1. Diagnosis of Differentiated Thyroid Cancers
Thyroid nodules are very common in the adult population, while the cancer rate among people with a thyroid nodule is rare. Accordingly, screening for thyroid cancer is not recommended
[1]. Ultrasound (US) is a first-line tool for the initial malignancy risk assessment
[2][3], and suspicious nodules are addressed with Fine Needle Aspiration (FNA). The main limitations of thyroid FNA, however, are “indeterminate” nodules. The rate of malignancy ranges from 10 to 30% in such cases, with histological examination a necessity to achieve the final diagnosis
[4]. Molecular imaging methods can fruitfully contribute to refining the preoperative diagnosis of indeterminate thyroid nodules
[5][6].
1.1. Molecular Imaging of Thyroid Nodules
Thyroid scintigraphy (TS), performed using either [
99mTc]Pertechnetate (Na[
99mTc]TcO4) or [
123I]Sodium-Iodide (Na[
123I]I) is the only method able to detect autonomously functioning thyroid nodules (AFTNs) and exclude malignancy with a 96–99% negative predictive value (NPV) even in the presence of low-normal TSH levels
[7]. Moreover, molecular imaging with [
99mTc]Tc-hexakis-(2-methoxy-2-isobutyl isonitrile ([
99mTc]Tc-MIBI) and [
18F]FDG may help to differentiate benign from malignant indeterminate nodules
[6][7].
1.1.1. [99mTc]Tc-MIBI Thyroid Scintigraphy
The [
99mTc]Tc-MIBI is a lipophilic cation able to cross the cell membrane. It penetrates into the cytoplasm in a reversible way and then irreversibly moves through the membrane of the mitochondria using a different electrical gradient regulated by a high negative inner membrane electric potential. Tumor cells are characterized by a higher negative inner membrane electric potential compared to normal cells. Consequently, it can be helpful to characterize the biological behavior of cytologically indeterminate nodules
[8]. A very high to absolute NPV was found in thyroid nodules with a [
99mTc]Tc-MIBI hypoactive pattern, while an increased [
99mTc]Tc-MIBI uptake conferred a significantly higher risk of cancer. However, while a NPV of >96 is achieved, the positive predictive value (PPV) is unsatisfactory as [
99mTc]Tc-MIBI uptake may also be recorded in benign nodules (especially follicular and oxyphilic adenomas)
[9][10]. Moreover, a semiquantitative approach to evaluate the tracer washout from the nodule (Washout Index (WOind)) was introduced, providing better results in comparison with qualitative analysis
[11].
1.1.2. [18F]FDG Positron Emission Tomography/Computed Tomography
[
18F]FDG uptake is related to an overexpression of the transmembrane glucose transporter proteins (GLUTs), which transport the tracer into the cell, and to the overactivation of hexokinases that phosphorylate [
18F]FDG to [
18F]FDG-6-phosphate and trap the tracer in the cell. Interestingly, a visually [
18F]FDG-negative indeterminate thyroid nodule has a negligible risk of malignancy, making [
18F]FDG PET/CT a suitable ruling-out test (as robustly demonstrated by meta-analyses
[12][13][14]). Moreover, [
18F]FDG PET/CT radiomics analysis preliminarily proved to increase specificity and PPV
[15].
Table 1 gives an overview of the different tracers and imaging methods to further evaluate thyroid nodules in a clinical setup.
Table 1. Molecular imaging to differentiate benign and malignant thyroid nodules.
| Method |
Tracer |
Indication |
Pattern |
Action |
| Scintigraphy |
Na[99mTc]TcO4 |
nodules-low TSH |
AFTNs |
Avoid FNA |
| Scintigraphy |
Na[123I]I |
nodules-low TSH |
AFTNs |
Avoid FNA |
| Scintigraphy |
[99mTc]Tc-MIBI |
nodules-CI |
[99mTc]Tc-MIBI- |
Avoid surgery |
| PET/CT |
[18F]FDG |
nodules-CI |
[18F]FDG- |
Avoid surgery |
Legend: PET/CT, positron emission tomography/computed tomography; [99mTc]Tc-MIBI, methoxy-isobutyl-isonitrile; [18F]FDG, fluorodeoxyglucose; TSH, thyrotropin; CI, cytologically indeterminate; AFTNs, autonomously functioning thyroid nodules; FNA, fine-needle aspiration.
2. Differentiated Thyroid Cancers
The current strategy for DTC management is a risk-stratified approach based on information from surgical histopathology and molecular markers, post-operative serum thyroglobulin (Tg) levels, and functional/anatomical imaging studies.
2.1. Surgical Treatment for DTC and Preoperative Staging
Traditionally, (near-) total thyroidectomy was performed in most DTC patients, even though the current American Thyroid Association (ATA) guidelines recommend lobectomy for patients with unifocal intrathyroidal low-risk DTC
[16]. Cervical lymph nodal metastases occur in 20–60% of patients with DTC, and central and/or lateral neck compartment dissection reduces the risk of local-regional recurrence. Prophylactic central neck dissection may improve regional control for invasive tumors (T3–T4), but it is discouraged for low-risk DTC because of potential associated morbidities are not justified by a significant clinical benefit
[16]. Preoperative neck US generally suffices to plan surgery, however additional cross-sectional imaging (i.e., contrast-enhanced computed tomography (ceCT), magnetic resonance imaging (MRI)) are reserved for patients with locally advanced disease or for those that are at a high risk of developing distant metastases
[17]. PET/CT with [
18F]FDG could be performed preoperatively in aggressive DTC and anaplastic thyroid cancer (see the specific section). Kim et al. retrospectively analyzed 60 patients with low- or intermediate-risk DTC who underwent [
18F]FDG PET/CT before thyroidectomy. They reported very low sensitivity and NPV in detecting lymph-node metastases (10% and 50%, respectively) but very high specificity (90%)
[18]. Some other studies compared the accuracy of PET with that of neck ultrasound and ceCT. The specificities of PET, ultrasonography, and ceCT in evaluating both the central and lateral neck regions were very high. However, the sensitivities were low (≤50%) for all three modalities. The overall diagnostic accuracy of [
18F]FDG PET/CT tended to be higher for lateral than for central lymph nodes
[19][20].
2.2. Postoperative 131I Therapy
Initial (near-)total thyroidectomy followed by
131I administration has remained the mainstay in achieving a cure in many DTC patients.
131I treatment of DTC is based on the principle of sodium iodide symporter (NIS) expressing thyroid cells with DTC cells having the ability of trapping circulating
131I. After surgery, the risk of structural disease recurrence and/or persistence is assessed using the three-tier (low, intermediate, high) stratification recommended by ATA
[16]. The goal of therapeutic
131I administration after total thyroidectomy is outlined based on standardized definitions as follows
[16][17].
-
Remnant ablation to eliminate normal thyroid tissue remnants in low risk patients, thereby ensuring undetectable or minimal serum Tg levels (in the absence of neoplastic tissue), which facilitates follow-up.
-
Adjuvant treatment to irradiate suspected but unproven sites of neoplastic cells in low-intermediate and intermediate risk patients, as determined by histopathologic features, thereby reducing the risk of disease recurrence.
-
Treatment of known disease to treat persistent or recurrent disease in patients with demonstrated metastatic disease.
Basically, two approaches to 131I therapy planning and delivery are adopted in clinical practice: (i) the approach based on clinical-pathologic factors and institutional protocols (i.e., risk-adapted approach), and (ii) the approach integrating postoperative radioiodine functional imaging (i.e., functional imaging-guided). No evidence data are available to allow recommending one strategy over the other and the local choice is based on local factors (i.e., surgery quality, laboratory and imaging availability, and multidisciplinary thyroid team), including physician–patient preferences.
2.3. The Role of Functional Imaging in Managing Radioiodine Therapy
Nuclear imaging of DTC takes advantage of the NIS-mediated uptake allowing characterization of iodine-avid versus noniodine-avid disease, as well as the localization and quantification of iodine-avid postoperative thyroid remnant.
2.3.1. Post-Therapy Whole-Body Scintigraphy (TxWBS)
When DTC patients receive
131I therapy based on clinical-pathological risk stratification a [
131I] post-treatment whole-body scintigraphy (TxWBS) is obtained 3–10 days after treatment. TxWBS is a highly sensitive and specific diagnostic tool useful to determine the location and extent of iodine-avid thyroid tissue. Consequently, it permits accurate disease restaging by detecting unknown loco-regional and/or distant metastases thus changing the initial risk stratification and customizing additional therapies and follow-up strategies. The diagnostic performance of TxWBS can be significantly improved using additional single-photon emission computed tomography/computed tomography (SPECT/CT). Compared to planar imaging, SPECT/CT images (i) reveal more foci of pathological radioiodine uptake located either in the cervical region or at distance (i.e., higher sensitivity), (ii) distinguish physiological uptakes from the foci of the disease (i.e., higher specificity), and (iii) detect metastatic lesions in unexpected sites
[21][22].
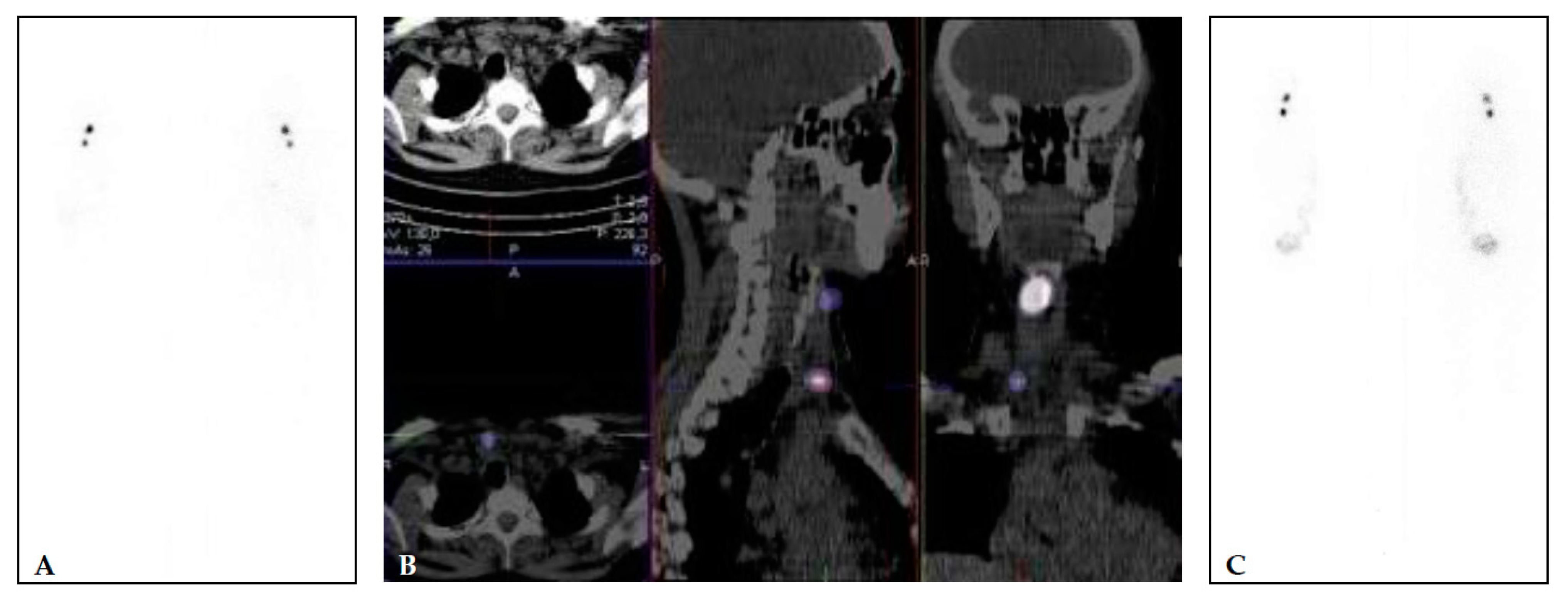
Figure 1 illustrates a patient that received both pre-ablational WBS with a diagnostic dose and its correlative after therapeutic dose (TxWBS).
Figure 1. (A,B) 40 years old female, with multifocal papillary microcarcinoma (left lobe 7 mm and 2 foci of 1 mm in the right lobe (pT1a(m)N0). TSH 67.83 mIU/L, Tg 11.83 μg/L (<2 μg/L), and TgA 2.2 kIU/L (<4.5 kIU/L). Diagnostic WBS and SPECT/CT (A,B) performed after application of 74 MBq of [131I] show two focuses of [131I] uptake—larger in the right upper part of the region VI and small focus lower in the region VI, right paratracheal. The patient was treated with 1665 MBq (45 mCi) of [131I]. Post-treatment [131I] WBS (C) shows an accumulation of the [131I] in the same two focuses.
2.3.2. Postoperative Diagnostic Whole-Body Scintigraphy (DxWBS)
When the functional-imaging guided approach is preferred by the local team, postoperative whole-body scintigraphy with diagnostic activities of different radioactive iodine isotopes ([
131I], [
123I], [
124I]) is performed. Theoretically, postoperative DxWBS leads to a significant improvement of risk stratification and staging of DTC patients and informs subsequent [
131I] therapy
[23]. As an example, visualization of metastatic lesions prompts risk re-stratification and, potentially, adjustment of [
131I] administered activity. Indeed, the suspicion of noniodine-avid disease (i.e., negative radioiodine WBS with elevated thyroglobulin (Tg) or Tg levels out of proportion to the WBS findings) may require additional studies (i.e., neck/chest computed tomography, CT; [
18F]FDG PET/CT). Finally, negative WBS results with undetectable Tg levels may rule out
131I therapy in low-risk DTC patients. Some authors, however, argue that TxWBS is more sensitive in identifying metastatic lesions (not initially seen on DxWBS), avoiding the so-called “stunning effect” of iodine-avid tissue
[24]. Notably, recent improvements in technology (i.e., SPECT/CT), image acquisition, and reconstruction protocols enabled the use of lower [
131I] activities
[25]. Accordingly, a systematic review of 14 original research articles describing the incremental value of [
131I] SPECT/CT demonstrated significant clinical benefit in terms of staging, risk stratification, and follow-up of DTC
[26].
Figure 2 and
Figure 3 show examples of [
131I] TxWBS in patients with lymphogenous and/or distant spread.
Figure 2. Post-treatment high-activity 131I whole-body scintigraphy. Large thyroid remnant with a small single iodine-avid lymph node metastasis (red arrows) at central neck compartment.
Figure 3. Post-treatment high-activity 131I whole-body scintigraphy. Multiple cervical and mediastinal iodine-avid metastatic lymph nodes and diffuse lung “miliariform” metastases.
In addition, [
123I] and/or [
124I] can be used instead of
131I to minimize the risk of stunning. Pre-ablation [
123I] WBS provided additional critical information in 25% of 122 patients, by revealing unsuspected regional or distant metastases and thus guiding the administration of higher
131I therapeutic activities, or revealing unexpected large thyroid remnants
[27].
Other authors also demonstrated that the information gained by [
123I] DxWBS changed the applied [
131I] activity in 49% of cases
[28]. Santhanam and colleagues, in a recent meta-analysis, described a 94% sensitivity of [
124I] PET/CT in postoperative detection of metastatic lesions amenable to RAI
[29]. In particular, it allows lesion-based evaluation of iodine uptake and clearance that is especially advisable to tailor [
131I] therapy in patients with advanced disease
[30][31]. Finally, new perspectives in the field are represented by NIS-imaging via [
18F]tetrafluoroborate ([
18F]TFB) and [
18F]fluorosulfate ([
18F]FSO3). The visualization of the expression of NIS through in vivo molecular imaging has been based for a number of years on diagnostic or post-therapeutic [
131I] WBS. The limitations of scintigraphic methods, including resolution and the possibility of target quantification, are well known. The technique of choice in the diagnostic setting could be [
124I] PET/CT, as it shows higher sensitivity compared to the [
131I] WBS, but also allows for dosimetric estimation in therapeutic cases. However, it is not easily available and for its intrinsic characteristic requires a long time to scan with (presently) suboptimal quality and resolution of images. Recently, new tracers such as [
18F]TFB or [
18F]FSO3 have shown promise as potential candidates for NIS visualization in preclinical studies and first preliminary human applications
[32]. From a technical point of view, the development of fluorinate tracers has the advantages of easier labeling, higher image quality, and high tumor to background contrast in animal and human studies. From a biological point of view [
18F]TFB and [
18F]FSO3 are iodine analogues, but with a main difference, the formers do not go into the iodine organification process in thyroid cells. These radiopharmaceuticals have been tested in healthy volunteers with promising biodistribution, in particular, low background uptake in the liver, muscle, and brain and high uptake in organs that normally express NIS (thyroid, salivary gland, and stomach)
[33]. Furthermore, a rapid blood clearance (20 min after injection) and stability up to 4 h after injection have been observed. In breast cancer mice models [
18F]TFB compared to [
123I] whole-body scan showed superior imaging characteristics related to a faster blood clearance, higher tumor/blood ratio, and higher sensitivity related to PET imaging
[34]. O’Doherty et al. evaluated [
18F]TFB uptake in 5 patients with intrathyroidal thyroid cancer while Samnik et al. compared [
18F]TFB to [
124I] PET in 9 patients after total thyroidectomy
[35][36]. In the first study, intra-thyroid tumor nodules showed lower uptake compared to normal thyroid tissue, while in the second the two tracers showed almost comparable performance. In only 2 patients, [
18F]TFB showed two more lesions compared to [
124I] PET and in all cases lower retention in thyroid tissue, probably due to the absence of the organification phase. Their applications in thyroid cancer patients still require further exploration and at this moment are under investigation in pilot studies (NCT03196518). Of note, any therapeutic [
131I] administration should be followed by a TxWBS to assess therapeutic [
131I] localization, which is routinely used together with a preablation Tg measurement, to complete post-operative staging and predict the patient’s outcome.
2.3.3. [18F]FDG Positron Emission Tomography/Computed Tomography (PET/CT)
Very promising results of [
18F]FDG PET/CT performed at the time of first postoperative staging were reported in patients with high-risk DTC and Hürthle cell and poorly differentiated histotypes. In particular, [
18F]FDG PET/CT might be very useful in correctly addressing disease aggressiveness and in detecting distant metastases (especially bone) in such cases
[37].
2.4. The Role of Functional Imaging in Response Assessment and Disease Monitoring
Thyroglobulin measurement and ultrasound are essential tools to monitor DTC after primary treatment
[16][17][38][39].
2.4.1. Radiodine Whole Body Scintigraphy
There is no consensus regarding the routine use of DxWBS during the follow-up of patients with DTC
[40]. Indeed, DxWBS remains a useful tool in selected patients with (i) higher risk of persistent/recurrent disease, (ii) extra-thyroid radioiodine uptake at TxWBS (i.e., loco-regional and/or distant metastases), (iii) poorly informative TxWBS (i.e., large remnants), or (iv) positive thyroglobulin antibody values. Gonzalez Carvalho and colleagues
[41] evaluated a very large cohort of DTC patients (
n = 1420) in all TNM categories in whom a life-long follow-up (up to 25 years) was regularly performed also using DxWBS. They concluded that in DTC patient follow-up, the use of DxWBS can still be justified at least until stimulated thyroglobulin is below functional sensitivity (in the absence of thyroglobulin antibodies) and DxWBS is negative (i.e., no evidence of radioiodine loco-regional and/or distant uptake). Importantly, the results of a large SEER database (28,220 patients diagnosed with DTC between 1998 and 2011) showed that follow-up DxWBS performed after primary treatment of DTC are the only imaging studies associated with improved disease-specific survival
[42].
2.4.2. Positron Emission Tomography
The main indication for [
18F]FDG PET/CT in DTC is during DTC follow-up in the case of high or increasing thyroglobulin levels, but negative or doubtful ultrasound, and negative diagnostic and post-therapeutic RAI imaging. In such a context, evidence supports the use of [
18F]FDG PET/CT, with reported pooled sensitivity and specificity of 80% and 90%, respectively
[43][44][45].
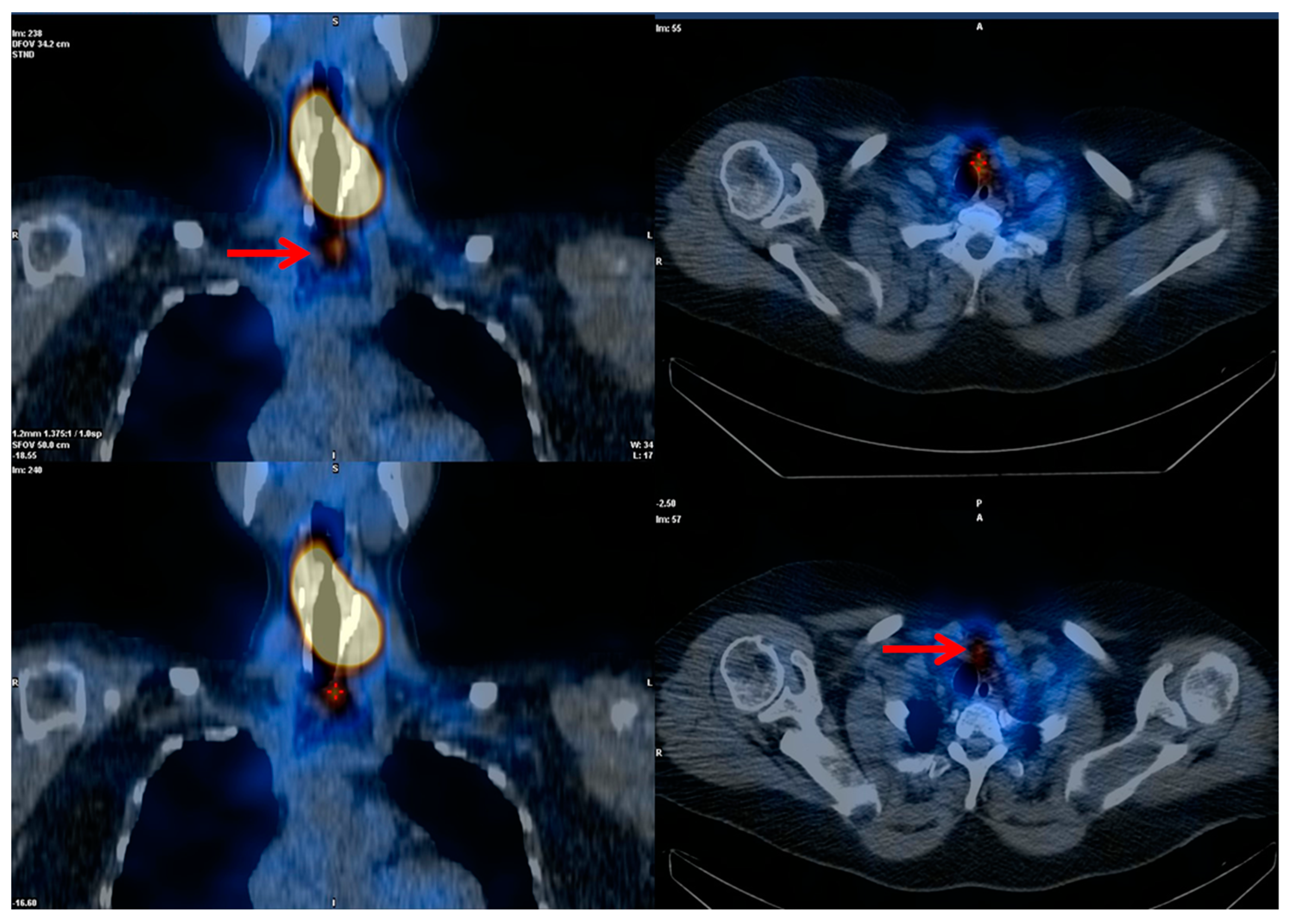
Figure 4 demonstrates a patient with this flip flop phenomenon (i.e., WBS negative and FDG positive). The current ATA guidelines suggest that [
18F]FDG PET/CT should be performed when stimulated thyroglobulin levels are >10 ng/mL. Further, although the positivity rate of [
18F]FDG PET/CT increases with higher serum thyroglobulin level, true-positive results have also been reported in 10–20% of DTC patients with serum thyroglobulin levels <10 ng/mL
[46][47]. Giovanella et al. suggested that shortened thyroglobulin doubling time (i.e., <1 year) independently predicted a positive [
18F]FDG PET/CT scan in patients with biochemical recurrence
[48]. Considering the relatively low sensitivity of diagnostic
131I WBS, one of the most important issues is the comparison of post-therapeutic
131I WBS with [
18F]FDG PET/CT. Leboulleux et al. evaluated the sensitivity of post-therapeutic [
131I] WBS versus [
18F]FDG PET/CT in patients with elevated serum thyroglobulin levels. The sensitivity in detecting DTC relapse was 88% for [
18F]FDG PET/CT and 16% for TxWBS (
p < 0.01), respectively. [
18F]FDG-PET/CT was abnormal in 22 patients, five of whom also had an abnormal TxWBS. Authors concluded that in patients with suspicion of recurrence based on thyroglobulin levels after a normal TxWBS, [
18F]FDG PET/CT is better able to localize disease than TxWBS
[49]. Similarly, Kim et al. reported that second empiric
131I therapy and TxWBS were neither diagnostically nor therapeutically useful in 39 patients with elevated stimulated thyroglobulin and negative [
18F]FDG PET/CT after initial treatment. These data suggest that the correct integration of radioiodine imaging and [
18F]FDG PET/CT may optimize additional administrations of high [
131I] activities and inform alternative strategies (i.e., surgical procedures or external beam radiotherapy)
[50].
Figure 4. DTC patient with increasing thyroglobulin and negative diagnostic whole-body scan after thyroidectomy and radioiodine therapy. [18F]FDG-avid relapsing disease.