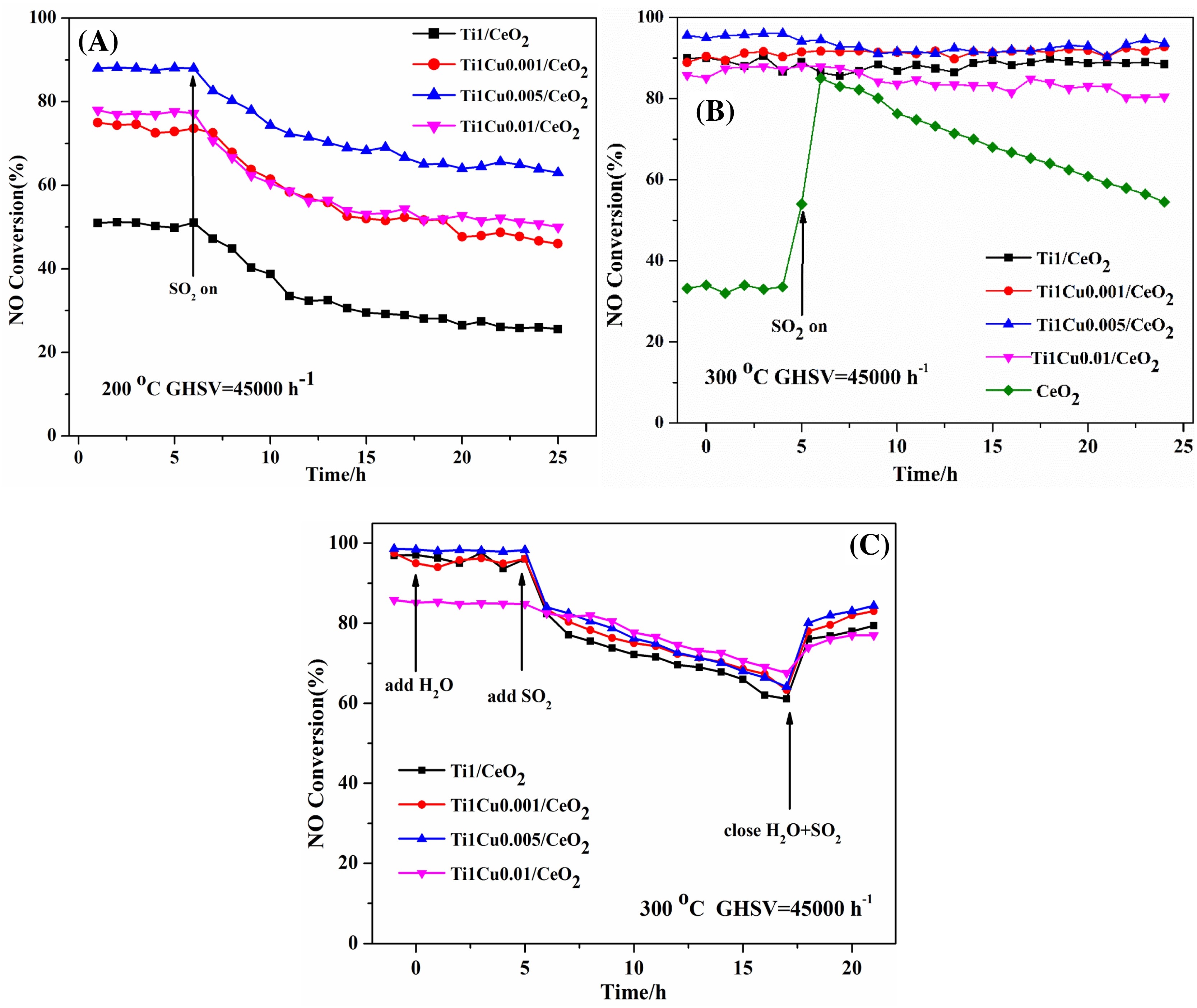
Figure 8. SO2 durability of catalysts (A) SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 catalysts at 200 °C (B) SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 catalysts at 300 °C. (C) H2O/H2O + SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 at 300 °C[10].
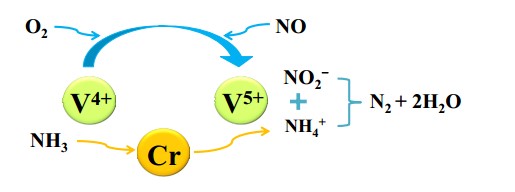
Figure 9. Mechanism of Cr doping improving sulfur resistance of Cr–V/TiO2 catalyst[11].
Above all, the content of oxygen adsorbed on the active material and catalyst surface—and even the number of acid sites—could be increased by adding Ce, Fe, Cu, W, and others to the active components, which would accelerate the rapid reaction of SCR and increase the catalytic performance of catalyst. Under the reaction conditions containing SO2, these additives can preferentially react with SO2 as the SO2 catchers to avoid the sulfation of active substances. Besides, the stability of NH4HSO4 and (NH4)2SO4 on the surface was reduced, and the low-temperature sulfur tolerance of catalyst was effectively improved. It is believed that transition metals and rare earth elements will have promising applications in improving sulfur resistance of catalyst at low temperatures. Figure 10 shows the typical SO2-tolerant modified catalyst at low temperatures for selective catalytic reduction (SCR) reaction.
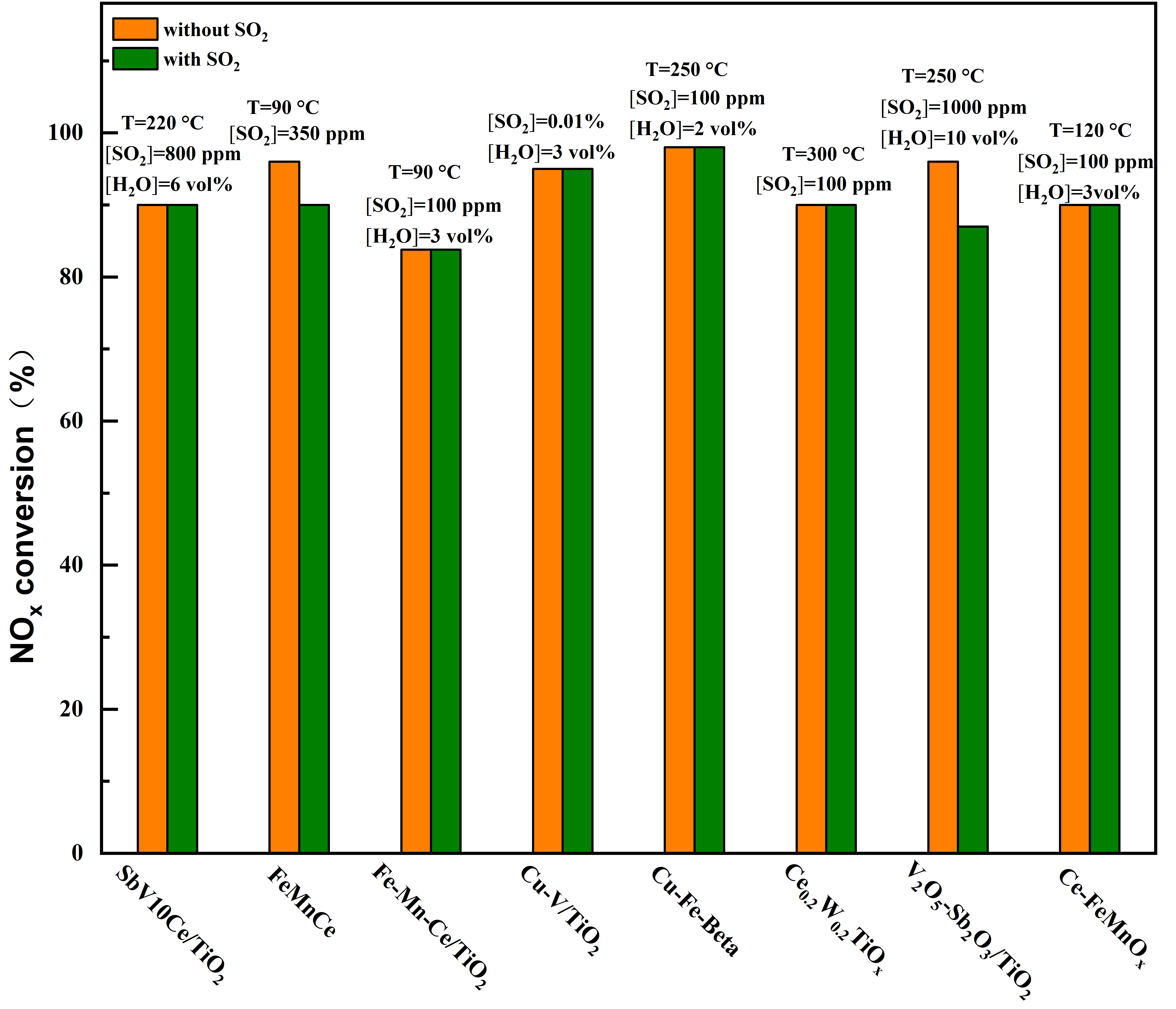
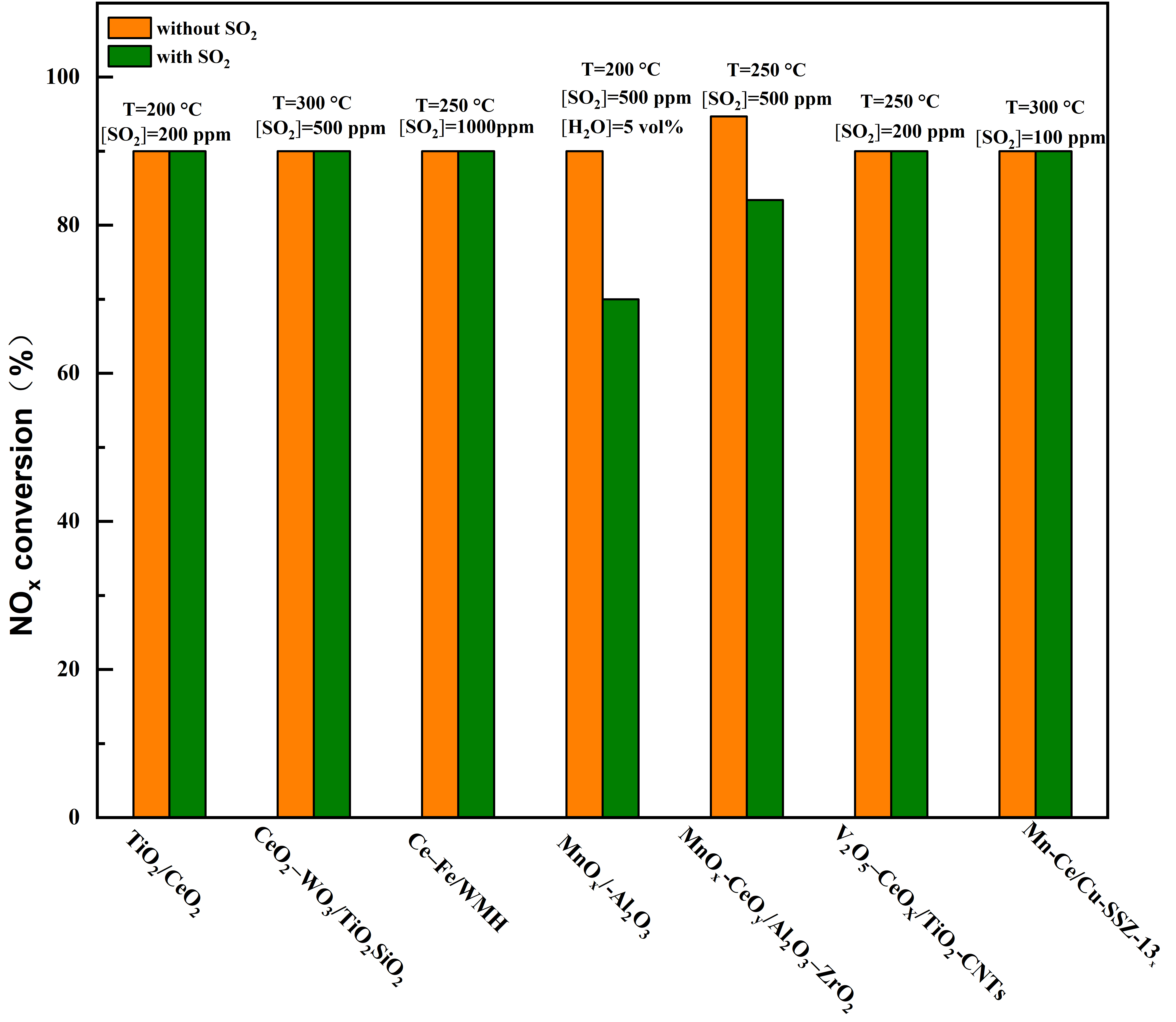
Figure 10. Typical SO2-tolerant modified catalyst at low temperatures for selective catalytic reduction (SCR) reaction[7][12][9][13][14][15][16][17].
3.2. Effects of Supports
The support of catalyst is of vital importance in the catalytic activity. Loading the active components onto the support contributes to improving the specific surface area, thermal resistance, and mechanical strength of catalyst. Especially, the supports can slow down SO2 poisoning on the catalytic activity of catalyst. As of now, there are TiO2, Al2O3, activated carbon and zeolites, and other conventional supports in practical application, as shown in Figure 11 and Figure 12. However, different supports show different catalytic activity and sulfur resistance, which makes the research and improvement of the support become a part of the emphasis of research to improve the low-temperature SO2 resistance of catalyst. Figure 13 shows the typical SO2-tolerant catalysts with different supports at low temperatures for SCR reaction.
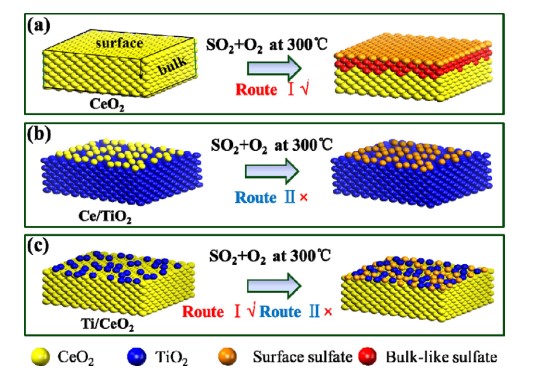
Figure 11. Sulfur resistance mechanism of (a) CeO2, (b) Ce/Ti and (c) Ti/Ce catalyst[18].
Figure 12. Reaction mechanism of Ce–Fe/WMH catalyst[19].
Figure 13. Typical SO2-tolerant catalysts with different supports at low temperatures for SCR reaction[18][20][19][21][22][23][24].
3.3. Composite Oxide Catalysts
In recent years, extensive work has been done in the area of composite oxide catalyst. Compared with the supported catalysts, these catalysts all use metal oxides and have no clear support or active components. Many achievements have been made in the research of the catalytic performance and sulfur tolerance of catalyst.
3.4. Other Strategies to Improve the SO2 Resistance
Among all the measures to enhance the sulfur resistance of the catalyst, some researchers have tried to find the influence of preparation methods,acidification,preparation and reaction conditions, further promoting the acidity and catalytic efficiency of catalyst.
4. Conclusions and Perspectives
Facing progressively strict legislation and policies to control NO emission, the research and design of low-temperature catalysts for NH3-SCR have received a great deal of attention. Although the poisoning mechanism of catalyst at low temperatures has been studied thoroughly, how to maintain the high catalytic efficiency of catalyst at low temperatures and promote the SO2-resistance-poisoning ability of catalyst to achieve practical application is still an urgent problem.
In the presence of O2, SO3 is easily formed on the catalyst due to the oxidation reaction of SO2, and further combined with NH3 to produce NH4HSO4 and/or (NH4)2SO4, which can deposit on the surface of catalyst and inhibit the reaction gas to be adsorbed on the catalyst to participate in the SCR reaction. Besides, sulfate of active components can be formed and cause irreversible deactivation of catalyst. Therefore, it is effective to adopt several measurements to improve the SO2 tolerance. Firstly, it is to reduce the adsorption of SO2 on the catalyst. The highly acidic catalysts are effective to prevent the SO2 adsorbing. Secondly, preventing the oxidation of SO2 to SO3 plays a significant role in high SO2 tolerance by reducing the redox ability of catalyst, which can cut off the oxidation of SO2 to some extent. Furthermore, the synergistic effect between catalyst components can also improve the sulfur resistance of catalyst, such as the construction of sacrificial sites, which is responsible for the reduction of active components sulfation. Along with these existing excellent sulfur resistant catalysts, it is expected that future studies will focus on optimizing the supports and preparation methods and concentrating on the application of new structures and technology, which are effective strategies to improve the low-temperature SO2 tolerance of SCR catalysts.
References
- Chuanmin Chen; Yue Cao; Songtao Liu; Jianmeng Chen; Wenbo Jia; Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chinese Journal of Catalysis 2018, 39, 1347-1365, 10.1016/s1872-2067(18)63090-6.
- Qi Zhao; Bingbing Chen; Jin Li; Xianbin Wang; Mark Crocker; Chuan Shi; Insights into the structure-activity relationships of highly efficient CoMn oxides for the low temperature NH3-SCR of NOx. Applied Catalysis B: Environmental 2020, 277, 119215, 10.1016/j.apcatb.2020.119215.
- Dongmei Meng; Qian Xu; Yunlei Jiao; Yun Guo; Yanglong Guo; Li Wang; Guanzhong Lu; Wang-Cheng Zhan; Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Applied Catalysis B: Environmental 2018, 221, 652-663, 10.1016/j.apcatb.2017.09.034.
- Xiaolong Tang; Yiran Shi; Fengyu Gao; Shunzheng Zhao; Honghong Yi; Zongli Xi; Promotional role of Mo on Ce0.3FeOx catalyst towards enhanced NH3-SCR catalytic performance and SO2 resistance. Chemical Engineering Journal 2020, 398, 125619, 10.1016/j.cej.2020.125619.
- Ya-Juan Shi; Hang Shu; Yu-Hua Zhang; Hong-Mei Fan; Ya-Ping Zhang; Linjun Yang; Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Fuel Processing Technology 2016, 150, 141-147, 10.1016/j.fuproc.2016.05.016.
- Ruiben Jin; Yue Liu; Yan Wang; Wanglai Cen; Zhongbiao Wu; Haiqiang Wang; Xiaole Weng; The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Applied Catalysis B: Environmental 2014, 148, 582-588, 10.1016/j.apcatb.2013.09.016.
- Liam John France; Qing Yang; Wan Li; Zhihang Chen; Jianyu Guang; Dawei Guo; Lefu Wang; Xuehui Li; Ceria modified FeMnO —Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Applied Catalysis B: Environmental 2017, 206, 203-215, 10.1016/j.apcatb.2017.01.019.
- Ziran Ma; Xiaodong Wu; Ya Feng; Zhichun Si; Duan Weng; Lei Shi; Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5–WO3/TiO2 catalyst. Progress in Natural Science: Materials International 2015, 25, 342-352, 10.1016/j.pnsc.2015.07.002.
- Fan Cao; Sheng Su; Jun Xiang; Pengying Wang; Song Hu; Lushi Sun; Anchao Zhangd; The activity and mechanism study of Fe–Mn–Ce/γ-Al 2 O 3 catalyst for low temperature selective catalytic reduction of NO with NH 3. Fuel 2015, 139, 232-239, 10.1016/j.fuel.2014.08.060.
- Lulu Li; Lei Zhang; Kaili Ma; Weixin Zou; Yuan Cao; Yan Xiong; Changjin Tang; Lin Dong; Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Applied Catalysis B: Environmental 2017, 207, 366-375, 10.1016/j.apcatb.2017.02.041.
- Rui Yang; Haifeng Huang; Yijie Chen; Xixiong Zhang; Hanfeng Lu; Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chinese Journal of Catalysis 2015, 36, 1256-1262, 10.1016/s1872-2067(15)60884-1.
- Kyung Ju Lee; Pullur Anil Kumar; Muhammad Salman Maqbool; Komateedi N. Rao; Kwang Ho Song; Heon Phil Ha; Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Applied Catalysis B: Environmental 2013, 142, 705-717, 10.1016/j.apcatb.2013.05.071.
- Boxiong Shen; Ting Liu; Ning Zhao; Xiaoyan Yang; Lidan Deng; Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3.. Journal of Environmental Sciences 2010, 22, 1447-1454, 10.1016/s1001-0742(09)60274-6.
- Xin Zhao; L. Huang; Hongrui Li; Hang Hu; Jin Han; Liyi Shi; Dengsong Zhang; Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chinese Journal of Catalysis 2015, 36, 1886-1899, 10.1016/s1872-2067(15)60958-5.
- Li Xu; Chuan Shi; Bingbing Chen; Qi Zhao; Yongjun Zhu; Hermann Gies; Feng-Shou Xiao; Dirk De Vos; Toshiyuki Yokoi; Xinhe Bao; et al.Ute KolbMathias FeyenStefan MaurerAhmad MoiniUlrich MüllerWeiping Zhang Improvement of catalytic activity over Cu--Fe modified Al-rich Beta catalyst for the selective catalytic reduction of NO with NH3. Microporous and Mesoporous Materials 2016, 236, 211-217, 10.1016/j.micromeso.2016.08.042.
- Wenpo Shan; Fudong Liu; Hong He; Xiaoyan Shi; Chang-Bin Zhang; A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Applied Catalysis B: Environmental 2012, 115, 100-106, 10.1016/j.apcatb.2011.12.019.
- Tengfei Xu; Xiaodong Wu; Yuxi Gao; Qiwei Lin; Jianfeng Hu; Duan Weng; Comparative study on sulfur poisoning of V 2 O 5 -Sb 2 O 3 /TiO 2 and V 2 O 5 -WO 3 /TiO 2 monolithic catalysts for low-temperature NH 3 -SCR. Catalysis Communications 2017, 93, 33-36, 10.1016/j.catcom.2017.01.021.
- Lei Zhang; Lulu Li; Yuan Cao; Xiaojiang Yao; Chengyan Ge; Bin Gao; Yu Deng; Changjin Tang; Lin Dong; Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Applied Catalysis B: Environmental 2015, 165, 589-598, 10.1016/j.apcatb.2014.10.029.
- Yun Shu; Tanana Aikebaier; Xie Quan; Shuo Chen; Hongtao Yu; Selective catalytic reaction of NOx with NH3 over Ce–Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Applied Catalysis B: Environmental 2014, 150, 630-635, 10.1016/j.apcatb.2014.01.008.
- Yue Peng; Caixia Liu; Xueying Zhang; Junhua Li; The effect of SiO2 on a novel CeO2–WO3/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Applied Catalysis B: Environmental 2013, 140, 276-282, 10.1016/j.apcatb.2013.04.030.
- Xiaojiang Yao; Tingting Kong; Shuohan Yu; Lulu Li; Fumo Yang; Lin Dong; Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Applied Surface Science 2017, 402, 208-217, 10.1016/j.apsusc.2017.01.081.
- Long Qu; Caiting Li; Guangming Zeng; Mengying Zhang; Mengfan Fu; Jinfeng Ma; Fuman Zhan; Diqiang Luo; Support modification for improving the performance of MnOx–CeOy/γ-Al2O3 in selective catalytic reduction of NO by NH3. Chemical Engineering Journal 2014, 242, 76-85, 10.1016/j.cej.2013.12.076.
- Qian Li; Xiaoxu Hou; Hangsheng Yang; Zhaoxia Ma; Junwei Zheng; Fu Liu; Xiaobin Zhang; Zhongyong Yuan; Promotional effect of CeOX for NO reduction over V2O5/TiO2-carbon nanotube composites. Journal of Molecular Catalysis A: Chemical 2012, 356, 121-127, 10.1016/j.molcata.2012.01.004.
- Qingling Liu; Zhenchao Fu; Lei Ma; Hejingying Niu; Caixia Liu; Junhua Li; Ziyin Zhang; MnO -CeO2 supported on Cu-SSZ-13: A novel SCR catalyst in a wide temperature range. Applied Catalysis A: General 2017, 547, 146-154, 10.1016/j.apcata.2017.08.024.