
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joel Imoukhuede Omage | -- | 3424 | 2022-04-13 17:13:10 | | | |
| 2 | Peter Tang | Meta information modification | 3424 | 2022-04-14 03:45:26 | | |
Video Upload Options
Cancer is a major cause of death worldwide. With the advantages of simplicity, rapid response, reusability, and a low cost, aptamer-based electrochemical biosensors have received considerable attention as a promising approach for the clinical diagnosis of early-stage cancer.
1. Introduction
2. Redox-Active Molecules
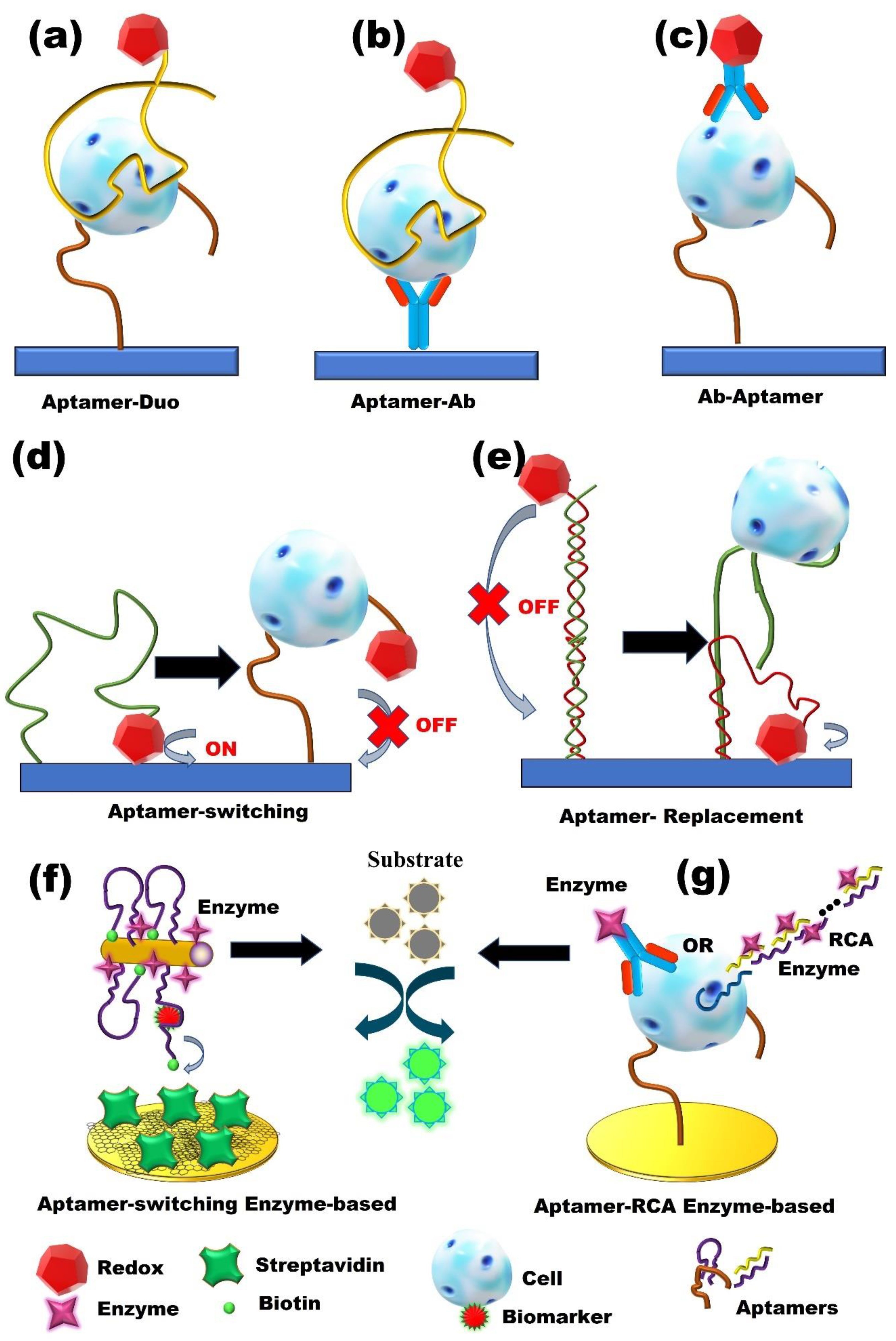
3. Enzyme-Based Aptasensors
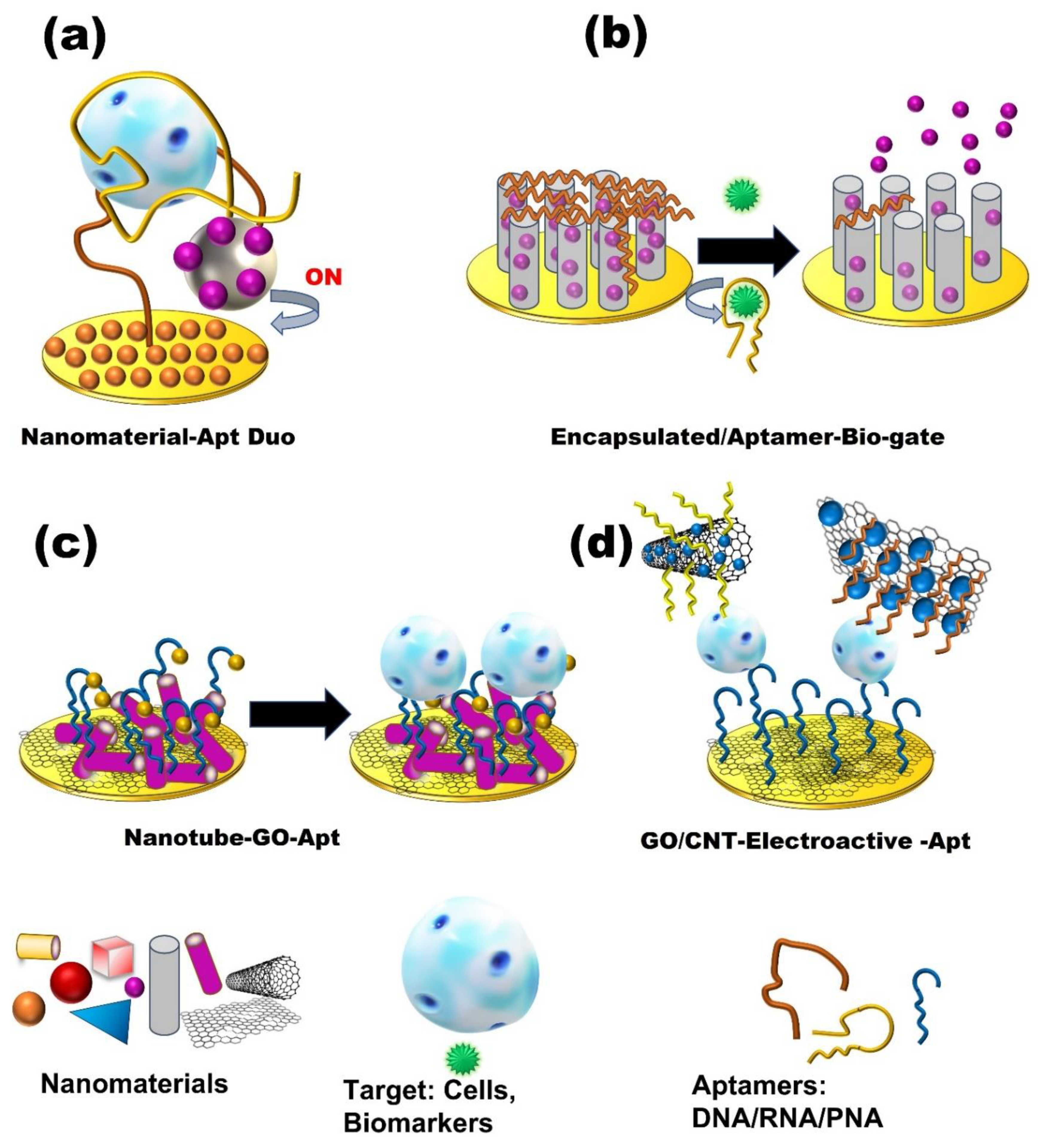
4. Nanomaterials-Based Aptasensors
|
Cancer Type |
Target |
Technique |
Sample |
Assay Time |
LOD |
Linear Range |
Reference |
|---|---|---|---|---|---|---|---|
|
Breast Cancer |
EGFR |
DPV |
Serum |
30 min |
50 pg/mL |
1–40 ng/mL |
[41] |
|
ER |
DPV |
Buffer |
10 min |
0.001 ng/mL |
0.001–1000 pg/µL |
[42] |
|
|
Exosomes |
CV |
buffer |
1 h |
96 particles/μL. |
1.12 × 102–1.12 × 108 particles/μL |
[43] |
|
|
Exosomes (MCF-7 cells) |
ECL |
Blood serum sample |
120 min |
7.41 × 104 particle/mL |
3.4 × 105 –1.7 × 108 particle/mL |
[44] |
|
|
HER2 |
stripping voltammetry |
Human serum |
20 min |
26 cells/mL |
50 to 20,000 cells/mL |
[32] |
|
|
HER2 |
EIS |
Buffer |
- |
0.047 pg/mL |
0.01 to 5 ng/mL |
[45] |
|
|
HER2 |
CV, EIS |
Serum |
2 h |
1 pM |
1 pM–100 nM |
[46] |
|
|
HER2 |
EIS |
Serum sample |
40 min |
50 fg/mL |
0.1 pg/mL–1 ng/mL |
[43] |
|
|
HER2 |
CV, DPV, EIS |
PBS buffer |
5–10 min |
0.001 ng/mL |
0.001–100 ng/mL |
[47] |
|
|
MCF-7 |
CC, CV, EIS |
Serum |
25 min |
47 cells/mL |
0–500 cells/mL |
[48] |
|
|
MCF-7 |
SWV, CV |
Human plasma |
2 h |
328 cells/mL |
328–593 cells/mL |
[49] |
|
|
MCF-7 |
CV, DPV |
Human serum |
60 min |
20 cells/mL |
50–106 cells/mL |
[50] |
|
|
MCF-7 Exosomes |
PEC |
Buffer |
110 min (total) |
1.38 × 103 particles/μL |
5.00 × 103 to 1.00 × 106 particles/mL |
[51] |
|
|
MDA-MB-231 |
DPV |
Blood Serum |
30 min |
5 cell/ mL |
10–1 × 103 cell/mL |
[52] |
|
|
MUC1 |
DPV |
Serum sample |
25 min |
0.79 fM |
1 fM–100 nM |
[53] |
|
|
MUC1 |
SWV, CV |
Buffer |
1 h |
0.33 pM |
1.0 pM–10 µM |
[54] |
|
|
MUC-1 |
EIS |
PBS buffer |
2 h |
38 cells/mL |
100 to 5.0 × 107 cells/mL |
[27] |
|
|
Nucleolin |
DPV |
Buffer |
1 h |
8 ± 2 cells ml/mL |
10–106 cells/mL |
[55] |
|
|
Nucleolin |
ECL |
Buffer |
10 min |
10 cells |
10–100 cells |
[56] |
|
|
Nucleolin |
EIS |
Buffer |
- |
40 cells/mL |
103–107 cells/mL |
[57] |
|
|
Nucleolin |
CV, EIS |
Phosphate buffer |
30 min |
4 cells/mL |
1 × 101–1 × 106 cells/mL |
[58] |
|
|
OPN |
CV, SWV |
Synthetic human plasma |
60 min |
1.3 ± 0.1 nM |
CV: 25 to 100 nM SWV: 12 to 100 nM |
[59] |
|
|
OPN |
CV |
PBS buffer |
60 min |
3.7 ± 0.6 nM |
25–200 nM |
[60] |
|
|
PDGF-BB, MCF-7 cells |
CV, SWV |
PBS buffer |
- |
PDGF-BB: 0.52 nM MCF-7: 328 cells/mL |
PDGF: 0.52–1.52 nM MCF-7: 328 to 593 cells/mL |
[49] |
|
|
Lung Cancer |
CEA, NSE |
CV, DPV |
Serum |
1 h |
CEA: 2 pg/mL NSE: 10 pg/mL |
CEA: 0.01–500 ng/mL NSE: 0.05–500 ng/mL |
[61] |
|
CEA |
DPV, EIS |
Human serum |
85 min (total) |
1.5 pg/mL |
5 pg/mL to 50 ng/mL |
[62] |
|
|
CEA |
EIS |
Buffer, serum |
- |
Buffer: 0.45 ng/mL Serum: 1.06 ng/mL |
0.77–14 ng/mL |
[63] |
|
|
Lung tumor |
EIS |
Blood plasma |
~25 min |
- |
- |
[64] |
|
|
Lung cancer tissues (proteins) |
SWV |
Blood plasma |
1 h |
0.023 ng/mL |
230 ng/mL to 0.023 ng/mL |
[65] |
|
|
VEGF165 |
CV, EIS |
Lung cancer Serum samples |
40 min |
1.0 pg/mL |
10.0–300.0 pg/mL |
[66] |
|
|
Lung cancer tumor |
CV, DPV, SWV, EIS |
Human blood |
- |
- |
- |
[14] |
|
|
Lung/Breast/ others cancer |
VEGF |
DPV |
Buffer |
45 min |
30 nmol/L |
0–250 nmol/L |
[35] |
|
CEA |
DPV |
Spiked Serum |
50 min |
0.9 pg/mL |
3 pg/mL to 40 ng/mL |
[29] |
|
|
CEA |
DPV, EIS, CV |
Human serum |
1 h |
0.34 fg/mL |
0.5 fg/mL to 0.5 ng/mL |
[38] |
|
|
CEA |
DPV, CV, EIS |
Serum |
1 h |
0.31 pg/mL |
1 pg/mL–80 ng/mL |
[67] |
|
|
CEA |
EIS |
Buffer/Blood sample |
1 h 30 min |
0.5 pg/mL |
1 pg/mL–10 ng/mL |
[68] |
|
|
CEA |
DPV |
Buffer |
1 h |
40 fg/mL |
0.0001–10 ng/mL |
[69] |
|
|
CEA |
PES |
Serum |
60 min |
0.39 pg/mL |
0.001–2.5 ng/mL |
[70] |
|
|
VEGF165 |
CV |
Buffer |
1 h |
30 fM |
100 fM to 10 nM |
[71] |
|
|
MUC 1 |
CV, SWV, EIS |
Buffer |
120 min |
4 pM |
10 pM to 1 μM |
[72] |
|
|
CEA |
CV, EIS |
Buffer |
1 h |
3.4 ng/mL |
5 ng/mL–40 ng/mL |
[73] |
|
|
CEA |
CV |
PBS/spiked human serum |
40 min |
6.3 pg/mL |
50 pg/mL to 1.0 μg/mL |
[11] |
|
|
CEA |
DPV |
Buffer/spiked human serum |
45 min |
0.84 pg/mL |
10 pg/mLto 100 ng/mL |
[74] |
|
|
CEA and CA153 |
PEC |
Serum samples |
20 min |
CEA: 2.85 pg/mL CA153: 0.0275 U/mL |
CEA: 0.005–10 ng mL, CA153: 0.05–100 U/mL |
[75] |
|
|
Prostate Cancer |
PSA |
EIS |
Buffer |
2 h |
0.5 pg/mL |
0.05 ng/mL to 50 ng/mL |
[5] |
|
PSA |
EIS |
Buffer |
2 h (total) |
1 pg/mL |
1 × 102 pg/mL–1 × 102 ng/mL |
[76] |
|
|
PSA |
DPV |
Serum samples |
40 min |
0.25 ng/ mL |
0.25 to 200 ng/mL |
[77] |
|
|
PSA |
SWV, EIS |
Spiked human serum |
- |
EIS: 10 pg/mL |
EIS: 10 pg/mL to 10 ng/mL |
[78] |
|
|
PSA |
DPV |
Blood serum |
30 min |
50 pg/mL |
0.125 to 128 ng/mL |
[79] |
|
|
PSA |
PEC |
Human serum |
- |
0.34 pg/mL |
0.001 to 80 ng/mL |
[80] |
|
|
PSA |
DPV |
Human serum |
30 min |
0.064 pg/mL |
1 pg/mL to 100 ng/mL |
[81] |
|
|
PSA |
DPV, EIS |
Serum sample |
40 min |
1.0 pg/ mL |
DPV: 0.005–20 ng/mL EIS: 0.005–100 ng/mL |
[82] |
|
|
PSA |
EIS |
Human serum |
2 h 30 min |
0.33 pg/mL |
5 to 2 × 104 pg/mL |
[83] |
|
|
PSA |
CV, SWV, EIS |
Buffer |
30 min |
0.028 * and 0.007 ** ng/mL |
0.5–7 ng/mL |
[84] |
|
|
PSA |
PEC |
PBS buffer/ spiked Serum |
40 min |
4.300 fg/mL |
1.000 × 10−5 to 500.0 ng/mL, |
[85] |
|
|
PSA |
SWV, EIS |
Serum sample |
4 h (total) |
2.3 fg/mL |
10 fg/mL–100 ng/mL |
[86] |
|
|
PSA |
PEC |
Human serum |
90 min |
0.52 pg/mL |
1.0 pg/mL to 8.0 ng/mL |
[87] |
|
|
PSA |
ECL |
Human serum |
60 min |
0.17 pg/mL |
0.5 pg/mL to 5.0 ng/mL |
[88] |
|
|
PSA |
DPV |
Spiked Urine Blood serum |
60 min |
280 pg/mL |
1 to 300 ng/mL |
[89] |
|
|
PSA |
DPV |
Human serum |
30 min |
6.2 pg/mL |
0.01–100 ng/mL |
[90] |
|
|
PSA, SAC |
SWV |
50% Human serum |
PSA: 2 h SAC: 1 h |
PSA: 2.5 fg/mL, SAC: 14.4 fg/mL |
PSA: 1 fg/mL to 500 ng/mL SAC: 1 fg/mL to 1 μg/mL |
[91] |
|
|
Blood cell cancer |
Ramos cell |
LSV |
Human serum |
3 h |
10 cells/mL |
1 × 101–1 × 106 cell/mL |
[37] |
|
Breast/ Liver cancer |
HeLa, MCF-7, HepG2. |
PEC |
Buffer |
4 h 20 min (total) |
19 cell/mL (HeLa) |
50–5 × 105 cell/mL (HeLa) |
[92] |
|
Breast/ Prostate cancer |
CTC HER2, PSMA, and MUC1 |
LSW |
Spiked in Blood |
1 h |
2 cells/sensor |
2–200 cells/sensor |
[93] |
|
PDGF-BB |
DPV |
PBS buffer |
40 min |
0.65 pM |
0.0007–20 nM |
[94] |
|
|
PDGF-BB |
CV, EIS |
ID water, 5% trehalose |
40 min |
CV: 7 pM EIS: 1.9 pM |
CV: 0.01–50 nM EIS: 0.005–50 nM |
[95] |
|
|
PDGF-BB |
DPV |
PBS buffer |
2 h |
0.034 pg/ mL |
0.0001 to 60 ng/mL |
[96] |
|
|
PDGF-BB |
EIS |
PBS buffer |
2 h |
0.82 pg/ mL |
1 pg/mL to 0.05 ng/mL |
[97] |
|
|
CAT |
HER2 |
EIS, CV |
Diluted human serum |
2 h 20 min (total) |
15 fM |
0.1 pM to 20 nM |
[98] |
|
Cervical cancer |
HeLa |
EIS |
Buffer |
2 h |
90 cells/mL |
2.4 × 102–2.4 × 105 cells/mL |
[99] |
|
Colon cancer |
MUC-1 |
EIS, CV |
Buffer |
120 min |
40 cells/mL |
1.25 × 102–1.25 × 106 cells/mL |
[100] |
|
CEA |
PES |
Human serum |
1 h |
1.9 pg/mL |
0.01 ng/mL to 2.5 ng |
[101] |
|
|
CEA |
PEC |
Serum |
90 min |
4.8 pg/ mL |
10.0 pg/mL–5.0 ng/mL |
[102] |
|
|
inflammation-associated carcinogenesis |
TNF-α |
SWV |
Human blood |
4 h |
10 ng/mL |
10–100 ng/mL |
[34] |
|
Leukemia, blood cancer |
CCRF-CEM |
SWV |
Buffer |
40 min |
10 cells/mL |
1.0 × 102–1.0 × 106 Cells/mL |
[103] |
|
K562 cells |
EIS |
Buffer |
40 min |
30 cells/mL |
1 × 102–1 × 107 cells/mL |
[104] |
|
|
Liver cancer |
HepG2 |
EIS |
Buffer |
2 h |
2 cells/mL |
1 × 102–1 × 106 cells/mL |
[22] |
|
HepG2 |
DPV, CV, EIS |
PBS buffer |
60 min |
15 cells/mL |
1 × 102–1 × 107 cell/mL |
[36] |
|
|
MEAR |
DPV, CV, EIS |
Diluted human blood |
60 min (Total) |
1 cell/mL |
1−14 Cells/mL |
[33] |
|
|
HepG2 |
CV |
buffer |
2 h |
2 cells/mL |
1 × 102–1 × 106 cells/mL |
[22] |
|
|
AFP |
EIS |
PBS/ diluted human serum |
30 min |
0.3 fg/mL |
1 fg/mL to 100 ng/mL |
[105] |
References
- Mathers, C.; Fat, D.M.; Boerma, J.T. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008.
- Available online: https://www.who.int/news/item/03-02-2022-world-cancer-day-closing-the-care-gap (accessed on 7 February 2022).
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564.
- Wu, J.; Fu, Z.; Yan, F.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem. 2007, 26, 679–688.
- Yang, Z.; Kasprzyk-Hordern, B.; Goggins, S.; Frost, C.G.; Estrela, P. A novel immobilization strategy for electrochemical detection of cancer biomarkers: DNA-directed immobilization of aptamer sensors for sensitive detection of prostate specific antigens. Analyst 2015, 140, 2628–2633.
- Wu, Y.; Zhou, H.; Wei, W.; Hua, X.; Wang, L.; Zhou, Z.; Liu, S. Signal amplification cytosensor for evaluation of drug-induced cancer cell apoptosis. Anal. Chem. 2012, 84, 1894–1899.
- Arya, S.K.; Estrela, P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors 2018, 18, 2010.
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2020, 122, 115701.
- Mohammadinejad, A.; Kazemi Oskuee, R.; Eivazzadeh-Keihan, R.; Rezayi, M.; Baradaran, B.; Maleki, A.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Development of biosensors for detection of alpha-fetoprotein: As a major biomarker for hepatocellular carcinoma. TrAC Trends Anal. Chem. 2020, 130, 115961.
- Thakare, S.; Shaikh, A.; Bodas, D.; Gajbhiye, V. Application of dendrimer-based nanosensors in immunodiagnosis. Colloids Surf. B Biointerfaces 2022, 209, 112174.
- Zhang, F.; Liu, Z.; Han, Y.; Fan, L.; Guo, Y. Sandwich electrochemical carcinoembryonic antigen aptasensor based on signal amplification of polydopamine functionalized graphene conjugate Pd-Pt nanodendrites. Bioelectrochemistry 2021, 142, 107947.
- Sohrabi, H.; Bolandi, N.; Hemmati, A.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Oroojalian, F.; Reza Majidi, M.; de la Guardia, M.; Mokhtarzadeh, A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review. Microchem. J. 2022, 177, 107248.
- Fernandez, L.; Bustos, R.H.; Zapata, C.; Garcia, J.; Jauregui, E.; Ashraf, G.M. Immunogenicity in Protein and Peptide Based-Therapeutics: An Overview. Curr. Protein Pept. Sci. 2018, 19, 958–971.
- Shabalina, A.V.; Sharko, D.O.; Glazyrin, Y.E.; Bolshevich, E.A.; Dubinina, O.V.; Kim, A.M.; Veprintsev, D.V.; Lapin, I.N.; Zamay, G.S.; Krat, A.V.; et al. Development of Electrochemical Aptasensor for Lung Cancer Diagnostics in Human Blood. Sensors 2021, 21, 7851.
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26.
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265.
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822.
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468.
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510.
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Design and construction of a label free aptasensor for electrochemical detection of sodium diclofenac. Biosens. Bioelectron. 2012, 33, 184–189.
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Aptamer-conjugated silver nanoparticles for electrochemical detection of adenosine triphosphate. Biosens. Bioelectron. 2012, 37, 94–98.
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A.; Wiechec, E.; Turner, A.P.; Tiwari, A. Ultrasensitive detection of human liver hepatocellular carcinoma cells using a label-free aptasensor. Anal. Chem. 2014, 86, 4956–4960.
- Mascini, M. Aptamers in Bioanalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009.
- Shangguan, D.; Cao, Z.; Meng, L.; Mallikaratchy, P.; Sefah, K.; Wang, H.; Li, Y.; Tan, W. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008, 7, 2133–2139.
- Lyu, Y.; Chen, G.; Shangguan, D.; Zhang, L.; Wan, S.; Wu, Y.; Zhang, H.; Duan, L.; Liu, C.; You, M.; et al. Generating Cell Targeting Aptamers for Nanotheranostics Using Cell-SELEX. Theranostics 2016, 6, 1440–1452.
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126.
- Yan, M.; Sun, G.; Liu, F.; Lu, J.; Yu, J.; Song, X. An aptasensor for sensitive detection of human breast cancer cells by using porous GO/Au composites and porous PtFe alloy as effective sensing platform and signal amplification labels. Anal. Chim. Acta 2013, 798, 33–39.
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249.
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K. A Novel Electrochemical Aptasensor for Carcinoembryonic Antigen Detection Based on Target-induced Bridge Assembly. Electroanalysis 2018, 30, 1734–1739.
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics 2015, 5, 322.
- Chen, X.; Zhang, Q.; Qian, C.; Hao, N.; Xu, L.; Yao, C. Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly (o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens. Bioelectron. 2015, 64, 485–492.
- Zhu, Y.; Chandra, P.; Shim, Y.-B. Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine–Au nanoparticle–aptamer bioconjugate. Anal. Chem. 2012, 85, 1058–1064.
- Qu, L.; Xu, J.; Tan, X.; Liu, Z.; Xu, L.; Peng, R. Dual-aptamer modification generates a unique interface for highly sensitive and specific electrochemical detection of tumor cells. ACS Appl. Mater. Interfaces 2014, 6, 7309–7315.
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326.
- Ravalli, A.; Rivas, L.; De La Escosura-Muñiz, A.; Pons, J.; Merkoçi, A.; Marrazza, G. A DNA Aptasensor for Electrochemical Detection of Vascular Endothelial Growth Factor. J. Nanosci. Nanotechnol. 2015, 15, 3411–3416.
- Sun, D.; Lu, J.; Zhong, Y.; Yu, Y.; Wang, Y.; Zhang, B.; Chen, Z. Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens. Bioelectron. 2016, 75, 301–307.
- Yi, Z.; Li, X.-Y.; Gao, Q.; Tang, L.-J.; Chu, X. Aptamer-aided target capturing with biocatalytic metal deposition: An electrochemical platform for sensitive detection of cancer cells. Analyst 2013, 138, 2032–2037.
- Huang, J.-Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, Y.-J.; Bao, T.; Xiong, H.-Y.; Zhang, X.-H.; Wang, S.-F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33.
- Lee, J.H. Conjugation approaches for construction of aptamer-modified nanoparticles for application in imaging. Curr. Top. Med. Chem. 2013, 13, 504–512.
- Gedi, V.; Kim, Y.-P. Detection and characterization of cancer cells and pathogenic bacteria using aptamer-based nano-conjugates. Sensors 2014, 14, 18302–18327.
- Ilkhani, H.; Sarparast, M.; Noori, A.; Bathaie, S.Z.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497.
- Ahirwar, R.; Dalal, A.; Sharma, J.G.; Yadav, B.K.; Nahar, P.; Kumar, A.; Kumar, S. An aptasensor for rapid and sensitive detection of estrogen receptor alpha in human breast cancer. Biotechnol. Bioeng. 2019, 116, 227–233.
- Rostamabadi, P.F.; Heydari-Bafrooei, E. Impedimetric aptasensing of the breast cancer biomarker HER2 using a glassy carbon electrode modified with gold nanoparticles in a composite consisting of electrochemically reduced graphene oxide and single-walled carbon nanotubes. Microchim. Acta 2019, 186, 495.
- Qiao, B.; Guo, Q.; Jiang, J.; Qi, Y.; Zhang, H.; He, B.; Cai, C.; Shen, J. An electrochemiluminescent aptasensor for amplified detection of exosomes from breast tumor cells (MCF-7 cells) based on G-quadruplex/hemin DNAzymes. Analyst 2019, 144, 3668–3675.
- Shen, C.; Zeng, K.; Luo, J.; Li, X.; Yang, M.; Rasooly, A. Self-Assembled DNA Generated Electric Current Biosensor for HER2 Analysis. Anal. Chem. 2017, 89, 10264–10269.
- Arya, S.K.; Zhurauski, P.; Jolly, P.; Batistuti, M.R.; Mulato, M.; Estrela, P. Capacitive aptasensor based on interdigitated electrode for breast cancer detection in undiluted human serum. Biosens. Bioelectron. 2018, 102, 106–112.
- Harahsheh, T.; Makableh, Y.F.; Rawashdeh, I.; Al-Fandi, M. Enhanced aptasensor performance for targeted HER2 breast cancer detection by using screen-printed electrodes modified with Au nanoparticles. Biomed. Microdevices 2021, 23, 46.
- Cai, S.; Chen, M.; Liu, M.; He, W.; Liu, Z.; Wu, D.; Xia, Y.; Yang, H.; Chen, J. A signal amplification electrochemical aptasensor for the detection of breast cancer cell via free-running DNA walker. Biosens. Bioelectron. 2016, 85, 184–189.
- Hasanzadeh, M.; Razmi, N.; Mokhtarzadeh, A.; Shadjou, N.; Mahboob, S. Aptamer based assay of plated-derived grow factor in unprocessed human plasma sample and MCF-7 breast cancer cell lysates using gold nanoparticle supported α-cyclodextrin. Int. J. Biol. Macromol. 2018, 108, 69–80.
- Liu, N.; Song, J.; Lu, Y.; Davis, J.J.; Gao, F.; Luo, X. Electrochemical Aptasensor for Ultralow Fouling Cancer Cell Quantification in Complex Biological Media Based on Designed Branched Peptides. Anal. Chem. 2019, 91, 8334–8340.
- Xia, Y.; Chen, T.; Chen, W.; Chen, G.; Xu, L.; Zhang, L.; Zhang, X.; Sun, W.; Lan, J.; Lin, X.; et al. A dual-modal aptasensor based on a multifunctional acridone derivate for exosomes detection. Anal. Chim. Acta 2022, 1191, 339279.
- Akhtartavan, S.; Karimi, M.; Sattarahmady, N.; Heli, H. An electrochemical signal-on apta-cyto-sensor for quantitation of circulating human MDA-MB-231 breast cancer cells by transduction of electro-deposited non-spherical nanoparticles of gold. J. Pharm. Biomed. Anal. 2020, 178, 112948.
- Bharti, A.; Rana, S.; Dahiya, D.; Agnihotri, N.; Prabhakar, N. An electrochemical aptasensor for analysis of MUC1 using gold platinum bimetallic nanoparticles deposited carboxylated graphene oxide. Anal. Chim. Acta 2020, 1097, 186–195.
- Wang, H.; Sun, J.; Lu, L.; Yang, X.; Xia, J.; Zhang, F.; Wang, Z. Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin1. Anal. Chim. Acta 2020, 1094, 18–25.
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sheibani, S. Signalling probe displacement electrochemical aptasensor for malignant cell surface nucleolin as a breast cancer biomarker based on gold nanoparticle decorated hydroxyapatite nanorods and silver nanoparticle labels. Microchim. Acta 2018, 185, 154.
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.-K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223.
- Safavipour, M.; Kharaziha, M.; Amjadi, E.; Karimzadeh, F.; Allafchian, A. TiO2 nanotubes/reduced GO nanoparticles for sensitive detection of breast cancer cells and photothermal performance. Talanta 2020, 208, 120369.
- Shafiei, F.; Saberi, R.S.; Mehrgardi, M.A. A label-free electrochemical aptasensor for breast cancer cell detection based on a reduced graphene oxide-chitosan-gold nanoparticle composite. Bioelectrochemistry 2021, 140, 107807.
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Electrochemical aptasensor for human osteopontin detection using a DNA aptamer selected by SELEX. Anal. Chim. Acta 2017, 987, 25–37.
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Development of an electrochemical RNA-aptasensor to detect human osteopontin. Biosens. Bioelectron. 2015, 71, 332–341.
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90.
- Wen, W.; Huang, J.-Y.; Bao, T.; Zhou, J.; Xia, H.-X.; Zhang, X.-H.; Wang, S.-F.; Zhao, Y.-D. Increased electrocatalyzed performance through hairpin oligonucleotide aptamer-functionalized gold nanorods labels and graphene-streptavidin nanomatrix: Highly selective and sensitive electrochemical biosensor of carcinoembryonic antigen. Biosens. Bioelectron. 2016, 83, 142–148.
- Yen, Y.-K.; Chao, C.-H.; Yeh, Y.-S. A Graphene-PEDOT:PSS Modified Paper-Based Aptasensor for Electrochemical Impedance Spectroscopy Detection of Tumor Marker. Sensors 2020, 20, 1372.
- Zamay, G.S.; Zamay, T.N.; Kolovskaya, O.S.; Krat, A.V.; Glazyrin, Y.E.; Dubinina, A.V.; Zamay, A.S. Development of a biosensor for electrochemical detection of tumor-associated proteins in blood plasma of cancer patients by aptamers. Dokl. Biochem. Biophys. 2016, 466, 70–73.
- Zamay, G.S.; Zamay, T.N.; Kolovskii, V.A.; Shabanov, A.V.; Glazyrin, Y.E.; Veprintsev, D.V.; Krat, A.V.; Zamay, S.S.; Kolovskaya, O.S.; Gargaun, A.; et al. Electrochemical aptasensor for lung cancer-related protein detection in crude blood plasma samples. Sci. Rep. 2016, 6, 34350.
- Amouzadeh Tabrizi, M.; Shamsipur, M.; Farzin, L. A high sensitive electrochemical aptasensor for the determination of VEGF165 in serum of lung cancer patient. Biosens. Bioelectron. 2015, 74, 764–769.
- Xue, S.; Yi, H.; Jing, P.; Xu, W. Dendritic Au nanowires as nanocarriers and signal enhancers for sensitive electrochemical detection of carcinoembryonic antigen. RSC Adv. 2015, 5, 77454–77459.
- Quan, H.; Zuo, C.; Li, T.; Liu, Y.; Li, M.; Zhong, M.; Zhang, Y.; Qi, H.; Yang, M. Electrochemical detection of carcinoembryonic antigen based on silver nanocluster/horseradish peroxidase nanocomposite as signal probe. Electrochim. Acta 2015, 176, 893–897.
- Liu, Z.; Wang, Y.; Guo, Y.; Dong, C. Label-free Electrochemical Aptasensor for Carcino-embryonic Antigen Based on Ternary Nanocomposite of Gold Nanoparticles, Hemin and Graphene. Electroanalysis 2016, 28, 1023–1028.
- Deng, W.; Shen, L.; Wang, X.; Yang, C.; Yu, J.; Yan, M.; Song, X. Using carbon nanotubes-gold nanocomposites to quench energy from pinnate titanium dioxide nanorods array for signal-on photoelectrochemical aptasensing. Biosens. Bioelectron. 2016, 82, 132–139.
- Da, H.; Liu, H.; Zheng, Y.; Yuan, R.; Chai, Y. A highly sensitive VEGF165 photoelectrochemical biosensor fabricated by assembly of aptamer bridged DNA networks. Biosens. Bioelectron. 2018, 101, 213–218.
- Wen, W.; Hu, R.; Bao, T.; Zhang, X.; Wang, S. An insertion approach electrochemical aptasensor for mucin 1 detection based on exonuclease-assisted target recycling. Biosens. Bioelectron. 2015, 71, 13–17.
- Wang, Q.-L.; Cui, H.-F.; Song, X.; Fan, S.-F.; Chen, L.-L.; Li, M.-M.; Li, Z.-Y. A label-free and lectin-based sandwich aptasensor for detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 260, 48–54.
- Niu, C.; Lin, X.; Jiang, X.; Guo, F.; Liu, J.; Liu, X.; Huang, H.; Huang, Y. An electrochemical aptasensor for highly sensitive detection of CEA based on exonuclease III and hybrid chain reaction dual signal amplification. Bioelectrochemistry 2022, 143, 107986.
- Zhong, Y.; Wang, X.; Zha, R.; Wang, C.; Zhang, H.; Wang, Y.; Li, C. Dual-wavelength responsive photoelectrochemical aptasensor based on ionic liquid functionalized Zn-MOFs and noble metal nanoparticles for the simultaneous detection of multiple tumor markers. Nanoscale 2021, 13, 19066–19075.
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer–MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016, 75, 188–195.
- Liu, B.; Lu, L.; Hua, E.; Jiang, S.; Xie, G. Detection of the human prostate-specific antigen using an aptasensor with gold nanoparticles encapsulated by graphitized mesoporous carbon. Microchim. Acta 2012, 178, 163–170.
- Jolly, P.; Zhurauski, P.; Hammond, J.L.; Miodek, A.; Liébana, S.; Bertok, T.; Tkáč, J.; Estrela, P. Self-assembled gold nanoparticles for impedimetric and amperometric detection of a prostate cancer biomarker. Sens. Actuators B Chem. 2017, 251, 637–643.
- Sattarahmady, N.; Rahi, A.; Heli, H. A signal-on built in-marker electrochemical aptasensor for human prostate-specific antigen based on a hairbrush-like gold nanostructure. Sci. Rep. 2017, 7, 11238.
- Hu, M.; Yang, H.; Li, Z.; Zhang, L.; Zhu, P.; Yan, M.; Yu, J. Signal-switchable lab-on-paper photoelectrochemical aptasensing system integrated triple-helix molecular switch with charge separation and recombination regime of type-II core-shell quantum dots. Biosens. Bioelectron. 2020, 147, 111786.
- Raouafi, A.; Sánchez, A.; Raouafi, N.; Villalonga, R. Electrochemical aptamer-based bioplatform for ultrasensitive detection of prostate specific antigen. Sens. Actuators B Chem. 2019, 297, 126762.
- Heydari-Bafrooei, E.; Shamszadeh, N.S. Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens. Bioelectron. 2017, 91, 284–292.
- Meng, F.; Sun, H.; Huang, Y.; Tang, Y.; Chen, Q.; Miao, P. Peptide cleavage-based electrochemical biosensor coupling graphene oxide and silver nanoparticles. Anal. Chim. Acta 2019, 1047, 45–51.
- Aayanifard, Z.; Alebrahim, T.; Pourmadadi, M.; Yazdian, F.; Dinani, H.S.; Rashedi, H.; Omidi, M. Ultra pH-sensitive detection of total and free prostate-specific antigen using electrochemical aptasensor based on reduced graphene oxide/gold nanoparticles emphasis on TiO(2)/carbon quantum dots as a redox probe. Eng. Life Sci. 2021, 21, 739–752.
- Xu, R.; Du, Y.; Ma, H.; Wu, D.; Ren, X.; Sun, X.; Wei, Q.; Ju, H. Photoelectrochemical aptasensor based on La(2)Ti(2)O(7)/Sb(2)S(3) and V(2)O(5) for effectively signal change strategy for cancer marker detection. Biosens. Bioelectron. 2021, 192, 113528.
- Zhao, J.; Ma, Z. Ultrasensitive detection of prostate specific antigen by electrochemical aptasensor using enzyme-free recycling amplification via target-induced catalytic hairpin assembly. Biosens. Bioelectron. 2018, 102, 316–320.
- Cai, G.; Yu, Z.; Ren, R.; Tang, D. Exciton–Plasmon Interaction between AuNPs/Graphene Nanohybrids and CdS Quantum Dots/TiO2 for Photoelectrochemical Aptasensing of Prostate-Specific Antigen. ACS Sens. 2018, 3, 632–639.
- Cao, J.-T.; Yang, J.-J.; Zhao, L.-Z.; Wang, Y.-L.; Wang, H.; Liu, Y.-M.; Ma, S.-H. Graphene nanorods-based multiple-assisted electrochemiluminescence signal amplification strategy for sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 92–98.
- Argoubi, W.; Sánchez, A.; Parrado, C.; Raouafi, N.; Villalonga, R. Label-free electrochemical aptasensing platform based on mesoporous silica thin film for the detection of prostate specific antigen. Sens. Actuators B Chem. 2018, 255, 309–315.
- Zhao, Y.; Liu, H.; Shi, L.; Zheng, W.; Jing, X. Electroactive Cu2O nanoparticles and Ag nanoparticles driven ratiometric electrochemical aptasensor for prostate specific antigen detection. Sens. Actuators B Chem. 2020, 315, 128155.
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous detection of dual biomarkers using hierarchical MoS(2) nanostructuring and nano-signal amplification-based electrochemical aptasensor toward accurate diagnosis of prostate cancer. Biosens. Bioelectron. 2022, 197, 113797.
- Zhu, J.H.; Gou, H.; Zhao, T.; Mei, L.P.; Wang, A.J.; Feng, J.J. Ultrasensitive photoelectrochemical aptasensor for detecting telomerase activity based on Ag(2)S/Ag decorated ZnIn(2)S(4)/C(3)N(4) 3D/2D Z-scheme heterostructures and amplified by Au/Cu(2+)-boron-nitride nanozyme. Biosens. Bioelectron. 2022, 203, 114048.
- Wan, Y.; Zhou, Y.G.; Poudineh, M.; Safaei, T.S.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. Highly Specific Electrochemical Analysis of Cancer Cells using Multi-Nanoparticle Labeling. Angew. Chem. 2014, 53, 13145–13149.
- Forouzanfar, S.; Khakpour, I.; Alam, F.; Pala, N.; Wang, C. Novel application of electrochemical bipolar exfoliated graphene for highly sensitive disposable label-free cancer biomarker aptasensors. Nanoscale Adv. 2021, 3, 5948–5958.
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. Highly sensitive label-free electrochemical aptasensors based on photoresist derived carbon for cancer biomarker detection. Biosens. Bioelectron. 2020, 170, 112598.
- Li, Y.; Liu, Z.; Lu, W.; Zhao, M.; Xiao, H.; Hu, T.; Ma, J.; Zheng, Z.; Jia, J.; Wu, H. A label-free electrochemical aptasensor based on the core–shell hybrid nanoarchitecture for the sensitive detection of PDGF-BB. Analyst 2021, 146, 979–988.
- Zhang, Z.; Guo, C.; Zhang, S.; He, L.; Wang, M.; Peng, D.; Tian, J.; Fang, S. Carbon-based nanocomposites with aptamer-templated silver nanoclusters for the highly sensitive and selective detection of platelet-derived growth factor. Biosens. Bioelectron. 2017, 89, 735–742.
- Zhang, T.; Song, Y.; Xing, Y.; Gu, Y.; Yan, X.; Liu, H.; Lu, N.; Xu, H.; Xu, Z.; Zhang, Z.; et al. The synergistic effect of Au-COF nanosheets and artificial peroxidase (NiPd) rhombic dodecahedra for signal amplification for biomarker detection. Nanoscale 2019, 11, 20221–20227.
- Wang, Z.; Chen, S.; Hu, C.; Cui, D.; Jia, N. An enhanced impedance cytosensor based on folate conjugated-polyethylenimine-carbon nanotubes for tumor targeting. Electrochem. Commun. 2013, 29, 4–7.
- Cao, H.; Ye, D.; Zhao, Q.; Luo, J.; Zhang, S.; Kong, J. A novel aptasensor based on MUC-1 conjugated CNSs for ultrasensitive detection of tumor cells. Analyst 2014, 139, 4917–4923.
- Han, Z.; Luo, M.; Weng, Q.; Chen, L.; Chen, J.; Li, C.; Zhou, Y.; Wang, L. ZnO flower-rod/g-C3N4-gold nanoparticle-based photoelectrochemical aptasensor for detection of carcinoembryonic antigen. Anal. Bioanal. Chem. 2018, 410, 6529–6538.
- Qiu, Z.; Shu, J.; Liu, J.; Tang, D. Dual-Channel Photoelectrochemical Ratiometric Aptasensor with up-Converting Nanocrystals Using Spatial-Resolved Technique on Homemade 3D Printed Device. Anal. Chem. 2019, 91, 1260–1268.
- Zhang, K.; Tan, T.; Fu, J.-J.; Zheng, T.; Zhu, J.-J. A novel aptamer-based competition strategy for ultrasensitive electrochemical detection of leukemia cells. Analyst 2013, 138, 6323–6330.
- Zhang, D.; Zhang, Y.; Zheng, L.; Zhan, Y.; He, L. Graphene oxide/poly-l-lysine assembled layer for adhesion and electrochemical impedance detection of leukemia K562 cancercells. Biosens. Bioelectron. 2013, 42, 112–118.
- Rahmati, Z.; Roushani, M.; Hosseini, H. Hierarchical nickel hydroxide nanosheets grown on hollow nitrogen doped carbon nanoboxes as a high-performance surface substrate for alpha-fetoprotein cancer biomarkers electrochemical aptasensing. Talanta 2022, 237, 122924.




