
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rita Azevedo | -- | 7231 | 2022-04-13 10:00:52 | | | |
| 2 | Rita Azevedo | + 22 word(s) | 7253 | 2022-04-13 10:01:43 | | | | |
| 3 | Rita Azevedo | -5233 word(s) | 2020 | 2022-04-13 10:21:51 | | |
Video Upload Options
Neurodegenerative diseases are incurable, heterogeneous, and age-dependent disorders that challenge modern medicine. A deeper understanding of the pathogenesis underlying neurodegenerative diseases is necessary to solve the unmet need for new diagnostic biomarkers and disease-modifying therapy and reduce these diseases’ burden. Specifically, post-translational modifications (PTMs) play a significant role in neurodegeneration. Due to its proximity to the brain parenchyma, cerebrospinal fluid (CSF) has long been used as an indirect way to measure changes in the brain. Mass spectrometry (MS) analysis in neurodegenerative diseases focusing on PTMs and in the context of biomarker discovery has improved and opened venues for analyzing more complex matrices such as brain tissue and blood. Notably, phosphorylated tau protein, truncated α-synuclein, APP and TDP-43, and many other modifications were extensively characterized by MS. Great potential is underlying specific pathological PTM-signatures for clinical application. This review focuses on PTM-modified proteins involved in neurodegenerative diseases and highlights the most important and recent breakthroughs in MS-based biomarker discovery.
1. Introduction
2. The Next Step in Neuroproteomics
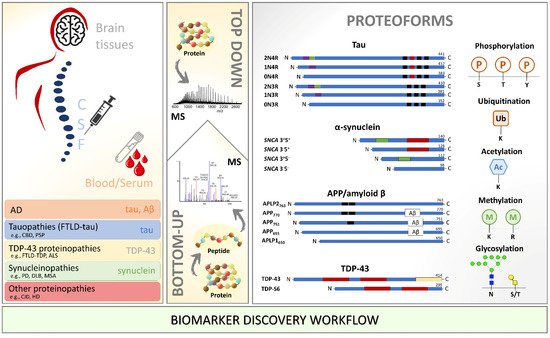
3. Concluding Remarks
References
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125.
- Armstrong, R. What Causes Neurodegenerative Disease? Folia Neuropathol. 2020, 58, 93–112.
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The Probabilistic Model of Alzheimer Disease: The Amyloid Hypothesis Revised. Nat. Rev. Neurosci. 2022, 23, 53–66.
- Poddar, M.K.; Chakraborty, A.; Banerjee, S. Neurodegeneration: Diagnosis, Prevention, and Therapy; IntechOpen: London, UK, 2021; ISBN 978-1-83880-901-0.
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect. Biol. 2018, 10, a033118.
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical Diagnosis of Alzheimer’s Disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496.
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria. Lancet Neurol. 2014, 13, 614–629.
- Oudart, J.-B.; Djerada, Z.; Nonnonhou, V.; Badr, S.; Bertholon, L.-A.; Dammak, A.; Jaidi, Y.; Novella, J.-L.; Pallet, N.; Gillery, P.; et al. Incremental Value of CSF Biomarkers in Clinically Diagnosed AD and Non-AD Dementia. Front. Neurol. 2020, 11, 560.
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and Blood Biomarkers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Lancet Neurol. 2016, 15, 673–684.
- Abeliovich, A.; Gitler, A.D. Defects in Trafficking Bridge Parkinson’s Disease Pathology and Genetics. Nature 2016, 539, 207–216.
- Canter, R.G.; Penney, J.; Tsai, L.-H. The Road to Restoring Neural Circuits for the Treatment of Alzheimer’s Disease. Nature 2016, 539, 187–196.
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206.
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186.
- Heemels, M.-T. Neurodegenerative Diseases. Nature 2016, 539, 179.
- Perry, D.C.; Brown, J.A.; Possin, K.L.; Datta, S.; Trujillo, A.; Radke, A.; Karydas, A.; Kornak, J.; Sias, A.C.; Rabinovici, G.D.; et al. Clinicopathological Correlations in Behavioural Variant Frontotemporal Dementia. Brain 2017, 140, 3329–3345.
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative Disease Concomitant Proteinopathies Are Prevalent, Age-Related and APOE4-Associated. Brain 2018, 141, 2181–2193.
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of Biomarkers across Different Neurodegenerative. Alzheimers Res. 2020, 12, 56.
- Marsh, A.P. Molecular Mechanisms of Proteinopathies across Neurodegenerative Disease: A Review. Neurol. Res. Pract. 2019, 1, 35.
- Dovidchenko, N.V.; Leonova, E.I.; Galzitskaya, O.V. Mechanisms of Amyloid Fibril Formation. Biochemistry 2014, 79, 1515–1527.
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A. The Role of Protein Misfolding and Tau Oligomers (TauOs) in Alzheimer’s Disease (AD). Int. J. Mol. Sci. 2019, 20, E4661.
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb Perspect. Med. 2012, 2, a009399.
- Bu, L.-L.; Huang, K.-X.; Zheng, D.-Z.; Lin, D.-Y.; Chen, Y.; Jing, X.-N.; Liang, Y.-R.; Tao, E.-X. Alpha-Synuclein Accumulation and Its Phosphorylation in the Enteric Nervous System of Patients Without Neurodegeneration: An Explorative Study. Front. Aging Neurosci. 2020, 12, 575481.
- Hansson, O. Biomarkers for Neurodegenerative Diseases. Nat. Med. 2021, 27, 954–963.
- Barthélemy, N.R.; Bateman, R.J.; Hirtz, C.; Marin, P.; Becher, F.; Sato, C.; Gabelle, A.; Lehmann, S. Cerebrospinal Fluid Phospho-Tau T217 Outperforms T181 as a Biomarker for the Differential Diagnosis of Alzheimer’s Disease and PET Amyloid-Positive Patient Identification. Alzheimers Res. 2020, 12, 26.
- Obrocki, P.; Khatun, A.; Ness, D.; Senkevich, K.; Hanrieder, J.; Capraro, F.; Mattsson, N.; Andreasson, U.; Portelius, E.; Ashton, N.J.; et al. Perspectives in Fluid Biomarkers in Neurodegeneration from the 2019 Biomarkers in Neurodegenerative Diseases Course-a Joint PhD Student Course at University College London and University of Gothenburg. Alzheimers Res. 2020, 12, 20.
- Smith, L.M.; Kelleher, N.L. Proteoform: A Single Term Describing Protein Complexity. Nat. Methods 2013, 10, 186–187.
- Martin, L.; Latypova, X.; Terro, F. Post-Translational Modifications of Tau Protein: Implications for Alzheimer’s Disease. Neurochem. Int. 2011, 58, 458–471.
- Olzscha, H. Posttranslational Modifications and Proteinopathies: How Guardians of the Proteome Are Defeated. Biol. Chem. 2019, 400, 895–915.
- Ramesh, M.; Gopinath, P.; Govindaraju, T. Role of Post-Translational Modifications in Alzheimer’s Disease. Chembiochem 2020, 21, 1052–1079.
- Ping, L.; Duong, D.M.; Yin, L.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Global Quantitative Analysis of the Human Brain Proteome in Alzheimer’s and Parkinson’s Disease. Sci. Data 2018, 5, 180036.
- Ping, L.; Kundinger, S.R.; Duong, D.M.; Yin, L.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Global Quantitative Analysis of the Human Brain Proteome and Phosphoproteome in Alzheimer’s Disease. Sci. Data 2020, 7, 315.
- Abreha, M.H.; Dammer, E.B.; Ping, L.; Zhang, T.; Duong, D.M.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Quantitative Analysis of the Brain Ubiquitylome in Alzheimer’s Disease. Proteomics 2018, 18, e1800108.
- Yu, L.; Huo, Z.; Yang, J.; Palma-Gudiel, H.; Boyle, P.A.; Schneider, J.A.; Bennett, D.A.; Zhao, J. Human Brain and Blood N-Glycome Profiling in Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias. Front. Aging Neurosci. 2021, 13, 765259.
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated Tau Interactome in the Human Alzheimer’s Disease Brain. Brain 2020, 143, 2803–2817.
- Crutchfield, C.A.; Thomas, S.N.; Sokoll, L.J.; Chan, D.W. Advances in Mass Spectrometry-Based Clinical Biomarker Discovery. Clin. Proteom. 2016, 13, 1.
- Huang, J.; Wang, F.; Ye, M.; Zou, H. Enrichment and Separation Techniques for Large-Scale Proteomics Analysis of the Protein Post-Translational Modifications. J. Chromatogr. A. 2014, 1372, 1–17.
- Lim, H.; Eng, J.; Yates, J.R.; Tollaksen, S.L.; Giometti, C.S.; Holden, J.F.; Adams, M.W.W.; Reich, C.I.; Olsen, G.J.; Hays, L.G. Identification of 2D-Gel Proteins: A Comparison of MALDI/TOF Peptide Mass Mapping to Mu LC-ESI Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom 2003, 14, 957–970.
- Mertins, P.; Tang, L.C.; Krug, K.; Clark, D.J.; Gritsenko, M.A.; Chen, L.; Clauser, K.R.; Clauss, T.R.; Shah, P.; Gillette, M.A.; et al. Reproducible Workflow for Multiplexed Deep-Scale Proteome and Phosphoproteome Analysis of Tumor Tissues by Liquid Chromatography-Mass Spectrometry. Nat. Protoc. 2018, 13, 1632–1661.
- Prus, G.; Hoegl, A.; Weinert, B.T.; Choudhary, C. Analysis and Interpretation of Protein Post-Translational Modification Site Stoichiometry. Trends Biochem. Sci. 2019, 44, 943–960.
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk With Proteomics. Mol. Cell Proteom. 2021, 20, 100129.
- Siuti, N.; Kelleher, N.L. Decoding Protein Modifications Using Top-down Mass Spectrometry. Nat. Methods 2007, 4, 817–821.
- Leney, A.C.; Heck, A.J.R. Native Mass Spectrometry: What Is in the Name? J. Am. Soc. Mass Spectrom 2017, 28, 5–13.
- Ruffini, N.; Klingenberg, S.; Schweiger, S.; Gerber, S. Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale. Cells 2020, 9, 2642.
- Simithy, J.; Sidoli, S.; Garcia, B.A. Integrating Proteomics and Targeted Metabolomics to Understand Global Changes in Histone Modifications. Proteomics 2018, 18, e1700309.




